Abstract
Myoinositol is an isomer of glucose that has chemopreventive activity in animal models of cancer. In a recent phase I clinical trial, myoinositol administration correlated with a statistically significant regression of preexisting bronchial dysplastic lesions in heavy smokers. To shed light on the potential mechanisms involved, activation of Akt and extracellular signal-regulated kinase (ERK), two kinases that control cellular proliferation and survival, was assessed in 206 paired bronchial biopsies from 21 patients who participated in this clinical trial. Before myoinositol treatment, strongly positive staining for activation of Akt was detected in 27% of hyperplastic/metaplastic lesions and 58% of dysplastic lesions (P = 0.05, χ2 test). There was also a trend toward increased activation of ERK (28% in regions of hyperplasia/metaplasia to 42% of dysplastic lesions). Following myoinositol treatment, significant decreases in Akt and ERK phosphorylation were observed in dysplastic (P < 0.01 and 0.05, respectively) but not hyperplastic/metaplastic lesions (P > 0.05). In vitro, myoinositol decreased endogenous and tobacco carcinogen–induced activation of Akt and ERK in immortalized human bronchial epithelial cells, which decreased cell proliferation and induced a G1-S cell cycle arrest. These results show that the phenotypic progression of premalignant bronchial lesions from smokers correlates with increased activation of Akt and ERK and that these kinases are targets of myoinositol. Moreover, they suggest that myoinositol might cause regression of bronchial dysplastic lesions through inhibition of active Akt and ERK.
Lung cancer is the leading cause of cancer mortality in the United States (1) and tobacco smoking is the greatest risk factor (2). Because current treatments for advanced disease are only palliative, prevention becomes an important strategy to mitigate the death and suffering due to lung cancer, particularly in high-risk populations of current and former smokers. The phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin pathway is an important signal transduction pathway that controls processes integral in the development of cancer, including protein translation, growth, metabolism, and survival. The clinical importance of Akt activation in lung cancer has been shown by showing it is detected in human bronchial dysplastic lesions and early-stage non–small cell lung cancer (NSCLC) specimens and confers a poor prognosis (3, 4). Akt is activated following activation of class I PI3K. The products of PI3K bind to the pleckstrin homology domain of Akt and induce its translocation to the plasma membrane, where it is phosphorylated on T308 by PDK-1 and on S473 by the rictor/mammalian target of rapamycin TORC2 complex as well as possibly by other proteins. Phosphorylation of both T308 and S473 is required for full kinase activity. Our previous studies have shown that evaluation of both sites of phosphorylation improves the prognostic value of Akt activation and that T308 alone was valuable and sufficient to indicate a poor prognosis for patients with stage I disease or with tumors ≤5 cm (4).
Tobacco components, such as nicotine or the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), activate the PI3K/Akt pathway in normal human bronchial and alveolar epithelial and NSCLC cells, which increases Akt-dependent proliferation and survival (5, 6). Nicotine or NNK can also lead to phosphorylation and activation of extracellular signal-regulated kinase (ERK) 1/2, a widely conserved member of the mitogen-activated protein kinase family (7–9). Activation of ERK1/2 is associated with advanced NSCLC tumors (10) and can lead to phosphorylation of a variety of nuclear substrates that are associated with proliferation and can result in malignant transformation both in vitro and in vivo (11, 12). These studies suggest that activation of Akt and ERK by tobacco carcinogens might contribute to the development of lung cancer in smokers and that activated Akt and/or ERK could have value as targets for lung cancer prevention.
Although inhibitors of the PI3K/Akt pathway or mitogen-activated protein/ERK kinase (MEK)/ERK pathway are being developed as cancer therapeutics, toxicity profiles are likely to make them less attractive for chemoprevention than agents such as myoinositol that are present in the human diet. Myoinositol is equivalent to the head group of phosphatidylinositol, the basic substrate for PI3K, and its structure is similar to D-3-deoxy-3-fluoro-PtdIns, a known PI3K inhibitor. It has been reported that blocking both PI3K and ERK is involved in inositol-mediated anticancer activity (13–15). Moreover, inositol hexaphosphate (IP6) can directly target PI3K in an in vitro assay (15, 16). Myoinositol inhibits tobacco carcinogen–induced lung tumorigenesis in A/J mice and is one of the few agents that can suppress tumorigenesis when administered either before or after the carcinogen (17). This compound and its phosphorylated derivatives have little toxicity in animal models (18). A recent phase I clinical trial showed that myoinositol was well tolerated and possibly effective in causing regression of preexisting bronchial dysplastic lesions in heavy smokers (19). However, the molecular mechanisms responsible for this effect are still unclear. Because of the promising results obtained in this trial, we hypothesized that myoinositol may have targeted Akt and/or ERK in the bronchial epithelium of the heavy smokers in this trial, which may have contributed to the observed regression of bronchial dysplastic lesions.
Materials and Methods
Tissue specimens
Two hundred and six paired bronchial biopsies acquired before or after myoinositol treatment were obtained from 21 smokers with smoking histories of ≥30 pack-years who enrolled in a phase I clinical trial conducted by the British Columbia Cancer Agency and the University of British Columbia, Canada (19). The details of the study population, treatment response, and toxicity in the myoinositol phase I trial were reported elsewhere (19). Briefly, current and former smokers between 40 and 74 y of age, with a ≥30 pack-year smoking history, and one or more sites of bronchial dysplasia, were enrolled in the phase I study of myoinositol as an agent for lung cancer chemoprevention. Based on the order of the entry to the study, participants received in a dose escalation schema one of the four dose levels (12, 18, 24, or 30 g) of myoinositol mixed with juice or water, divided into two doses daily. The current study was reviewed and approved by the National Cancer Institute Institutional Review Board.
Immunohistochemistry
Immunohistochemical analysis of phosphorylated Akt (P-Akt) and phosphorylated ERK (P-ERK) was done using phospho-specific antibodies (P-Akt Thr308, P-ERK1/ERK2 Thr202/Tyr204; Cell Signaling, Inc.). The tissue slides were deparaffinized in xylenes and rehydrated in ethanol to water. Antigen retrieval was done using Dako target retrieval solution in a rice cooker with boiling water. Immunohistochemical staining was done using a Vectastain avidin-biotin complex method kit according to the manufacturer’s instructions (Vector Laboratories). Primary antibodies (1:100 dilution) were incubated at 4°C overnight. 3,3′-Diaminobenzidine (Sigma Chemical Co.) was used as the chromogen.
Scoring and statistical analysis
Entire bronchial epithelial lesions were scored blinded to the treatment arm or outcome. The intensity of staining was scored using the following scale: 0, negative; 1, weak; 2, moderate; 3, strong staining. A staining index for each biopsy was achieved by summing the products of the fraction of positive cells from each intensity category and then multiplying this by the intensity. A staining index >2 is defined as strongly positive, a staining index >1.5 is considered moderately positive, and a staining index <1.5 is regarded as negative. Each patient’s score is the average staining index from all of the biopsies analyzed. The χ2 test was used to test the statistical significance of the association between P-Akt or P-ERK expression in hyperplastic/metaplastic and dysplastic lesions. Immunohistochemical scoring was reviewed by two independent blinded researchers from two different laboratories who agreed. P-Akt and P-ERK levels were compared before and after myoinositol treatment using a paired, two-sided t test.
In vitro assays
Immortalized human bronchial epithelial cells (HBEC) and HBEC stably transfected with mutant K-ras [HBEC(R)] were a gift from Dr. John D. Minna (University of Texas Southwestern Medical Center, Dallas, TX) and were cultured in keratinocyte serum-free medium containing 50 μg/mL bovine pituitary extract and 5 ng/mL epidermal growth factor (Invitrogen) at 37°C in a 5% CO2 atmosphere (20, 21). The H322 NSCLC cell line was obtained from Navy Medical Oncology (Bethesda, MD) and was cultured in RPMI 1640 + 10% fetal bovine serum. For studies of signaling pathways, HBECs were preincubated with 5 mmol/L myoinositol for 72 h before stimulation with 1 μmol/L nicotine. LY294002 and U0126 were purchased from Calbiochem/EMD. Activated protein kinases were detected by immunoblotting using phospho-specific antibodies to P-Thr308 Akt and P-Thr202/Tyr204 ERK1/ERK2. Antibodies against P-Akt and total Akt and ERK were from Cell Signaling. For cell proliferation studies, HBECs (5 × 104) were seeded onto 12-well plates and incubated with myoinositol for 72 h. Cell proliferation was measured by Coulter counter (Beckman Coulter Corp.) and flow cytometry using a Becton Dickinson FACSort (BD Biosciences). Flow cytometry was done as previously described (22). Proliferation and immunoblot experiments were repeated thrice with reproducible results.
Results
Expression of active Akt and ERK in bronchial tissues
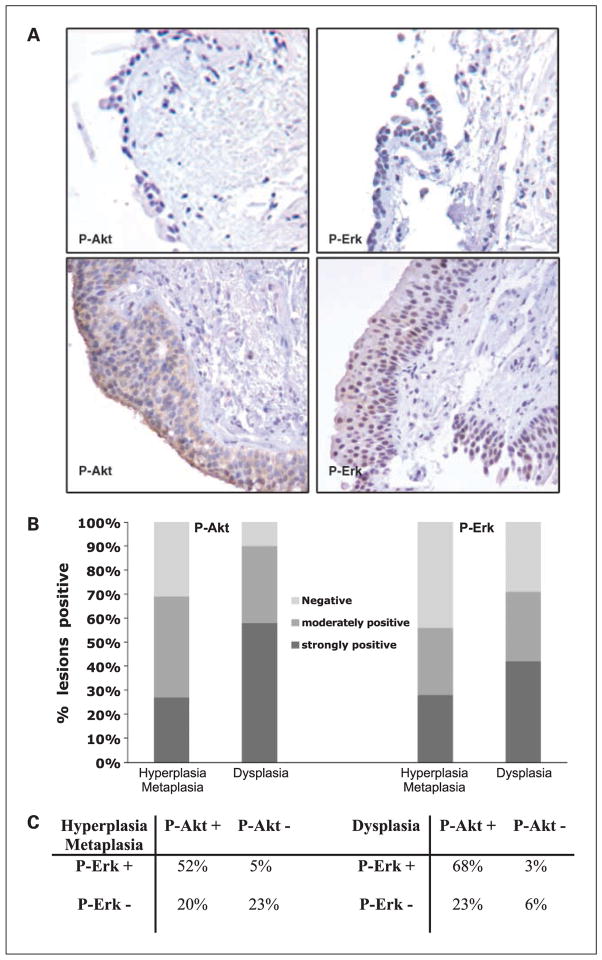
Two hundred and six bronchial biopsies from 21 smokers that participated in the myoinositol phase I clinical trial were composed of 103 paired, pre- and post-myoinositol samples that included 5 normal specimens, 67 specimens displaying hyper-plasia/metaplasia, and 31 dysplastic specimens (19). Baseline levels of P-Akt and P-ERK were assessed in the pretreatment samples by immunohistochemistry. Staining was barely evident in normal specimens (Fig. 1A, top). In dysplastic specimens, P-Akt displayed nuclear and cytoplasmic expression patterns (Fig. 1A, bottom left), and P-ERK showed mainly nuclear localization and less cytoplasmic expression (Fig. 1A, bottom right). Active Akt was detected in 90% of the dysplastic specimens, with 58% staining strongly positive and 32% staining moderately positive (Fig. 1B). Active Akt was also observed in 71% of the hyperplastic/metaplastic specimens, including 27% that were strongly positive and 44% that were moderately positive. The increased frequency of strong P-Akt expression from hyperplasia/metaplasia to dysplasia was statistically significant (P = 0.05, χ2 test; Fig. 1B, left). P-ERK displayed the same trend of increased expression from hyperplasia/metaplasia (56%) to dysplasia (71%); however, this increase did not reach statistical significance (P = 0.06, χ2 test). All normal bronchial epithelial specimens displayed either weakly positive or negative expression for P-Akt and P-ERK (data not shown). The expression of active Akt and ERK was coordinate in that 52% of hyperplastic/metaplastic lesions and 68% of the dysplastic lesions showed positive staining for both P-Akt and P-ERK (Fig. 1C).
Fig. 1.
Immunohistochemical analysis of bronchial biopsies from smokers (including 31 dysplastic and 67 hyperplastic/metaplastic sites). A, photomicrographs showing representative immunohistochemical staining for P-Akt (T308) and P-ERK (T202/Y204) in normal lung (top) and dysplastic lesions (bottom). B, percentage of lesions positive for P-Akt (T308) and P-ERK (T202/Y204). C, association between activated Akt and ERK in hyperplastic/metaplastic and dysplastic lesions.
Effect of myoinositol on activation of Akt and ERK in bronchial tissues
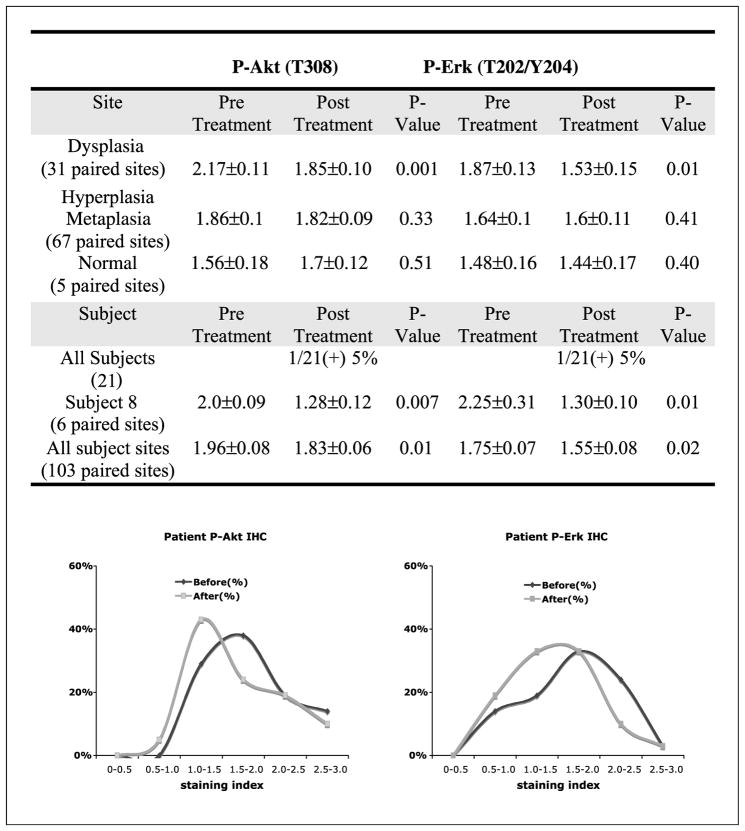
To assess if myoinositol administration could modulate levels of active Akt and ERK in the bronchial epithelium of heavy smokers, levels of P-Akt and P-ERK were assessed in both pretreatment and posttreatment bronchial biopsies by immunohistochemistry. Lesions were scored and P-Akt or P-ERK staining indices were compared in paired, pretreatment, and posttreatment samples in a site-specific manner. Following myoinositol treatment, there was a significant decrease in both P-Akt and P-ERK expression levels in dysplastic lesions (P = 0.001 and 0.01, respectively) but not in hyperplastic/metaplastic lesions (P > 0.05; Fig. 2). There were only five normal specimens available for immunohistochemistry. During the study, these normal sites progressed to hyper-plastic/metaplastic lesions. Following myoinositol, two of five increased P-Akt and one of five increased P-ERK staining, but these changes were not statistically significant. When all pre-treatment and posttreatment sites within a given individual were grouped together, only 1 of 21 subjects (5%) showed a decrease in both Akt and ERK phosphorylation. This patient (subject 8) had a 48% decrease in Akt phosphorylation and a 43% decrease in ERK phosphorylation. Comparing all pre-treatment and posttreatment biopsies from all patients, levels of P-Akt and P-ERK were significantly decreased (P = 0.01 and 0.02, respectively) following myoinositol treatment (Fig. 2, bottom). Interestingly, there was no relationship between dose of myoinositol and inhibition of Akt or ERK. These data show that myoinositol decreased Akt and ERK phosphorylation to the greatest extent in dysplastic lesions.
Fig. 2.
Effect of myoinositol on the levels of P-Akt and P-ERK in bronchial lesions analyzed with immunohistochemistry by per-site and subject analyses. Total baseline bronchial sites (103) include 31 dysplastic sites, 67 hyper/metaplastic sites, and 5 normal sites. Top, the staining index was expressed as mean ± SE; bottom, changes in staining indices for P-Akt and P-ERK from all pretreatment and posttreatment bronchial epithelial sites.
Myoinositol decreases P-Akt and P-ERK expression and inhibits proliferation in HBECs in vitro
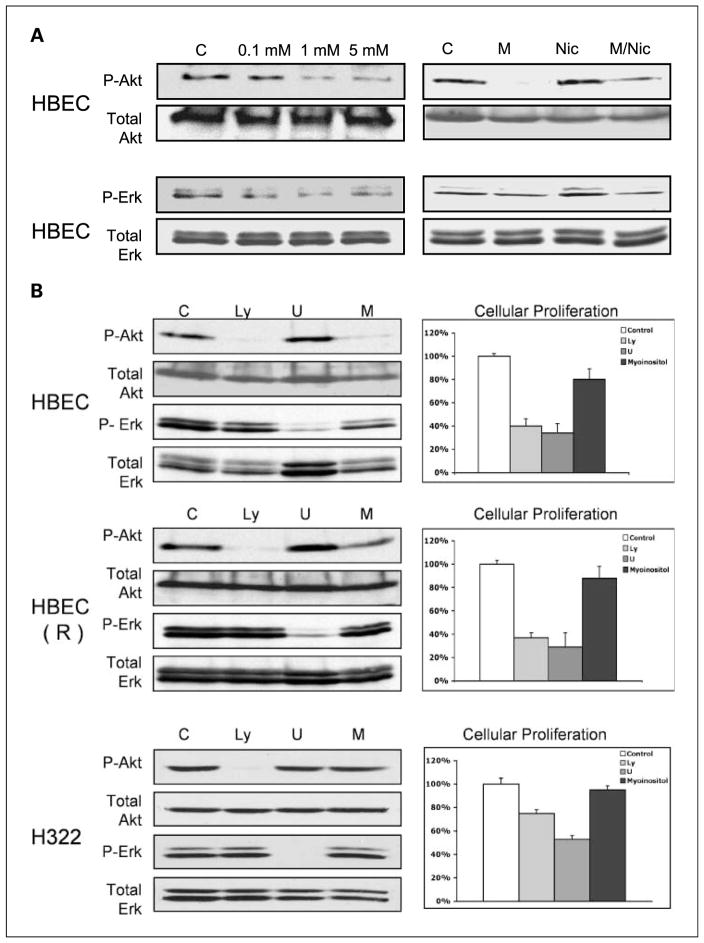
To further assess the relationship between myoinositol, activation of Akt and ERK, and cellular proliferation, a series of in vitro studies were done with HBEC and HBEC(R) and a human lung cancer cell line (H322). Myoinositol decreased endogenous levels of Akt and ERK phosphorylation in HBECs in a dose-dependent manner (Fig. 3A, top left). Maximal inhibition occurred at 1 mmol/L. Because the phase I trial of myoinositol was done in subjects with heavy smoking histories, we assessed whether myoinositol would inhibit activation of Akt or ERK after nicotine exposure. HBECs were pretreated with myoinositol and exposed to nicotine. Myoinositol decreased basal levels of P-Akt and P-ERK. Nicotine only modestly increased levels of P-Akt and P-ERK in HBECs. In the presence of myoinositol, levels of Akt and ERK phosphorylation induced by nicotine were less than that observed in the absence of myoinositol (Fig. 3A, top right). Inhibitors of Akt activation (LY294002) or ERK activation (U0126) were used to correlate inhibition of these signaling pathways with cellular proliferation (Fig. 3B). In HBEC, these inhibitors decreased phosphorylation of their intended targets and decreased proliferation by >60%. Myoinositol was less effective in decreasing Akt and ERK phosphorylation and only inhibited cellular proliferation by 24%. Inhibition of proliferation was associated with induction of a G1 cell cycle arrest (data not shown). Similar results were observed with HBEC(R), except that myoinositol did not inhibit Akt or ERK phosphorylation as well as LY294002 or U0126, respectively. In fully transformed H322 cells, LY294002 and U0126 effectively inhibited activation of Akt and ERK but inhibited proliferation by only 22% and 48%, respectively. Myoinositol did not inhibit Akt or ERK and did not inhibit proliferation. These studies establish a link between inhibition of Akt and ERK and cellular proliferation in immortalized but not transformed cells and support the selective activity of myoinositol toward premalignant lesions.
Fig. 3.
Effect of myoinositol on endogenous and nicotine-induced P-Akt and P-ERK. A, top left, dose-dependent inhibition of Akt and ERK phosphorylation by myoinositol in immortalized HBECs, as assessed by immunoblotting; top right, effect of myoinositol pretreatment on nicotine-induced P-Akt and P-ERK in HBEC analyzed by immunoblotting. B, a PI3K inhibitor, a MEK inhibitor, and myoinositol all reduce proliferation in immortalized HBEC and HBEC(R) cells, but not in fully transformed H322 cells. HBEC, HBEC(R), or H322 cells were incubated with 15 μmol/L LY294002, 15 μmol/L U0126, or 5 mmol/L myoinositol. Left, immunoblots showing the effects of the compounds after 2 h; right, effect of compounds on cell proliferation after 72 h. Cell proliferation was normalized to vehicle-treated cells. The experiments were repeated thrice with triplicate samples.
Discussion
In the present study, we were able to successfully do immunohistochemistry on small bronchial biopsies and show that increased Akt and ERK activation is characteristic of premalignant lesions in smokers. The progression of hyper-plastic or metaplastic lesions to dysplastic lesions was associated with increased activation of Akt and possibly ERK. Myoinositol, which had previously been shown to be effective in reducing the number of dysplastic lesions, reduced the levels of P-Akt and P-ERK in these lesions. These clinical data correlated with in vitro studies that showed that myoinositol inhibited endogenous and nicotine-induced activation of Akt and ERK in immortalized HBECs and decreased cellular proliferation.
How could smoking be related to increased activation of Akt and ERK in bronchial lesions? Tobacco components can activate Akt and ERK through nicotinic acetylcholine receptors (5, 23). Exposure to tobacco smoke or tobacco carcinogens can also lead to lung tumorigenesis primarily by inducing K-Ras mutations (24, 25). Activating K-Ras mutations stimulate the PI3K/Akt and MEK/ERK pathways (26). Although the K-Ras status of these lesions is not clear, we found positive P-Akt (T308) expression in a high percentage (90%) of bronchial dysplastic lesions from heavy smokers. This is the first report of high P-Akt (T308) expression in premalignant bronchial lesions as well as an increased frequency of strong P-Akt (T308) expression in the transition from hyperplastic/metaplastic lesions to dysplastic lesions (P = 0.05, χ2 test). Our data measuring phosphorylation of Akt at T308 correlate well with a report that showed that 88% of dysplastic lesions from smokers showed activation of Akt as assessed by S473 phosphorylation (3). Although technically more difficult to measure, phosphorylation of T308 may be more valuable as a single marker for Akt activation than S473. For example, cells that lack PDK-1 (the kinase that phosphorylates T308) that are stimulated with insulin have normal levels of S473 phosphorylation but no T308 phosphorylation and no kinase activity (27). Thus, evaluation of S473 phosphorylation alone may overestimate Akt activation. Our group previously observed that phosphorylation of T308 alone is associated with a worse prognosis for stage I NSCLC patients or tumors ≤5 cm (4). The current study shows that enhanced T308 phosphorylation is an early event in tobacco-related lung cancer development, and the feasibility of detecting T308 phosphorylation in small bronchial biopsies highlights the potential of T308 phosphorylation as a biomarker for lung cancer prevention studies.
In addition to Akt activation, ERK activation was observed in 71% of the dysplastic lesions. Activation of ERK by nicotine can inhibit apoptosis of lung cancer cells (28), and the fact that ERK activation was observed in premalignant lesions suggests that it may play a crucial role in lung cancer progression by inhibiting apoptosis of damaged pre-malignant cells that could ultimately undergo full transformation. Our data support the hypothesis that dual inhibition of Akt and ERK might have greater potential than targeting either kinase alone.
Are the concentrations of myoinositol used in vitro relevant to the levels of myoinositol achieved in humans? We observed that myoinositol inhibited Akt and ERK phosphorylation at relatively high concentrations (≥1 mmol/L). However, myoinositol is well tolerated in humans, and an average North American diet provides 1 g of mixed inositol daily (29). In the phase I clinical trial, subjects took doses of 12, 18, 24, or 30 g of myoinositol for 1 month, and subjects without grade ≥2 toxicity were allowed to dose escalate once for an additional month. Once the maximum tolerated dose was established, 10 new participants received the maximum tolerated dose of 18 g of oral myoinositol daily for 3 months. Despite these high doses of myoinositol administration, myoinositol levels were not measured in this phase I study, so a direct correlation between levels in the clinical trial and our in vitro studies cannot be made. However, pharmacokinetic analysis using lower doses of myoinositol has been done by Groenen et al. (30). In women, blood plasma levels of 100 μmol/L were achieved after a single oral dose of 100 mg/kg myoinositol (for a 150-lb subject, this equates to an oral dose of 6.8 g). Furthermore, it has been reported that in most mammalian cells or tissues, the content of myoinositol is 5- to 500-fold higher than the level in plasma due to an effective, active transport system for myoinositol (31). Because the phase I trial with heavy smokers used myoinositol at doses 2- to 3-fold higher than that used by Groenen et al. for 2 to 3 months (during which time accumulation may have occurred), it is plausible that the concentrations of myoinositol used in vitro are relevant to that achieved in humans.
Myoinositol was most effective in decreasing Akt and ERK phosphorylation in lesions at highest risk to undergo transformation. The fact that only one patient showed inhibition of both Akt and ERK phosphorylation suggests that myoinositol exerts independent effects on these pathways. Because myoinositol is the head group of phosphatidylinositol, it has been hypothesized that Akt inhibition may be through direct effects on PI3K, whereas ERK inhibition has been suggested to be through protein kinase Cδ (32). In fact, myoinositol may work through multiple mechanisms to inhibit tumor progression. Dong et al. (33) reported that IP6 strongly inhibited epidermal growth factor–induced and 12-O-tetradecanoylphorbol-13-acetate–induced cell transformation, activator protein-1 and ERK activation, as well as PI3K activity. Myoinositol has been shown to reduce tumor incidence in animal models (19, 34, 35) but only modestly reduces cellular proliferation in vitro. This suggests that myoinositol has other effects that contribute to the inhibition of tumorigenesis in vivo. Myoinositol is reported to have antioxidant, anti-inflammatory, and immune-enhancing activities that may also contribute to its cancer-preventive properties (16). For example, cigarette smoke decreases NK cell activity and causes lung inflammation (28). IP6 and inositol augment NK cell activity in vitro. In vivo, these compounds normalized the carcinogen-induced depression of NK cell activity and lowered tumor incidence (19). Thus, in vitro analysis likely underestimates the activity of myoinositol as a chemo-preventive agent. An additional advantage of myoinositol for lung cancer prevention is that it might be useful as an aid in smoking cessation due to its possible efficacy against depression (36).
In conclusion, Akt and ERK are activated in early premalignant lesions from smokers. Activation of these kinases was associated with phenotypic progression to dysplastic lesions, which was inhibited by myoinositol treatment. Akt and ERK are therefore potential targets for lung cancer prevention and could be used as biomarkers for future lung cancer prevention trials using myoinositol.
Acknowledgments
We thank the NIH Fellows Editorial Board for editorial assistance.
Grant support: Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, and federal funds from the National Cancer Institute, NIH, under contract National Cancer Institute Contract NO1-CO-12400 and N01-CN85188.
Footnotes
Note: The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.van Zandwijk N, Hirsch FR. Chemoprevention strategies for non-small cell lung cancer. Curr Opin Oncol. 2002;14:185–90. doi: 10.1097/00001622-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Tsao AS, McDonnell T, Lam S, et al. Increased phospho-AKT (Ser(473)) expression in bronchial dysplasia: implications for lung cancer prevention studies. Cancer Epidemiol Biomarkers Prev. 2003;12:660–4. [PubMed] [Google Scholar]
- 4.Tsurutani J, Fukuoka J, Tsurutani H, et al. Evaluation of two phosphorylation sites improves the prognostic significance of Akt activation in non-small-cell lung cancer tumors. J Clin Oncol. 2006;24:306–14. doi: 10.1200/JCO.2005.02.4133. [DOI] [PubMed] [Google Scholar]
- 5.West KA, Brognard J, Clark AS, et al. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsurutani J, Castillo SS, Brognard J, et al. Tobacco components stimulate Akt-dependent proliferation and NFκB-dependent survival in lung cancer cells. Carcinogenesis. 2005;26:1182–95. doi: 10.1093/carcin/bgi072. [DOI] [PubMed] [Google Scholar]
- 7.Hoshino S, Yoshida M, Inoue K, et al. Cigarette smoke extract induces endothelial cell injury via JNK pathway. Biochem Biophys Res Commun. 2005;329:58–63. doi: 10.1016/j.bbrc.2005.01.095. [DOI] [PubMed] [Google Scholar]
- 8.Laag E, Majidi M, Cekanova M, Masi T, Takahasi T, Schuller HM. NNK activates ERK1/2 and CREB/ATF-1 via β-1-AR and EGFR signaling in human lung adenocarcinoma and small airway epithelial cells. Int J Cancer. 2006;119:1547–52. doi: 10.1002/ijc.21987. [DOI] [PubMed] [Google Scholar]
- 9.Roman J, Ritzenthaler JD, Gil-Acosta A, Rivera HN, Roser-Page S. Nicotine and fibronectin expression in lung fibroblasts: implications for tobacco-related lung tissue remodeling. FASEB J. 2004;18:1436–8. doi: 10.1096/fj.03-0826fje. [DOI] [PubMed] [Google Scholar]
- 10.Vicent S, Lopez-Picazo JM, Toledo G, et al. ERK1/2 is activated in non-small-cell lung cancer and associated with advanced tumours. Br J Cancer. 2004;90:1047–52. doi: 10.1038/sj.bjc.6601644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webb CP, Van Aelst L, Wigler MH, Woude GF. Signaling pathways in Ras-mediated tumorigenicity and metastasis. Proc Natl Acad Sci U S A. 1998;95:8773–8. doi: 10.1073/pnas.95.15.8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blackhall FH, Pintilie M, Michael M, et al. Expression and prognostic significance of kit, protein kinase B, mitogen-activated protein kinase in patients with small cell lung cancer. Clin Cancer Res. 2003;9:2241–7. [PubMed] [Google Scholar]
- 13.Huang C, Ma WY, Hecht SS, Dong Z. Inositol hexaphosphate inhibits cell transformation and activator protein 1 activation by targeting phosphatidylinositol-3′ kinase. Cancer Res. 1997;57:2873–8. [PubMed] [Google Scholar]
- 14.Bozinovski S, Vlahos R, Hansen M, Liu K, Anderson GP. Akt in the pathogenesis of COPD. Int J Chron Obstruct Pulmon Dis. 2006;1:31–8. doi: 10.2147/copd.2006.1.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piccolo E, Vignati S, Maffucci T, et al. Inositol pentakisphosphate promotes apoptosis through the PI 3-K/Akt pathway. Oncogene. 2004;23:1754–65. doi: 10.1038/sj.onc.1207296. [DOI] [PubMed] [Google Scholar]
- 16.Vucenik I, Shamsuddin AM. Protection against cancer by dietary IP6 and inositol. Nutr Cancer. 2006;55:109–25. doi: 10.1207/s15327914nc5502_1. [DOI] [PubMed] [Google Scholar]
- 17.Hecht SS, Kenney PM, Wang M, Upadhyaya P. Dose-response study of myo-inositol as an inhibitor of lung tumorigenesis induced in A/J mice by benzo. Cancer Lett. 2001;167:1–6. doi: 10.1016/s0304-3835(01)00454-2. [DOI] [PubMed] [Google Scholar]
- 18.Clements RS, Jr, Darnell B. Myo-inositol content of common foods: development of a high-myo-inositol diet. Am J Clin Nutr. 1980;33:1954–67. doi: 10.1093/ajcn/33.9.1954. [DOI] [PubMed] [Google Scholar]
- 19.Lam S, McWilliams A, LeRiche J, MacAulay C, Wattenberg L, Szabo E. A phase I study of myo-inositol for lung cancer chemoprevention. Cancer Epidemiol Biomarkers Prev. 2006;15:1526–31. doi: 10.1158/1055-9965.EPI-06-0128. [DOI] [PubMed] [Google Scholar]
- 20.Ramirez RD, Sheridan S, Girard L, et al. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res. 2004;64:9027–34. doi: 10.1158/0008-5472.CAN-04-3703. [DOI] [PubMed] [Google Scholar]
- 21.Sato M, Vaughan MB, Girard L, et al. Multiple oncogenic changes (K-RAS(V12), p53 knockdown, mutant EGFRs, p16 bypass, telomerase) are not sufficient to confer a full malignant phenotype on human bronchial epithelial cells. Cancer Res. 2006;66:2116–28. doi: 10.1158/0008-5472.CAN-05-2521. [DOI] [PubMed] [Google Scholar]
- 22.Castillo SS, Brognard J, Petukhov PA, et al. Preferential inhibition of Akt and killing of Akt-dependent cancer cells by rationally designed phosphatidylinositol ether lipid analogues. Cancer Res. 2004;64:2782–92. doi: 10.1158/0008-5472.can-03-1530. [DOI] [PubMed] [Google Scholar]
- 23.Nakayama H, Numakawa T, Ikeuchi T, Hatanaka H. Nicotine-induced phosphorylation of extracellular signal-regulated protein kinase and CREB in PC12h cells. J Neurochem. 2001;79:489–98. doi: 10.1046/j.1471-4159.2001.00602.x. [DOI] [PubMed] [Google Scholar]
- 24.Ahrendt SA, Decker PA, Alawi EA, et al. Cigarette smoking is strongly associated with mutation of the K-ras gene in patients with primary adenocarcinoma of the lung. Cancer. 2001;92:1525–30. doi: 10.1002/1097-0142(20010915)92:6<1525::aid-cncr1478>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Zhang Z, Lubet R, You M. Tobacco smoke-induced lung tumorigenesis in mutant A/J mice with alterations in K-ras, p53, or Ink4a/Arf. Oncogene. 2005;24:3042–9. doi: 10.1038/sj.onc.1208390. [DOI] [PubMed] [Google Scholar]
- 26.Liu L, Zhu S, Gong Z, Low BC. K-ras/PI3K-Akt signaling is essential for zebrafish hematopoiesis and angiogenesis. PLoS ONE. 2008;3:e2850. doi: 10.1371/journal.pone.0002850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams MR, Arthur JS, Balendran A, et al. The role of 3-phosphoinositide-dependent protein kinase 1 in activating AGC kinases defined in embryonic stem cells. Curr Biol. 2000;10:439–48. doi: 10.1016/s0960-9822(00)00441-3. [DOI] [PubMed] [Google Scholar]
- 28.Heusch WL, Maneckjee R. Signalling pathways involved in nicotine regulation of apoptosis of human lung cancer cells. Carcinogenesis. 1998;19:551–6. doi: 10.1093/carcin/19.4.551. [DOI] [PubMed] [Google Scholar]
- 29.Holub BJ. Metabolism and function of myo-inositol and inositol phospholipids. Annu Rev Nutr. 1986;6:563–97. doi: 10.1146/annurev.nu.06.070186.003023. [DOI] [PubMed] [Google Scholar]
- 30.Groenen PM, Merkus HM, Sweep FC, Wevers RA, Janssen FS, Steegers-Theunissen RP. Kinetics of myo-inositol loading in women of reproductive age. Ann Clin Biochem. 2003;40:79–85. doi: 10.1258/000456303321016213. [DOI] [PubMed] [Google Scholar]
- 31.Berry GT, Prantner JE, States B, Yandrasitz JR. The effect of glucose and galactose toxicity on myo-inositol transport and metabolism in human skin fibroblasts in culture. Pediatr Res. 1994;35:141–7. doi: 10.1203/00006450-199402000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Vucenik I, Ramakrishna G, Tantivejkul K, Anderson LM, Ramljak D. Inositol hexaphosphate (IP6) blocks proliferation of human breast cancer cells through a PKCδ-dependent increase in p27Kip1 and decrease in retinoblastoma protein (pRb) phosphorylation. Breast Cancer Res Treat. 2005;91:35–45. doi: 10.1007/s10549-004-6456-5. [DOI] [PubMed] [Google Scholar]
- 33.Dong Z, Huang C, Ma WY. PI-3 kinase in signal transduction, cell transformation, and as a target for chemoprevention of cancer. Anticancer Res. 1999;19:3743–7. [PubMed] [Google Scholar]
- 34.Liao J, Seril DN, Yang AL, Lu GG, Yang GY. Inhibition of chronic ulcerative colitis associated adenocarcinoma development in mice by inositol compounds. Carcinogenesis. 2007;28:446–54. doi: 10.1093/carcin/bgl154. [DOI] [PubMed] [Google Scholar]
- 35.Hecht SS, Upadhyaya P, Wang M, Bliss RL, McIntee EJ, Kenney PM. Inhibition of lung tumori-genesis in A/J mice by N-acetyl-S-(N-2-phenethylthiocarbamoyl)-L-cysteine and myo-inositol, individually and in combination. Carcinogenesis. 2002;23:1455–61. doi: 10.1093/carcin/23.9.1455. [DOI] [PubMed] [Google Scholar]
- 36.Levine J. Controlled trials of inositol in psychiatry. Eur Neuropsychopharmacol. 1997;7:147–55. doi: 10.1016/s0924-977x(97)00409-4. [DOI] [PubMed] [Google Scholar]