Abstract
Purpose
Bcl-2 is a critical regulator of apoptosis that is overexpressed in the majority of small cell lung cancers (SCLC). Nativoclax (ABT-263) is a potent and selective inhibitor of Bcl-2 and Bcl-xL. The primary objectives of this phase IIa study included safety at the recommended phase II dose and preliminary, exploratory efficacy assessment in patients with recurrent and progressive SCLC after at least one prior therapy.
Experimental Design
Thirty-nine patients received navitoclax 325 mg daily, following an initial lead-in of 150 mg daily for 7 days. Study endpoints included safety and toxicity assessment, response rate, progression-free and overall survival (PFS and OS), as well as exploratory pharmacodynamic correlates.
Results
The most common toxicity associated with navitoclax was thrombocytopenia, which reached grade III–IV in 41% of patients. Partial response was observed in one (2.6%) patient and stable disease in 9 (23%) patients. Median PFS was 1.5 months and median OS was 3.2 months. A strong association between plasma pro–gastrin-releasing peptide (pro-GRP) level and tumor Bcl-2 copy number (R = 0.93) was confirmed. Exploratory analyses revealed baseline levels of cytokeratin 19 fragment antigen 21-1, neuron-specific enolase, pro-GRP, and circulating tumor cell number as correlates of clinical benefit.
Conclusion
Bcl-2 targeting by navitoclax shows limited single-agent activity against advanced and recurrent SCLC. Correlative analyses suggest several putative biomarkers of clinical benefit. Preclinical models support that navitoclax may enhance sensitivity of SCLC and other solid tumors to standard cytotoxics. Future studies will focus on combination therapies.
Introduction
Recurrent metastatic small cell lung cancer (SCLC) has a dismal prognosis and few therapeutic options (1). The only therapy for recurrent SCLC currently approved for use in the United States is topotecan, based on a study that showed improvement in adverse symptoms and similar outcome relative to combination chemotherapy (2). The clinical benefit of topotecan is generally limited to patients with objective response or stable disease persistent for at least 3 months following completion of first-line chemotherapy. For patients with refractory SCLC, defined as progressive disease during or within 3 months after first-line therapy, there are no effective therapies, and topotecan shows a response rate of only 2% to 6% (2, 3). There are no U.S. Food and Drug Administration (FDA)-approved therapies for disease progression following topotecan.
In contrast to the marked heterogeneity of non-SCLCs, SCLCs are characterized by mutations or aberrant expression of several key oncogenes and tumor suppressors (TP53, RB, BCL2) in a large majority of cases (4). Bcl-2 is a central apoptotic inhibitor, overexpression of which is associated with both malignant transformation and chemotherapeutic resistance (5). Overexpression of Bcl-2 has been reported in up to 80% of SCLCs, associated with gene amplification of the BCL2 locus on chromosome 18q21 (6-9).
Navitoclax is a selective high-affinity small-molecule inhibitor of Bcl-2 and the related apoptotic inhibitor Bcl-xL (10, 11). Navitoclax binds to and inhibits Bcl-2 in a protein domain similar to that of naturally occurring Bcl-2 inhibitors such as Bax or Bak. Treatment of mice bearing any of several SCLC cell line xenograft tumors with single-agent navitoclax, or the closely related parent molecule ABT-737, resulted in dramatic tumor responses (11-14). The primary toxicity of ABT-737 and navitoclax in preclinical models was a dose-dependent rapid thrombocytopenia, an on-target effect due to inhibition of Bcl-xL in platelets (15, 16).
We initiated a phase I/II study of single-agent navitoclax, with the phase I portion of this study open to patients with all solid tumors. Key observations from the phase I portion of the study included confirmation of rapid and dose-dependent thrombocytopenia, a plasma half-life of approximately 15 hours, and preliminary evidence of anti-cancer activity including a durable partial response in a patient with SCLC (17). The gene encoding pro–gastrin-releasing peptide (pro-GRP) is in close proximity to BCL2 on 18q21, and data from the phase I study suggested a correlation between BCL2 gene copy number and plasma pro-GRP levels, as well as between the percentage change in pro-GRP over the first 35 days of continuous treatment and reduction in tumor size (17). Exploration of doses and schedules in phase I suggested a recommended phase II dose of 325 mg daily and showed that the severity of initial thrombocytopenia could be attenuated by a 7-day lead-in of 150 mg daily. This regimen was further explored in a phase II expansion, limited to patients with relapsed or refractory SCLC. Here, we summarize the clinical outcome data in this cohort of patients with SCLC, present confirmatory data about exploratory correlates from the phase I, and extend these data to include evaluation of potential prognostic markers previously reported in SCLC, including cytokeratin 19 fragment antigen 21-1 (CYFRA 21-1), neuron-specific enolase (NSE), and circulating tumor cell (CTC) enumeration (18-25).
Materials and Methods
Patient population
Eligible candidates for the phase II portion of this study (NCT00445198) were adults with histologically or cytologically confirmed SCLC, with progressive disease after at least one prior chemotherapy regimen. Any number of prior therapies were allowed. Eligible patients had an Eastern Cooperative Oncology Group (ECOG) performance status of ≤1 and had adequate bone marrow, renal, and hepatic function. Patients were excluded if they had underlying or predisposing condition of bleeding (history of nonchemotherapy-induced thrombocytopenia with bleeding within 1 year, active peptic ulcer or hemorrhagic esophagitis/gastritis, active immune thrombocytopenic purpura).
This study was conducted according to the Declaration of Helsinki and with approval from Institutional Review Boards of all participating study sites. All participants provided written informed consent before participating.
Study design
The phase II component of this clinical trial was an open-label, single-arm study of patients with recurrent and progressive SCLC after at least one prior therapy. On the basis of the dose and schedule determined in phase I, patients were treated with navitoclax 150 mg daily for 1 week and 325 mg daily thereafter. Cycle duration was defined as 21 days. Subjects could remain on therapy indefinitely, until disease progression or intolerable toxicity.
Assessments
Safety
Safety assessments included history and physical examinations, vital signs, ECOG performance status, adverse events, blood chemistry, and complete blood counts with differential. Safety assessments were conducted at screening, weekly during cycle 1, and at the start of subsequent cycles. Adverse event severity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 3.0 (26). Relationships of adverse events to navitoclax (definitely, probably, possibly, unlikely, or unrelated) were assessed by the principal investigator at each site.
Efficacy
Tumor response was assessed using Response Evaluation Criteria in Solid Tumors (RECIST) after every 2 cycles of therapy (27). Additional efficacy variables included progression-free and overall survival (PFS and OS).
Pharmacodynamic correlates
Blood specimens for analyzing CTCs were collected at screening, on day 14 of cycles 1 and 2, and at final study visit for U.S. patients only. CTC detection was conducted as previously described (28), using the CellSearch System (Veridex, Raritan). Samples enriched for CTCs were removed from the Veridex cartridge after imaging and enumeration, washed in PBS, pelleted, resuspended in 100 μL PBS, dropped, and then dried on a positively charged slide (Biogenex). Slides were rinsed in water before imaging using the BioView Imaging System to identify and record loci of CTCs. FISH was conducted as previously described (29) using a Vysis LSI Bcl-2 (orange) probe and chromosome 18 probe (green) developed by Abbott Molecular. The slides were returned to the BioView imaging system and the previously identified CTCs assessed for DNA copy number.
Serum and plasma samples collected at the same intervals were stored at −70°C or colder until analyzed for quantitative assessment of tumor markers. Plasma CYFRA 21-1 and pro-GRP were measured using automated ARCHITECT chemiluminescent microparticle immunoassay (Abbott Diagnostics), and NSE was measured in plasma using automated electrochemoluminescent assays on Elecsys 2010 (Roche Diagnostics) in the laboratory of Petra Stieber (Institute of Clinical Chemistry, University Hospital Munich, Munich, Germany). Serum samples were analyzed for cleaved and full-length cytokeratin 18 fragment (M30 and M65) ELISAs (Peviva) using previously described assays validated to good clinical laboratory practice in the laboratory of C. Dive (30, 31). These determinants have been previously explored as diagnostic, prognostic, or predictive biomarkers in patients with cancer (18-21).
Statistical analysis
All subjects enrolled were included in clinical outcome analyses. Descriptive statistics were used to summarize demographic variables. PFS and OS were computed using Kaplan–Meier methodology and 95% confidence intervals (CI) were provided. PFS was defined as the number of days from the date the subject started study drug to the date the subject experienced disease progression or death. Overall survival was defined as the number of days from the date the subject started study drug to the date of death. A sample size of 40 subjects was chosen to provide approximately 16 patients with chemosensitive disease and 16 with chemoresistant disease. This sample size provided 90% confidence that the true response rate was within 20% to 25% of the observed response rate.
Correlations between median BCL2 copy number and pro-GRP level were conducted by the Pearson correlation. M30 and change in CTC number from baseline comparisons among pro-GRP groups were conducted by one-way ANOVA using JMP 8.0 statistical software. Optimized thresholds for the tumor markers were obtained using BATTing (Bootstrapping and Aggregating Thresholds from Trees) method (32, 33). BATTing uses tree-based model for threshold estimation. However, a single tree may be unstable and not robust enough against small perturbations in the distribution of the data. In addition, single tree-based models are known be prone to overfitting and have poor prediction power. Those issues are addressed in BATTing by aggregating the thresholds from multiple trees to get a more robust estimate. Each tree is built using a bootstrap random sample drawn from the original population and provides its own cutoff point. The final estimate of the threshold is calculated as the median from the distribution of cutoff points generated from the multiple trees. This procedure was implemented using the R statistical software.
Results
Patient characteristics and study drug dosing
A total of 39 patients participated in the phase II study. The majority of patients had performance status 1. The median number of prior therapeutic regimens was 2 (range, 1–6). A summary of patient demographics is shown in Table 1. All patients were started at the planned dose and schedule, including dose escalation to 325 mg in week 2. The primary reasons for study discontinuation included disease progression (N = 26, 67%) and adverse events (N = 11, 28%). The median number of treatment cycles was 2 (range, 1–11) and the median treatment duration was 1.3 months (range, 0.2–7.3 months).
Table 1.
Patient demographics
| Demographic category | N = 39 |
|---|---|
| Median age (range), y | 64 (45–78) |
| Female, n (%) | 21 (54) |
| Refractory disease, n (%) | 21 (54) |
| Sensitive disease, n (%) | 17 (44) |
| ECOG performance status, n (%) | |
| 0 | 13 (33) |
| 1 | 25 (64) |
| Missing | 1 (3) |
| Prior therapeutic regimens, n (%) | |
| 1–2 | 28 (72) |
| ≥3 | 11 (28) |
Safety and tolerability
Navitoclax-related adverse events are summarized in Table 2. The most common adverse event was thrombocytopenia; however, this was not associated with bleeding sequelae. A total of 17 of 39 patients (46.3%) required dose interruption and 7 (17.9%) required dose reduction on study. The most common reasons for dose interruption were thrombocytopenia (12.8%), fatigue (7.7%), and nausea (7.7%), all of which reversed with drug discontinuation. Eleven (28%) subjects discontinued for adverse event. Of these, 4 (10%) were considered possibly or probably related to ABT-263.
Table 2.
Treatment-related adverse events occurring in ≥10% of patients
| Adverse event | Any grade, N (%) | Grade III–IV, N (%) |
|---|---|---|
| Thrombocytopenia | 24 (62) | 16 (41) |
| Diarrhea | 19 (49) | 2 (5) |
| Nausea | 18 (46) | 1 (3) |
| Fatigue | 14 (36) | 2 (5) |
| Decreased appetite | 7 (18) | — |
| AST elevation | 7 (18) | 3 (8) |
| Neutropenia | 6 (15) | 4 (10) |
| ALT elevation | 6 (15) | 3 (8) |
| Dehydration | 4 (10) | 2 (5.1) |
| Dyspepsia | 4 (10) | — |
| Dysgeusia | 4 (10) | — |
NOTE: Adverse events possibly, probably, or definitely related are included.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Efficacy
A waterfall plot representing best percentage change from baseline in identified target lesions is shown in Fig. 1. Only one confirmed partial response was observed (2.6%). Nine patients (23.1%) experienced stable disease as best response. Sixteen patients (41%) had disease progression and another 13 (33.3%) were not evaluable for response. These 13 did not complete 2 cycles of therapy and did not have posttreatment tumor assessments on protocol. The majority of these patients discontinued therapy due to disease progression or decline in performance status. Median PFS was 1.5 months (95% CI, 1.4–1.7) and median OS was 3.2 months (95% CI, 2.3–8.1; Table 3).
Figure 1.
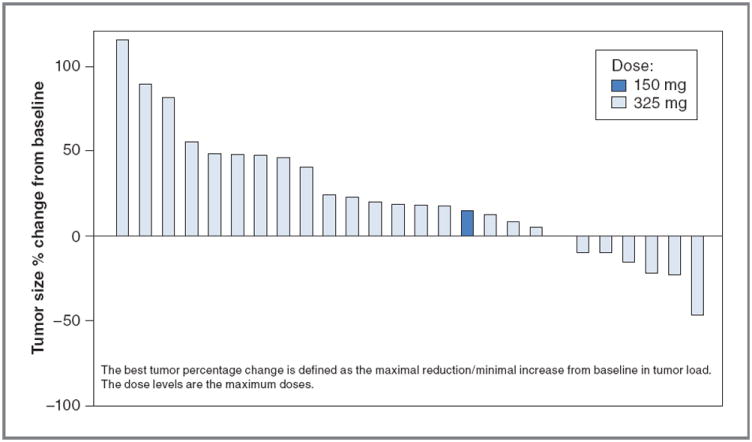
Waterfall plot of best fractional change in tumor size relative to baseline. The best tumor size percentage change from baseline is defined as the maximal reduction or minimal increase in sum of longest dimensions of target lesions relative to pretreatment assessment.
Table 3.
Efficacy data summary
| Efficacy endpoint | Patients (N = 39) |
|---|---|
| Median PFS, mo (95% CI) | 1.5 (1.4–1.7) |
| Median OS, mo (95% CI) | 3.2 (2.3–8.1) |
| ORR, % (95% CI) | 2.6 (0.1–13.5) |
| Best response, n (%) | |
| PR | 1 (2.6) |
| SD | 9 (23.1) |
| PD | 16 (41.0) |
| Inevaluablea | 13 (33.3) |
Abbreviation: ORR, overall response rate.
Baseline tumor data only.
Pharmacodynamic correlates
Several exploratory correlative biomarkers were included in this study, including both CTC enumeration and plasma protein markers associated with SCLC. The biomarker analysis included all phase II patients; to increase the sample size, patients with SCLC dosed with ≥325 mg navitoclax on the phase I portion of this study were also included in the analysis. Biomarkers correlating with outcome that are assessable before treatment are of particular potential use, consequently we determined optimized thresholds prognostic for patient outcome using BATTing for several tumor markers including CYFRA 21-1, NSE, and CTCs at baseline and on cycle 1 day 14. A summary of pretreatment biomarkers evaluated is presented in Table 4. Interestingly, baseline levels of CTC, and of both plasma biomarkers, appear to be associated with both PFS and OS in patients treated with navitoclax. CTC levels at cycle 1 day 14 were also significantly associated with outcome (data not shown).
Table 4.
Baseline biomarker summary
| Baseline biomarker | Threshold | Median PFS
|
Median OS
|
||||
|---|---|---|---|---|---|---|---|
| Above threshold, d (N) | Below threshold, d (N) | P | Above threshold, d (N) | Below threshold, d (N) | P | ||
| CYFRA 21-1 | 2.3 ng/mL | 44 (18) | 57 (14) | 0.0016 | 72 (18) | 242 (14) | 0.007 |
| NSE | 15 ng/mL | 44 (20) | 68 (12) | 0.0044 | 90 (20) | 242 (12) | 0.0088 |
| CTC | 12/7.5 mL | 37 (16) | 59 (12) | 0.0183 | 67 (16) | NR (12) | 0.0057 |
Abbreviations: N, number of patients; NR, not reached.
By grouping patients above these thresholds for both NSE and CYFRA in the analysis, we identified a population of patients with poor prognosis [median PFS: 41 days (n = 16) vs. 55 days (n = 23); Fig. 2A; P = 0.0006; median OS: 61 days (n = 15) vs. 242 days (n = 23); Fig. 2B; P = 0.0009).
Figure 2.
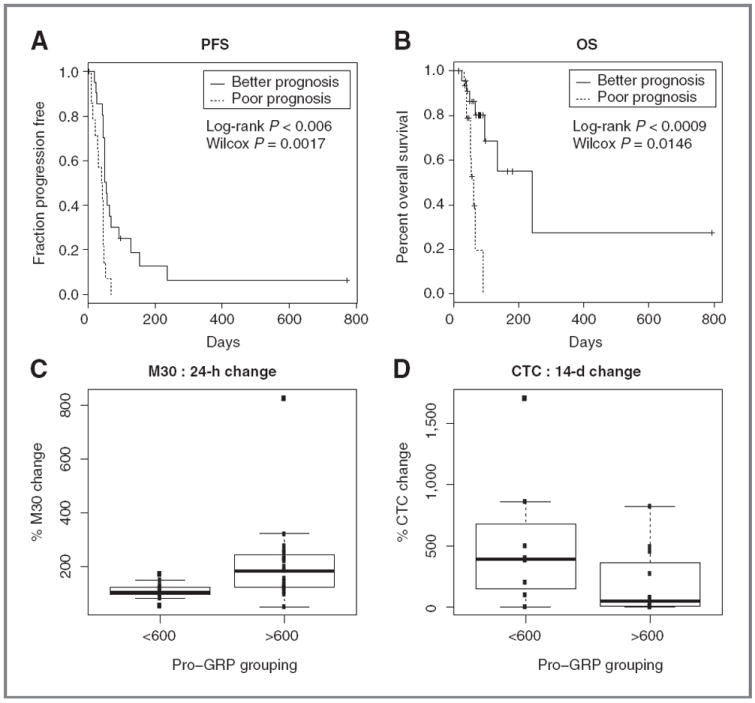
Pharmacodynamic biomarker assessment. NSE and CYFRA thresholds define a patient population with markedly poorer prognosis as assessed by PFS (A) and OS(B). Patients with high plasma pro-GRP (correlating with BCL2 gene amplification) showed increased apoptosis as assessed by the M30 ELISA assay (C) and did not show an increase in CTC from baseline to day 14, in contrast to patients with low plasma pro-GRP(D).
Pro-GRP was of particular interest with regard to navitoclax, as the GRP gene is in close chromosomal proximity to BCL2, the gene encoding the primary target for this drug. BCL2 copy number correlates with relative sensitivity to navitoclax in SCLC cell lines. Plasma pro-GRP levels may correlate with tumor BCL2 copy number and were implicated in our phase I study as a relatively noninvasive means of assessing tumor BCL2 gene amplification (17). To further evaluate this association, we assessed plasma Pro-GRP, and BCL2 copy number by FISH in CTC from patients on this study (n = 10). A strong correlation was confirmed (Pearson correlation, 0.93; P <0.0001). We identified an optimal pro-GRP threshold linked with amplification of Bcl-2 at 600 pg/mL and compared the activity of navitoclax between patients above and below this threshold using 2 measurements of activity.
First, we examined the early activation of the apoptotic pathway in patient serum, with the M30 ELISA assay. This assay measures the release of caspase-cleaved cytokeratin 18 following apoptotic cell death. M30 concentrations were significantly increased in patients in the high pro-GRP group (n = 19) when compared with those with low pro-GRP (n = 13; 212% vs. 109%; Fig. 2C; P = 0.0178). Second, we examined the changes in CTC numbers and found that the median CTC number increased more in the low pro-GRP group (n = 8) by 518% of baseline than in the high pro-GRP group (n = 12; 186% of baseline; Fig. 2D; P = 0.0433).
Discussion
A primary objective of this phase IIa study was to assess safety at recommended phase II dose. As anticipated from the phase I study, the most common grade III–IV toxicity was thrombocytopenia (17). However, this was not associated with clinically significant bleeding events and was reversible with interruption of study drug. Thrombocytopenia represents an expected and on-target toxicity of this drug, due to potent inhibition of Bcl-xL, a close functional homolog of Bcl-2 that is expressed in platelets (15). Bcl-xL inhibition has been shown to result in platelet apoptosis, with a bias toward apoptosis of older platelets in circulation (16).
The level of single-agent activity of navitoclax in recurrent SCLC was disappointing, given its remarkable preclinical in vivo activity as a single agent in multiple SCLC cell line xenograft models (12). This activity was in keeping with our earlier report using the closely related parental drug, ABT-737, in both in vitro and in vivo testing of multiple cell lines (13). In sharp contrast to these results, evaluation of ABT-737 activity in primary xenograft SCLC tumor models, derived from direct transfer of human tumors into recipient mice, showed low-level single-agent activity, varying from no effect relative to control to inhibition of growth trajectory without evident tumor reduction (34). Gene expression patterns in primary SCLC xenografts more closely mimic the human tumors of origin than do related cancer cell lines (35). In retrospect, the clinical data presented here support the predictive value of the primary xenograft model as a tool in cancer drug development and, more broadly, underline the importance of evaluating potential therapeutics across a spectrum of relevant preclinical disease models.
Multiple preclinical models, including primary xenografts, in fact do support that ABT-737 and navitoclax, through lowering of the tumor apoptotic threshold, enhance the efficacy of standard cytotoxic agents against SCLC and other solid tumors (36-42). Thus, combinatorial regimens involving navitoclax with chemotherapy or radiation may be of particular interest. This strategy is being actively tested in a variety of solid tumors.
The correlative biomarkers evaluated in this study are currently exploratory in nature. The relationship between tumor size and these markers has not been explored for first-line treatments in SCLC, and the independent prognostic role of the markers is not clear. We hypothesize that some of these endpoints, including CTC enumeration and tumor marker expression, may represent prognostic biomarkers, associated with poor outcome across a variety of therapeutic strategies and in a variety of clinical contexts (reviewed in ref. 43). NSE, CYRFA 21-1, CTCs, and M30 have been reported as poor prognostic factors in newly diagnosed SCLC (18, 22-24). Plasma pro-GRP, in contrast, we hypothesize may reflect tumor dependence on Bcl-2 and may be more closely linked to activity of potent and specific Bcl-2 inhibitors. If pro-GRP is confirmed as a predictive factor for sensitivity to Bcl-2–targeted inhibition, this could represent a strategy for selective treatment of patients for future clinical trials of navitoclax. In contrast, hypothesized prognostic markers (CYFRA 21-1, NSE, and CTCs), if confirmed in other settings, could be considered as stratification factors for future clinical research in SCLC. Evaluation of these putative biomarkers in future clinical trials in SCLC will better define their use as prognostic markers for SCLC or predictive markers for treatment with navitoclax.
Translational Relevance.
Bcl-2 is a key apoptotic resistance gene and is upregulated in the majority of small cell lung cancers (SCLC). Navitoclax, or ABT-263, is an orally bioavailable small molecule with selective and potent inhibition of Bcl-2, resulting in single-agent activity in preclinical SCLC models. This article describes the first phase II evaluation of navitoclax in patients with recurrent and metastatic SCLC. Navitoclax administration at this dose and schedule was feasible, but the observed response rate in this context was low. This result is in contrast to results using SCLC cell line xenograft models but consistent with prior observations in primary tumor xenograft SCLC models. Finally, the study validates a number of correlative biomarkers previously associated with SCLC prognosis or implicated as possible predictive correlates of benefit from Bcl-2–directed therapy.
Acknowledgments
The authors thank Saul Rosenberg and Steve Elmore for insightful discussions; Raymond Knight, Melissa Shah, Julianne Dziubinski, Michelle Pedersen, Diane D’Amico, Michael Dawson, and Renee Greco for operational support; Di Li and Joseph Beason for statistical analyses; and Ai Lockard for editorial assistance. All are employees of Abbott Laboratories.
Footnotes
Disclosure of Potential Conflicts of Interest
C.M. Rudin is a consultant/advisory board member for Genentech; D.R. Camidge, D. Khaira, and S.S. Ramalingam are consultants/advisory board members for Abbott; M.R. Ranson, C. Dive, E.M. McKeegan, T.A. Busman, and M.H. Mabry have ownership interest in Abbott. No potential conflicts of interest were disclosed by the other authors.
References
- 1.Hann CL, Rudin CM. Management of small-cell lung cancer: incremental changes but hope for the future. Oncology (Williston Park) 2008;22:1486–92. [PMC free article] [PubMed] [Google Scholar]
- 2.vonPawel J, Schiller JH, Shepherd FA, Fields SZ, Kleisbauer JP, Chrysson NG, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999;17:658–67. doi: 10.1200/JCO.1999.17.2.658. [DOI] [PubMed] [Google Scholar]
- 3.Schiller JH, Adak S, Cella D, DeVore RF, III, Johnson DH. Topotecan versus observation after cisplatin plus etoposide in extensive-stage small-cell lung cancer: E7593–a phase III trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:2114–22. doi: 10.1200/JCO.2001.19.8.2114. [DOI] [PubMed] [Google Scholar]
- 4.D’Angelo SP, Pietanza MC. The molecular pathogenesis of small cell lung cancer. Cancer Biol Ther. 2010;10:1–10. doi: 10.4161/cbt.10.1.12045. [DOI] [PubMed] [Google Scholar]
- 5.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–37. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikegaki N, Katsumata M, Minna J, Tsujimoto Y. Expression of bcl-2 in small cell lung carcinoma cells. Cancer Res. 1994;54:6–8. [PubMed] [Google Scholar]
- 7.Ikegaki N, Katsumata M, Tsujimoto Y, Nakagawara A, Brodeur GM. Relationship between bcl-2 and myc gene expression in human neuroblastoma. Cancer Lett. 1995;91:161–8. doi: 10.1016/0304-3835(95)03746-j. [DOI] [PubMed] [Google Scholar]
- 8.Jiang SX, Sato Y, Kuwao S, Kameya T. Expression of bcl-2 oncogene protein is prevalent in small cell lung carcinomas. J Pathol. 1995;177:135–8. doi: 10.1002/path.1711770206. [DOI] [PubMed] [Google Scholar]
- 9.Stefanaki K, Rontogiannis D, Vamvouka C, Bolioti S, Chaniotis V, Sotsiou F, et al. Immunohistochemical detection of bcl2, p53, mdm2 and p21/waf1 proteins in small-cell lung carcinomas. Anticancer Res. 1998;18:1167–73. [PubMed] [Google Scholar]
- 10.Park CM, Bruncko M, Adickes J, Bauch J, Ding H, Kunzer A, et al. Discovery of an orally bioavailable small molecule inhibitor of prosurvival B-cell lymphoma 2 proteins. J Med Chem. 2008;51:6902–15. doi: 10.1021/jm800669s. [DOI] [PubMed] [Google Scholar]
- 11.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–8. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 12.Shoemaker AR, Mitten MJ, Adickes J, Ackler S, Refici M, Ferguson D, et al. Activity of the Bcl-2 family inhibitor ABT-263 in a panel of small cell lung cancer xenograft models. Clin Cancer Res. 2008;14:3268–77. doi: 10.1158/1078-0432.CCR-07-4622. [DOI] [PubMed] [Google Scholar]
- 13.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 14.Tahir SK, Yang X, Anderson MG, Morgan-Lappe SE, Sarthy AV, Chen J, et al. Influence of Bcl-2 family members on the cellular response of small-cell lung cancer cell lines to ABT-737. Cancer Res. 2007;67:1176–83. doi: 10.1158/0008-5472.CAN-06-2203. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Nimmer PM, Tahir SK, Chen J, Fryer RM, Hahn KR, et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ. 2007;14:943–51. doi: 10.1038/sj.cdd.4402081. [DOI] [PubMed] [Google Scholar]
- 16.Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–86. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 17.Gandhi L, Camidge DR, Ribeiro de Oliveira M, Bonomi P, Gandara D, Khaira D, et al. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol. 2011;29:909–16. doi: 10.1200/JCO.2010.31.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holdenrieder S, von Pawel J, Dankelmann E, Duell T, Faderl B, Markus A, et al. Nucleosomes, ProGRP, NSE, CYFRA 21-1, and CEA in monitoring first-line chemotherapy of small cell lung cancer. Clin Cancer Res. 2008;14:7813–21. doi: 10.1158/1078-0432.CCR-08-0678. [DOI] [PubMed] [Google Scholar]
- 19.Molina R, Filella X, Auge JM, Fuentes R, Bover I, Rifa J, et al. Tumor markers (CEA, CA 125, CYFRA 21-1, SCC and NSE) in patients with non-small cell lung cancer as an aid in histological diagnosis and prognosis. Comparison with the main clinical and pathological prognostic factors. Tumour Biol. 2003;24:209–18. doi: 10.1159/000074432. [DOI] [PubMed] [Google Scholar]
- 20.Molina R, Auge JM, Bosch X, Escudero JM, Vinolas N, Marrades R, et al. Usefulness of serum tumor markers, including progastrin-releasing peptide, in patients with lung cancer: correlation with histology. Tumour Biol. 2009;30:121–9. doi: 10.1159/000224628. [DOI] [PubMed] [Google Scholar]
- 21.Rudin CM, Mauer A, Smakal M, Juergens R, Spelda S, Wertheim M, et al. Phase I/II study of pemetrexed with or without ABT-751 in advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2011;29:1075–82. doi: 10.1200/JCO.2010.32.5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ando S, Suzuki M, Yamamoto N, Iida T, Kimura H. The prognostic value of both neuron-specific enolase (NSE) and Cyfra21-1 in small cell lung cancer. Anticancer Res. 2004;24:1941–6. [PubMed] [Google Scholar]
- 23.Bremnes RM, Sundstrom S, Aasebo U, Kaasa S, Hatlevoll R, Aamdal S. The value of prognostic factors in small cell lung cancer: results from a randomised multicenter study with minimum 5 year follow-up. Lung Cancer. 2003;39:303–13. doi: 10.1016/s0169-5002(02)00508-1. [DOI] [PubMed] [Google Scholar]
- 24.Hou JM, Greystoke A, Lancashire L, Cummings J, Ward T, Board R, et al. Evaluation of circulating tumor cells and serological cell death biomarkers in small cell lung cancer patients undergoing chemotherapy. Am J Pathol. 2009;175:808–16. doi: 10.2353/ajpath.2009.090078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou J-M, Krebs MG, Lancashire L, Sloane R, Swain R, Backen A, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small cell lung cancer. J Clin Oncol. 2012;30:525–32. doi: 10.1200/JCO.2010.33.3716. [DOI] [PubMed] [Google Scholar]
- 26.Common Terminology Criteria for Adverse Events. Version 3.0. Bethesda, MD: NCI; [2012 Apr 25]. Available from: http://ctep.cancer.gov. [Google Scholar]
- 27.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 28.Benson AB, Kindler HL, Jodrell D, Hagey A, Coates AI, Meek KA, et al. Phase 2 study of ABT-751 in patients with refractory metastatic colorectal carcinoma (CRC) J Clin Oncol. 2005;23:255s. suppl; abstr 3537. [Google Scholar]
- 29.Olejniczak ET, Van Sant C, Anderson MG, Wang G, Tahir SK, Sauter G, et al. Integrative genomic analysis of small-cell lung carcinoma reveals correlates of sensitivity to bcl-2 antagonists and uncovers novel chromosomal gains. Mol Cancer Res. 2007;5:331–9. doi: 10.1158/1541-7786.MCR-06-0367. [DOI] [PubMed] [Google Scholar]
- 30.Cummings J, Ward TH, LaCasse E, Lefebvre C, St-Jean M, Durkin J, et al. Validation of pharmacodynamic assays to evaluate the clinical efficacy of an antisense compound (AEG 35156) targeted to the X-linked inhibitor of apoptosis protein XIAP. Br J Cancer. 2005;92:532–8. doi: 10.1038/sj.bjc.6602363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cummings J, Ranson M, Lacasse E, Ganganagari JR, St-Jean M, Jayson G, et al. Method validation and preliminary qualification of pharmacodynamic biomarkers employed to evaluate the clinical efficacy of an antisense compound (AEG35156) targeted to the X-linked inhibitor of apoptosis protein XIAP. Br J Cancer. 2006;95:42–8. doi: 10.1038/sj.bjc.6603220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Breiman L. Bagging predictors. Machine Learning. 1996;26:123–40. [Google Scholar]
- 33.Devanarayan V, Cummins DJ, Tanzer LR, et al. Application of GAM and tree models for assessing the role of drug resistance proteins in leukemia chemotherapy. Proceedings of the American Statistical Association Joint Statistical Meetings; 1999 Aug 10; Baltimore, MD. [Google Scholar]
- 34.Hann CL, Daniel VC, Sugar EA, Dobromilskaya I, Murphy SC, Cope L, et al. Therapeutic efficacy of ABT-737, a selective inhibitor of BCL-2, in small cell lung cancer. Cancer Res. 2008;68:2321–8. doi: 10.1158/0008-5472.CAN-07-5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daniel VC, Marchionni L, Hierman JS, Rhodes JT, Devereux WL, Rudin CM, et al. A primary xenograft model of small-cell lung cancer reveals irreversible changes in gene expression imposed by culture in vitro. Cancer Res. 2009;69:3364–73. doi: 10.1158/0008-5472.CAN-08-4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shoemaker AR, Oleksijew A, Bauch J, Belli BA, Borre T, Bruncko M, et al. A small-molecule inhibitor of Bcl-XL potentiates the activity of cytotoxic drugs in vitro and in vivo. Cancer Res. 2006;66:8731–9. doi: 10.1158/0008-5472.CAN-06-0367. [DOI] [PubMed] [Google Scholar]
- 37.Li R, Zang Y, Li C, Patel NS, Grandis JR, Johnson DE. ABT-737 synergizes with chemotherapy to kill head and neck squamous cell carcinoma cells via a Noxa-mediated pathway. Mol Pharmacol. 2009;75:1231–9. doi: 10.1124/mol.108.052969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim KW, Moretti L, Mitchell LR, Jung DK, Lu B. Combined Bcl-2/mammalian target of rapamycin inhibition leads to enhanced radiosensitization via induction of apoptosis and autophagy in non-small cell lung tumor xenograft model. Clin Cancer Res. 2009;15:6096–105. doi: 10.1158/1078-0432.CCR-09-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hikita H, Takehara T, Shimizu S, Kodama T, Shigekawa M, Iwase K, et al. The Bcl-xL inhibitor, ABT-737, efficiently induces apoptosis and suppresses growth of hepatoma cells in combination with sorafenib. Hepatology. 2010;52:1310–21. doi: 10.1002/hep.23836. [DOI] [PubMed] [Google Scholar]
- 40.Jain HV, Meyer-Hermann M. The molecular basis of synergism between carboplatin and ABT-737 therapy targeting ovarian carcinomas. Cancer Res. 2011;71:705–15. doi: 10.1158/0008-5472.CAN-10-3174. [DOI] [PubMed] [Google Scholar]
- 41.Zall H, Weber A, Besch R, Zantl N, Hacker G. Chemotherapeutic drugs sensitize human renal cell carcinoma cells to ABT-737 by a mechanism involving the Noxa-dependent inactivation of Mcl-1 or A1. Mol Cancer. 2010;9:164. doi: 10.1186/1476-4598-9-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan N, Malek M, Zha J, Yue P, Kassees R, Berry L, et al. Navitoclax enhances the efficacy of taxanes in non-small cell lung cancer models. Clin Cancer Res. 2011;17:1394–404. doi: 10.1158/1078-0432.CCR-10-2353. [DOI] [PubMed] [Google Scholar]
- 43.Krebs MG, Hou JM, Ward TH, Blackhall FH, Dive C. Circulating tumour cells: their utility in cancer management and predicting outcomes. Ther Adv Med Oncol. 2010;2:351–65. doi: 10.1177/1758834010378414. [DOI] [PMC free article] [PubMed] [Google Scholar]


