Abstract
Therapies that disrupt or repair blood–brain barrier integrity can result in major changes in MRI images even when the tumor volume remains constant. Thus, a reliable blood-based tumor biomarker could significantly improve clinical care and research studies in these patients. This study was performed to assess plasma concentrations of glial fibrillary acidic protein (GFAP) in patients with high- and low-grade gliomas before and after debulking surgery. Pre-operative plasma was collected from 33 patients with radiation- and chemotherapy-naïve gliomas. Additional plasma was collected 24–48 h postoperatively from 23 of these patients. Plasma GFAP (pGFAP) concentrations were determined using an electrochemiluminescent immunoassay and were analyzed as a function of tumor grade, tumor GFAP expression, the integrity of the blood-brain barrier, and post-operative status. Detectable pGFAP levels (≥0.04 ng/mL) were found pre-operatively in 52 % of patients and post-operatively in 96 %. Detectable pGFAP was more common in patients with WHO grade IV (100 %) than WHO grade III (56 %) or WHO grade II gliomas (20 %). No patient with undetectable GFAP had WHO grade IV glioma. Higher pGFAP concentrations were also associated with contrast enhancement but not related to tumor GFAP expression. GFAP is commonly detected in the plasma of patients with high-grade gliomas. pGFAP levels rise rather than fall following debulking surgery which is probably a result of surgical trauma. GFAP remains a potentially informative plasma biomarker for gliomas. Longitudinal studies are required to correlate pGFAP levels with patient outcomes.
Keywords: Glial fibrillary acidic protein, Glioma, Glioblastoma, Biomarker, Circulating tumor marker
Background
A major challenge in the treatment of human gliomas is the lack of an accurate measure of disease burden and response to therapy. Radiologic imaging of high-grade gliomas relies on contrast enhanced magnetic resonance imaging (MRI) scans that assess changes in blood–brain barrier (BBB) integrity rather than actual tumor volume. Treatment with radiation and temozolomide frequently results in increased contrast enhancement, edema, and mass effect which is unrelated to tumor progression [1, 2]. Similarly, treatment with agents that improve BBB integrity, such as glucocorticoids or bevacizumab, can lead to dramatic improvements on MRI scan without real changes in tumor size. These “pseudo-progressions” and “pseudo-responses” can result in premature changes to medical therapy, unnecessary invasive procedures, or significant treatment delays [3]. As a result, alternative means to determine tumor burden would be extremely helpful.
A number of potential blood-based markers have been explored in the past, including caldesmon [4], cathepsin D [5], MMP-9 [6], and YKL-40 [6]; however, they have not yet been accepted in clinical practice due to a lack of specificity or sensitivity. Another prominently discussed candidate biomarker for malignant gliomas is glial fibrillary acidic protein (GFAP). GFAP is a 55 kDa intermediate filament protein that is highly expressed in astrocytes of the central nervous system. GFAP is a member of the cytoskeletal protein family which is expressed in astroglial cells and functionally maintains astrocytic structure and stability. This brain-specific protein is currently being studied as a plasma biomarker for a variety of neurological conditions including acute stroke [7, 8], subarachnoid hemorrhage [9], traumatic brain injury [10], and sickle cell disease [11]. Previous studies demonstrated that GFAP could be detected in serum of patients with glioblastoma(GBM) with a sensitivity and specificity of 76 % and 100 %, respectively [12], and suggested that GFAP levels correlate with tumor volume [13]. In addition, it was reported that GFAP could not be detected in the serum of lower grade gliomas [12]. The goals of this study were (1) to evaluate pre- and post-surgical levels of GFAP in plasma samples from newly diagnosed patients with high- and low-grade gliomas to further assess detectability of GFAP in the blood of these patients and (2) to evaluate the effect of tumor debulking on GFAP levels.
Methods
Patient selection
Patients above the age of 18 with a suspected glioma on imaging who were scheduled to undergo surgical resection were asked to participate in an IRB-approved study on circulating biomarkers in solid tumor malignancies. Patients whose pathology revealed diagnosis of a glioma (WHO grade II, III or IV) and who had not previously been treated with chemotherapy or radiation to the brain were included in this analysis.
Specimen collection
Peripheral blood was collected in tubes containing EDTA (PPT, BD Vacutainer, Franklin Lakes, NJ) preoperatively and, if possible, 24–48 hrs after surgical resection. The samples were transferred to the laboratory within 30 min and centrifuged at 814 g for 10 min. The supernatant was collected and centrifuged at 18,000 g for 10 min at room temperature. 1 mL aliquots of plasma were then stored at −80 °C.
Analysis of the GFAP concentration in plasma
50 μl of plasma were used for GFAP measurements using an electrochemiluminescent immunoassay developed on the Meso Scale Discovery platform (Meso Scale Discovery, Gaithersburg, MD) at Johns Hopkins University. The assay procedures are outlined in published studies [8]. After experiments to determine the optimal antibody concentrations, plate type, and blocking material, the final assay for GFAP values had a standard curve with a linear range of quantification from 0.040 to 40.0 ng/mL. The GFAP assay had a lower limit of detection (LLOD) of 0.011 ng/mL as defined by two SDs above the background of blank wells. The lower limit of quantification (LLOQ) was defined as a GFAP concentration of 0.040 ng/mL, at which results are expected to be accurate within ±20 % of the true value. Validation of the GFAP assay was conducted using discarded diagnostic specimens from normal and positive controls. This GFAP assay validation study was part of a separate IRB-approved protocol.
Immunohistochemistry
10 μm representative tissue slides from formalin-fixed paraffin-embedded (FFPE) tissue from study-associated pathology specimens were stained for GFAP and reviewed independently by a neuropathologist who was unaware of the corresponding plasma GFAP (pGFAP) concentrations. The degree of GFAP within tumor was graded based on the relative degree of GFAP expression within tumor versus the surrounding brain tissue. Slides that stained positive for GFAP in surrounding tissue, but not in tumor, were graded as 0; slides staining both in tumor and surrounding tissues were graded as +, and strongly or very strongly staining tumors as ++ or +++, respectively. The amount of tumor was not evaluated.
Demographic data, imaging and pathology
Information on the age of patients at time of surgery, gender, the pathological diagnosis (WHO grades II, III, and IV), as well as type of surgeries were collected. Presurgical MRI scans were evaluated for presence or absence of any contrast enhancement within the tumor and graded in a binary fashion; contrast enhancement within the tumor was either present (yes) or absent (no).
Statistical considerations
A pGFAP concentration of less than 0.040 ng/mL, i.e., below the LLOQ, was considered ‘undetectable’ and a concentration of greater than and equal to 0.040 ng/mL was considered ‘detectable’. Data were summarized using percentages or median values according to their discrete or continuous nature. Chi-square statistic or Fisher exact test were used for proportion comparison. Comparison between the pre- and post-surgical pGFAP concentration was performed on 23 patients using paired t-test. 10 of the 33 patients did not have a post-surgical specimen available and were not included in this analysis. All p-values are reported as two-sided, and all analyses were conducted using the SAS software (version 9.2, SAS Institute).
Results
Plasma samples from a total of 33 patients with malignant gliomas (9 glioblastoma, i.e., WHO grade IV; 9 anaplastic gliomas, i.e., WHO grade III; and 15 low-grade gliomas, i.e., WHO grade II) were analyzed (Table 1). The median age of patients with WHO grade IV, III and II tumors were 58, 58, and 33 years (range 39–78, 32–78, 18–55 years), respectively. Both pre- and post-operative samples were available in 23 (70 %) of these patients. Reasons for not obtaining a postsurgical sample included discharge of patients form the hospital prior to the planned second blood draw, difficulty with venous access, and refusal of patients to have additional blood drawn. Nineteen patients (58 %; 6 with WHO grade IV, 5 with WHO grade III and 8 with WHO grade II) had tumor tissue available to be immunohistochemically analyzed for GFAP.
Table 1.
Synopsis of the histology, clinical data, GFAP immunohistochemostry (IHC) and GFAP plasma levels (pGFAP) of the 33 patients studied, grouped according to WHO grade
| Patient no. | Histology | Age and gender | Surgery | Tissue IHC GFAP | Contrast enhancement | Pre-operative pGFAP (ng/mL) | Post-operative pGFAP (ng/mL) |
|---|---|---|---|---|---|---|---|
| 1 | GBM | 45 F | STR | 0 | yes | 0.15 | 0.07 |
| 2 | GBM | 58 M | STR | n.a. | yes | 0.12 | n.a. |
| 3 | GBM | 62 M | STR | + | yes | 0.11 | 0.32 |
| 4 | GBM | 71 M | GTR | ++ | yes | 0.10 | 0.07 |
| 5 | GBM | 56 F | GTR | n.a. | yes | 0.09 | 0.04 |
| 6 | GBM | 48 F | GTR | + | yes | 0.08 | 0.19 |
| 7 | GBM | 74 M | STR | n.a. | yes | 0.08 | n.a. |
| 8 | GBM | 78 F | GTR | + | yes | 0.07 | n.a. |
| 9 | GBM | 39 F | STR | + | no | 0.07 | n.a. |
| 10 | AA | 40 M | GTR | + | yes | 0.07 | 0.16 |
| 11 | AA | 58 M | GTR | n.a. | no | 0.04 | 2.01 |
| 12 | AA | 32 M | SNB | ++ | no | 0.04 | n.a. |
| 13 | AA | 54 M | GTR | +++ | yes | <0.04 | 0.35 |
| 14 | AA | 60 M | STR | n.a. | no | <0.04 | n.a. |
| 15 | AO | 73 F | GTR | n.a.a | yes | <0.04 | 0.19 |
| 16 | AG | 58 F | GTR | n.a. | yes | 0.05 | n.a. |
| 17 | AG | 78 F | GTR | + | yes | 0.58 | 3.16 |
| 18 | OD/OS | 57 F | STR | n.a. | yes | <0.04 | n.a. |
| 19 | A | 53 F | STR | + | no | <0.04 | 3.32 |
| 20 | A | 34 F | GTR | + | no | <0.04 | 0.83 |
| 21 | A | 21 F | GTR | n.a. | no | <0.04 | 0.42 |
| 22 | A | 45 F | STR | ++ | no | <0.04 | 0.38 |
| 23 | A | 33 M | STR | n.a. | no | <0.04 | 0.2 |
| 24 | A | 27 F | STR | ++ | no | <0.04 | n.a. |
| 25 | A | 26 F | GTR | n.a. | no | <0.04 | <0.04 |
| 26 | OD | 23 M | GTR | n.a. | no | 0.22 | 0.63 |
| 27 | OD | 35 F | STR | 0 | yes | 0.08 | 9.65 |
| 28 | OD | 55 M | GTR | n.a. | no | 0.04 | 1.13 |
| 29 | OD | 31 M | STR | 0 | no | <0.04 | 0.20 |
| 30 | OD | 18 M | STR | n.a. | yes | <0.04 | 0.11 |
| 31 | OD | 43 M | GTR | 0 | no | <0.04 | 0.05 |
| 32 | OD | 24 F | STR | n.a. | no | <0.04 | n.a. |
| 33 | OA | 43 F | GTR | 0 | no | <0.04 | 0.07 |
Grading of IHC for tissue GFAP: 0 = GFAP stains in brain tissue but not tumor
+ GFAP stains in tumor and brain tissue; ++ strongly staining positive in tumor; +++ very strongly staining positive in tumor. N.a. not analyzed GBM glioblastoma (WHO grade IV), AA anaplastic astrocytoma (WHO grade III), AO anaplastic oligodendroglioma (WHO grade III), AG anaplastic glioma (WHO grade III), A low-grade astrocytoma (WHO grade II), OD oligodendroglioma (WHO grade II), OA low-grade oligoastrocytoma (WHO grade II), OS oligosarcoma. F = female, M male, GFAP glial fibrillary acidic protein, GTR gross total resection, STR subtotal or partial resection, SNB stereotactic needle biopsy
(patient 15) staining was inadequate. pGFAP levels of <0.04 ng/mL were below the lower limit of quantification (LLOQ) of the assay
Detectable pGFAP levels (≥0.04 ng/mL) were found pre-operatively in 52 % (95 % CI: 34–69 %) of patients (Table 1; Fig. 1). The median pGFAP level of the 17 patients with pGFAP above the LLOQ (≥0.04 ng/mL) was 0.08 ng/mL (range 0.04–0.58 ng/mL). The proportion of detectable pGFAP was significantly higher in patients with WHO grade IV (100 %) compared to WHO grade III (56 %; p = 0.02) or compared to WHO grade II gliomas (20 %; p = 0.0001) (Fig. 1). As expected, patients with higher grade gliomas had more evidence of BBB dysfunction, as measured by contrast enhancement, than patients with lower grade gliomas. Contrast enhancement was noted in 89 % of WHO grade IV, 67 % of WHO grade III, and 13 % of WHO grade II gliomas. Immunohistochemistry (IHC) staining for GFAP in tissue was positive in 13 of 19 samples where the tumor tissue was available. However, the expression of GFAP in the tumor was not associated with pGFAP concentrations (Fig. 1).
Fig. 1.
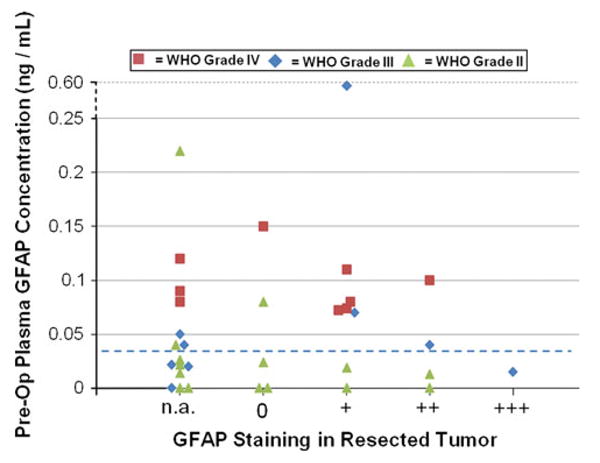
Synopsis of pre-operative pGFAP levels, tumor grade, and corresponding tumor GFAP expression as detected by IHC. Lower dotted line = LLOQ of 0.04 ng/mL
Post-operative pGFAP levels from 24 to 48 hrs after surgery were elevated compared to the baseline in 96 % of patients in whom plasma was available at this time point (n = 23), independent of tumor grade. The proportion of patients with detectable pGFAP status post resection was 100 % for WHO grade IV, 100 % for WHO grade III and 92 % for WHO grade II gliomas. Post-surgical pGFAP was detected in 12 of 13 patients who had undetectable pGFAP prior to surgery (2 with WHO grade III and 10 with WHO grade II tumors; Fig. 2). The average increase in pGFAP levels between pre- and post-surgical time points was 0.95 ng/mL (±2.07 standard deviations) which was statistically significant (p = 0.039).
Fig. 2.
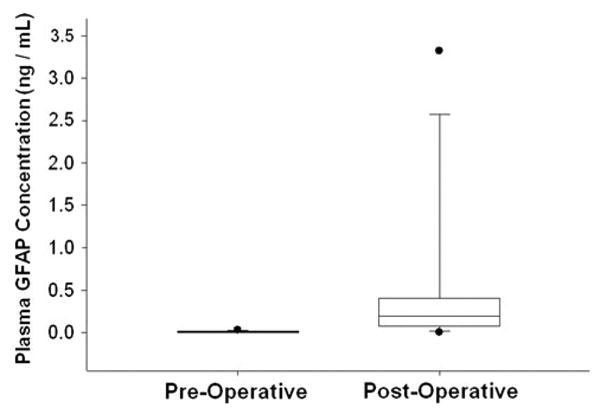
Box-and-whisker plots of pre- and post-surgical pGFAP levels in 12 patients with at baseline pGFAP levels below the lower limit of quantification (<0.04 ng/mL), illustrating that significant amounts of GFAP can be shed into the blood stream by injury (surgery), even in lower grade gliomas
Discussion
This study was conducted to assess plasma concentrations of GFAP in patients with high- and low-grade gliomas before and after their initial debulking surgery. The ultimate goal was to determine if this candidate blood-based tumor biomarker for gliomas deserves further investigation. Previous studies suggested that GFAP could be detected in the serum of patients with glioblastoma, but not of patients with lower grade gliomas [12]. Our study demonstrates that circulating GFAP is most commonly detected in the blood of patients with treatment-naïve glioblastomas but that this is also found in some patients with lower grade gliomas.
In addition to tumor grade, there appears to be a relationship between disruption of the BBB, as measured by the extent of contrast enhancement in the tumor by MRI, and the detectability of pre-operative pGFAP levels. This is unlikely to be an independent variable given the known association between tumor grade and contrast enhancement. However, disruption of the BBB may contribute to GFAP's ability to leave the brain and enter the bloodstream.
The increase in pGFAP levels after extensive debulking surgery is of interest. This appears to be independent of tumor grade and the degree of GFAP expression as detected by IHC staining of the tumor tissue. As tumor burden was substantially reduced at the time of the second blood draw and elevations were noted even in low-grade gliomas where pGFAP was initially undetectable, it is likely that the post-surgical increase in pGFAP was a result of surgical injury to the tumor and surrounding normal brain tissue. This might be expected as GFAP is a structural protein of normal astroglial and tumor cells with a plasma half-life of several days.
Based on the previously published data and the results of this study, GFAP should be evaluated further as a blood-based biomarker for high-grade gliomas. Several clinically available and widely used blood-based tumor markers have some of the potential limitations that we have found using pGFAP in patients with primary brain tumors. For example, prostate specific antigen (PSA) levels are frequently elevated post-operatively due to injury to normal prostate tissue. As a result, baseline PSA levels are measured weeks after surgery when the injury-related PSA elevation has resolved. In addition, carcinoembryonic antigen (CEA) is not elevated in every patient with colorectal cancer. However, in the selected patients who do have high CEA levels it may be a valuable blood-based biomarker. Further studies of pGFAP in patients with high-grade gliomas should follow patients longitudinally to determine whether changes in pGFAP concentrations over time are related to response, progression, and survival. A reliable blood-based tumor marker would have utility for the clinical management and the design and conduct of clinical trials in this patient population.
Acknowledgments
The authors would like to acknowledge the Robert H. Gross Memorial Fund for supporting this study. Matthias Holdhoff is recipient of the 2010 ASCO Young Investigator Award by the Conquer Cancer Foundation. These data were in part presented at the 2011 Annual Meeting of the American Society of Clinical Oncology in Chicago, IL.
Contributor Information
Hatim Husain, Brain Cancer Program, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Johns Hopkins University School of Medicine, Cancer Research Building II, 1550 Orleans Street, 1M-16, Baltimore, MD 21287, USA; Department of Oncology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
William Savage, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Stuart A. Grossman, Brain Cancer Program, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Johns Hopkins University School of Medicine, Cancer Research Building II, 1550 Orleans Street, 1M-16, Baltimore, MD 21287, USA; Department of Oncology, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Xiaobu Ye, Brain Cancer Program, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Johns Hopkins University School of Medicine, Cancer Research Building II, 1550 Orleans Street, 1M-16, Baltimore, MD 21287, USA; Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Peter C Burger, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Allen Everett, Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Chetan Bettegowda, Brain Cancer Program, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Johns Hopkins University School of Medicine, Cancer Research Building II, 1550 Orleans Street, 1M-16, Baltimore, MD 21287, USA; Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA; Ludwig Center for Cancer Genetics and Therapeutics at Johns Hopkins, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Luis A. Diaz, Jr., Department of Oncology, Johns Hopkins University School of Medicine, Baltimore, MD, USA; Ludwig Center for Cancer Genetics and Therapeutics at Johns Hopkins, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Cherie Blair, Ludwig Center for Cancer Genetics and Therapeutics at Johns Hopkins, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Katharine E. Romans, Ludwig Center for Cancer Genetics and Therapeutics at Johns Hopkins, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Matthias Holdhoff, Email: mholdho1@jhmi.edu, Brain Cancer Program, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Johns Hopkins University School of Medicine, Cancer Research Building II, 1550 Orleans Street, 1M-16, Baltimore, MD 21287, USA; Department of Oncology, Johns Hopkins University School of Medicine, Baltimore, MD, USA; Ludwig Center for Cancer Genetics and Therapeutics at Johns Hopkins, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
References
- 1.Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9:453–461. doi: 10.1016/S1470-2045(08)70125-6. [DOI] [PubMed] [Google Scholar]
- 2.Brandes AA, Franceschi E, Tosoni A, Blatt V, Pession A, Tallini G, Bertorelle R, Bartolini S, Calbucci F, Andreoli A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26:2192–2197. doi: 10.1200/JCO.2007.14.8163. [DOI] [PubMed] [Google Scholar]
- 3.Holdhoff M, Grossman SA. Controversies in the adjuvant therapy of high-grade gliomas. Oncologist. 2011;16:351–358. doi: 10.1634/theoncologist.2010-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng PP, Hop WC, Sillevis Smitt PA, van den Bent MJ, Avezaat CJ, Luider TM, Kros JM. Low-molecular weight caldesmon as a potential serum marker for glioma. Clin Cancer Res. 2005;11:4388–4392. doi: 10.1158/1078-0432.CCR-04-2512. [DOI] [PubMed] [Google Scholar]
- 5.Fukuda ME, Iwadate Y, Machida T, Hiwasa T, Nimura Y, Nagai Y, Takiguchi M, Tanzawa H, Yamaura A, Seki N. Cathepsin D is a potential serum marker for poor prognosis in glioma patients. Cancer Res. 2005;65:5190–5194. doi: 10.1158/0008-5472.CAN-04-4134. [DOI] [PubMed] [Google Scholar]
- 6.Hormigo A, Gu B, Karimi S, Riedel E, Panageas KS, Edgar MA, Tanwar MK, Rao JS, Fleisher M, DeAngelis LM, et al. YKL-40 and matrix metalloproteinase-9 as potential serum biomarkers for patients with high-grade gliomas. Clin Cancer Res. 2006;12:5698–5704. doi: 10.1158/1078-0432.CCR-06-0181. [DOI] [PubMed] [Google Scholar]
- 7.Wunderlich MT, Wallesch CW, Goertler M. Release of glial fibrillary acidic protein is related to the neurovascular status in acute ischemic stroke. Eur J Neurol. 2006;13:1118–1123. doi: 10.1111/j.1468-1331.2006.01435.x. [DOI] [PubMed] [Google Scholar]
- 8.Bembea MM, Savage W, Strouse JJ, Schwartz JM, Graham E, Thompson CB, Everett A. Glial fibrillary acidic protein as a brain injury biomarker in children undergoing extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2011;12:572–579. doi: 10.1097/PCC.0b013e3181fe3ec7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vos PE, van Gils M, Beems T, Zimmerman C, Verbeek MM. Increased GFAP and S100beta but not NSE serum levels after subarachnoid haemorrhage are associated with clinical severity. Eur J Neurol. 2006;13:632–638. doi: 10.1111/j.1468-1331.2006.01332.x. [DOI] [PubMed] [Google Scholar]
- 10.Vos PE, Jacobs B, Andriessen TM, Lamers KJ, Borm GF, Beems T, Edwards M, Rosmalen CF, Vissers JL. GFAP and S100B are biomarkers of traumatic brain injury: an observational cohort study. Neurology. 2010;75:1786–1793. doi: 10.1212/WNL.0b013e3181fd62d2. [DOI] [PubMed] [Google Scholar]
- 11.Savage WJ, Barron-Casella E, Fu Z, Dulloor P, Williams L, Crain BJ, White DA, Jennings JM, Van Eyk JE, Debaun MR, et al. Plasma glial fibrillary acidic protein levels in children with sickle cell disease. Am J Hematol. 2011;86:427–429. doi: 10.1002/ajh.21995;10.1002/ajh.21995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung CS, Foerch C, Schanzer A, Heck A, Plate KH, Seifert V, Steinmetz H, Raabe A, Sitzer M. Serum GFAP is a diagnostic marker for glioblastoma multiforme. Brain. 2007;130:3336–3341. doi: 10.1093/brain/awm263. [DOI] [PubMed] [Google Scholar]
- 13.Brommeland T, Rosengren L, Fridlund S, Hennig R, Isaksen V. Serum levels of glial fibrillary acidic protein correlate to tumour volume of high-grade gliomas. Acta Neurol Scand. 2007;116:380–384. doi: 10.1111/j.1600-0404.2007.00889.x. [DOI] [PubMed] [Google Scholar]


