Abstract
Our previous studies have shown that HOXB7 mRNA is overexpressed in ~50% of invasive breast carcinomas and promotes tumor progression in breast cancer cells grown as xenografts in mice. In silico analysis of published microarray data showed that high levels of HOXB7 predict a poor outcome in HER-2–positive (P = 0.046), but not in HER-2–negative breast cancers (P = 0.94). To study the function of HOXB7 in vivo in the context of HER-2 overexpression, we generated mouse mammary tumor virus (MMTV)-Hoxb7 transgenic mice, and then crossed them with MMTV-HER-2/neu transgenic mice. In the mice carrying both Hoxb7 and HER-2/neu transgenes, Hoxb7 plays a dual role in mammary tumorigenesis. In double transgenic mice, overexpression of Hoxb7 delayed tumor onset and lowered tumor multiplicity. However, consistent with the clinical data, once the tumors appeared, their growth was faster and metastasis to the lungs occurred at a higher frequency. Our data show, for the first time, that deregulated expression of Hoxb7 in mammary tumor cells can significantly modulate HER-2/neu-oncogene induced tumorigenesis in vivo.
Introduction
HOX genes were initially discovered in Drosophila where they control segment identity through regulating cellular proliferation, differentiation, and death (1). In humans, there are at least 39 HOX genes, organized in four clusters (A, B, C, and D) located on chromosomes 7, 17, 2, and 12, respectively (1). A potential role for HOX genes in neoplasia was first documented in leukemia (2). Since then, alterations in HOX gene expression have been detected in a variety of human tumors and derivative cell lines (3–5). Although many in vitro cell culture and xenograft injection studies have shown that deregulated HOX genes can promote cellular transformation and tumor progression, thus far, no evidence has been presented that their gain or loss of function can directly cause tumor formation in vivo (6, 7).
In recent years, a small number of studies have suggested that HOXB7, a member of the HOX gene family, plays a role in tumorigenesis. First, frequent overexpression of HOXB7 was reported in melanoma, ovarian, and breast cancer cell lines, as well as primary tumors (8, 9). Secondly, overexpression of HOXB7 in the breast cancer cell line SKBR3 increased proliferation and angiogenesis by up-regulating basic fibroblast growth factor (bFGF; refs. 8, 10, 11). Recently, our data has shown that overexpression of HOXB7 in breast cancer cells induced epithelial-mesenchymal transition (12), a critical step for metastasis. These results pointed to a potential oncogenic role for HOXB7 in breast cancer.
To investigate the role of HOXB7 in breast tumorigenesis in vivo, we generated a mouse mammary tumor virus (MMTV)-Hoxb7 FVB/N transgenic mouse model where expression of Hoxb7 is regulated by the MMTV promoter. Our preliminary data showed that overexpression of Hoxb7 alone in this strain of mouse was insufficient to induce tumor formation. To test whether over-expression of Hoxb7 potentiates tumorigenesis induced by other oncogenes, we crossed the MMTV-Hoxb7 with MMTV-HER-2/neu transgenic mice, which are known to develop mammary tumors at 6 to 12 months of age. Interestingly, we found that overexpressed Hoxb7 in the mammary gland played a dual role in HER-2/neu– induced tumorigenesis: it delayed tumor onset but promoted metastatic tumor progression.
Materials and Methods
In silico microarray data analysis
The clinical effect of the gene expression profiles of HOXB7 was evaluated using a published data set of breast cancer patients (13). This data set includes 286 lymph node–negative breast cancer patients who received no adjuvant treatment. The mean expression value was used as the cutoff to classify HOXB7 expression as high or low. Recurrence-free survival was estimated using the Kaplan-Meier method and compared with log-rank tests. The HER-2 expression status was statistically classified as described previously (14). All statistical tests were two-sided, and differences were considered statistically significant at P < 0.05. All analyses were performed using SAS (version 9.1) and R (version 2.4.1).
Generation and identification of transgenic mice
The full-length mouse Hoxb7 cDNA was amplified by PCR and inserted into a pMMTV vector (a kind gift from Dr. Jeffrey Rosen at Baylor College of Medicine)4 to generate the plasmid pMMTV-Hoxb7. Microinjections were performed by the Transgenic Mouse Core Facility at National Cancer Institute. Transgenic progeny were identified by Southern blot analysis, and PCR using genomic DNA was isolated from a tail biopsy. In brief, the tip of the tail was cut from 4-week-old mice. The tail DNA was extracted by digesting with proteinase K in 300 µL of lysis buffer overnight, followed by heating at 95°C for 10 min to inactivate the enzyme. After centrifugation, 1 µL of the supernatant was used for PCR reaction. The two pairs of primers used to detect the intact Hoxb7 gene were KCR1 (5′-TTC TGG CTG GCG TGG AAA TA-3′) and Hoxb7.2 (5′-GAA GCA AAG GCG CAA GAA GT-3′) for detection of the 5′ end sequence of Hoxb7 gene, and polyA3 (5′-CCC AGA ATA GAA TGA CAC CT-3′) and Hoxb7.7 (5′-ACA GAT CAA GAT CTG GTT TC-3′) for detection of the 3′ end sequence.
Mouse breeding. The mice were housed and treated in accordance with NIH Guide to Humane Use of Animals in Research. All surgical procedures were approved by the Johns Hopkins University Animal Care and Use Committee. The MMTV-HER-2/neu mice are purchased from The Jackson Laboratory. Both MMTV-Hoxb7 and MMTV-HER-2/neu mice were on the FVB background. The hemizygous MMTV-Hoxb7 and MMTV-HER-2/neu mice were intercrossed to generate progeny of following genotypes: wild type (wt), MMTV-neu, MMTV-Hoxb7, and MMTV-Hoxb7/MMTV-neu. Age-matched virgin mice were used for phenotypic and genotypic alterations.
Whole mount staining analysis of mammary gland
Mammary glands were stained as previously described (15). Inguinal mammary fad pads were excised from euthanized mice and stretched on a histologic glass slide. The fat pads were placed in 10% formalin for at least 24 h and then defatted in acetone for 2 d. The fat pads were rehydrated in a graded series of alcohols to distilled water and stained with hematoxylin for 4 to 6 h. The stained slides were washed in tap water and then dehydrated in a graded series of alcohols and transferred to xylene, and coverslips were mounted with Permount mounting media (Fisher Scientific). Five mice per genotype were analyzed.
Reverse transcription-PCR analysis
Reverse transcription–PCR was performed as described previously for the genes queried using primers Hoxb7F2 (5′-ACC GAG TTC CTT CAA CAT GC-3′) and Hoxb7R2 (5′-CCG AGT CAG GTA GCG ATT GT-3′; ref. 16).
Western blot analysis
Western blot analysis was done using standard procedure (16). HER-2/neu antibody was purchased from Santa Cruz Biotechnology, Inc.
Histologic assay
Mammary tumor formation was monitored in nulliparous mice by weekly physical palpation. Mammary tumors and lung tissue were harvested from mice bearing tumors for 70 d. Histologic services were provided by the Department of Pathology at the Johns Hopkins School of Medicine.
Tumors were graded according to the modified Elston and Ellis histologic grading system (17). In brief, three tumor characteristics were evaluated: tubule formation, nuclear pleomorphism, and mitotic counts. A numerical scoring system of 1 to 3 was used to ensure that each factor was assessed individually. The three values were added together to produce scores of 3 to 9, to which the grade was assigned as follows: grade 1, well differentiated (3–5 points); grade 2, moderately differentiated (6–7 points); and grade 3, poorly differentiated (8–9 points). Mitotic cells were counted in random 10 high-power fields (×400) for each tumor sample. Lung metastases identified by microscopic analysis were called “micrometastasis,” and the total number of foci in a section of the entire lung was counted.
Assessment of angiogenesis, proliferation, and apoptosis
Immunohistochemical analysis was performed as previously described (18). Angiogenesis, proliferation, and apoptotic index were assessed by immunochemical staining analysis of primary tumors using antibody against CD34 (Dako), active caspase-3 (BD Sciences), and Ki67 (BD PharMingen), respectively. Angiogenesis was evaluated by investigators blinded to the identity of the mice by counting blood vessels in three areas of the section at 200× magnification. Microvessel counts were expressed as the mean number of vessels in the three areas. To assess proliferation and apoptotic rate, 1,000 tumor cells were counted, and the percentage of positive staining cells is presented in the figures.
Results
The expression status of HOXB7 predicts clinical outcome in HER-2–positive breast cancer
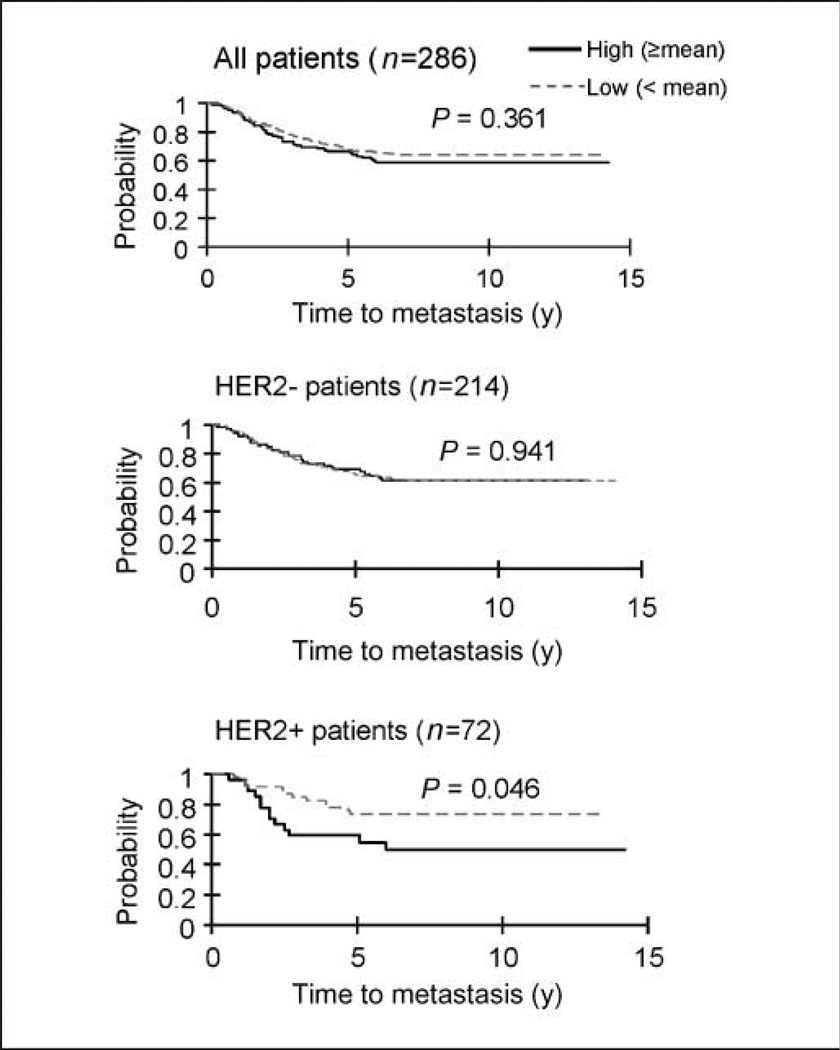
Our previous data showed that HOXB7 was overexpressed in the majority of invasive breast carcinomas (12). To test whether overexpression of HOXB7 has clinical consequences in breast cancer, we performed statistical analyses in silico using published microarray data on 286 lymph node–negative breast cancers (13). Absence of any therapy, pre-surgery or postsurgery, permitted the evaluation of the prognostic role of the marker, without the confounding effects of therapy (13). In this cohort, HOXB7 expression alone did not significantly predict clinical outcome. However, when the patients were divided into HER-2–positive (n = 72) and HER-2–negative (n = 214) subgroups, we found that the expression status of HOXB7 was a strong prognostic biomarker for development of metastasis in the HER-2– positive subgroup (P = 0.046). High HOXB7 expression in 27 of 72 HER-2–positive cancers (37%) was significantly associated with decreased distant metastasis-free survival (P = 0.046). Compared with HER-2–positive patients with low HOXB7 expression (n = 54) or HER-2–negative patients (n = 214), the HER-2–positive patients with high HOXB7 expression displayed the worst clinical outcome. The HER-2–negative group of patients showed very similar metastasis-free survival rate regardless of HOXB7 expression status (P = 0.94; Fig. 1). Seventy-four of 214 HER-2–negative tumors (35%) expressed high levels of HOXB7, which was very similar to the percentage of HER-2–positive tumors with high HOXB7 expression (37%). Thus, in all likelihood, HOXB7 expression is not associated with the expression status of HER-2. These data suggested that HOXB7 can serve as an independent prognostic marker to identify a subgroup of HER-2–positive patients with worse clinical outcome. Furthermore, it raised the possibility that HOXB7 may have the potential to modulate HER-2–induced mammary tumorigenesis.
Figure 1.
Expression status of HOXB7 predicts clinical outcome. The 286 lymph node–negative breast cancer patients were divided into three subgroups based on their HER-2 expression status: All (n = 286), HER-2+ (n = 72), and HER-2– (n = 214) groups. The mean expression value was used to set the cutoff that classified HOXB7 expression as high or low. HER-2-positive patients (n = 72), 27 patients with high HOXB7 expression versus 45 patients with low HOXB7 expression; HER-2–negative group (n = 214), 74 patients with high HOXB7 expression versus 140 patients with low HOXB7 expression. The Y axis represents the probability of metastasis-free survival.
Overexpression of Hoxb7 impairs HER-2/neu–induced mammary tumor onset
To study the role of Hoxb7 in mammary tumorigenesis in vivo, we generated an MMTV-Hoxb7 transgenic mouse model (Supplementary Fig. S1–S3). Overexpression of Hoxb7 alone was insufficient to form tumors in the MMTV-Hoxb7 transgenic mice during the 2 years of observation. Because our clinical data analysis suggested that overexpression of HOXB7 may render tumors aggressive and promote metastatic features in HER-2/neu–positive breast cancer, we crossed the MMTV-Hoxb7 transgenic mice with MMTV-HER-2/neu transgenic mice carrying the wt rat HER-2/neu gene. MMTV-HER-2/neu transgenic mice develop mammary carcinomas and lung metastasis stochastically with a long latency (19), suggesting that overexpression of wt HER-2/neu alone is not sufficient to initiate tumorigenesis and another genetic or epigenetic event is required for HER-2/neu–mediated tumorigenesis. We theorized that overexpression of Hoxb7 and HER-2/neu in mammary epithelial cells could result in an acceleration of tumorigenesis and an increase in metastatic progression due to cooperation of the two oncogenes, or alternatively, in an inhibition of tumorigenesis due to premature senescence and differentiation (20, 21).
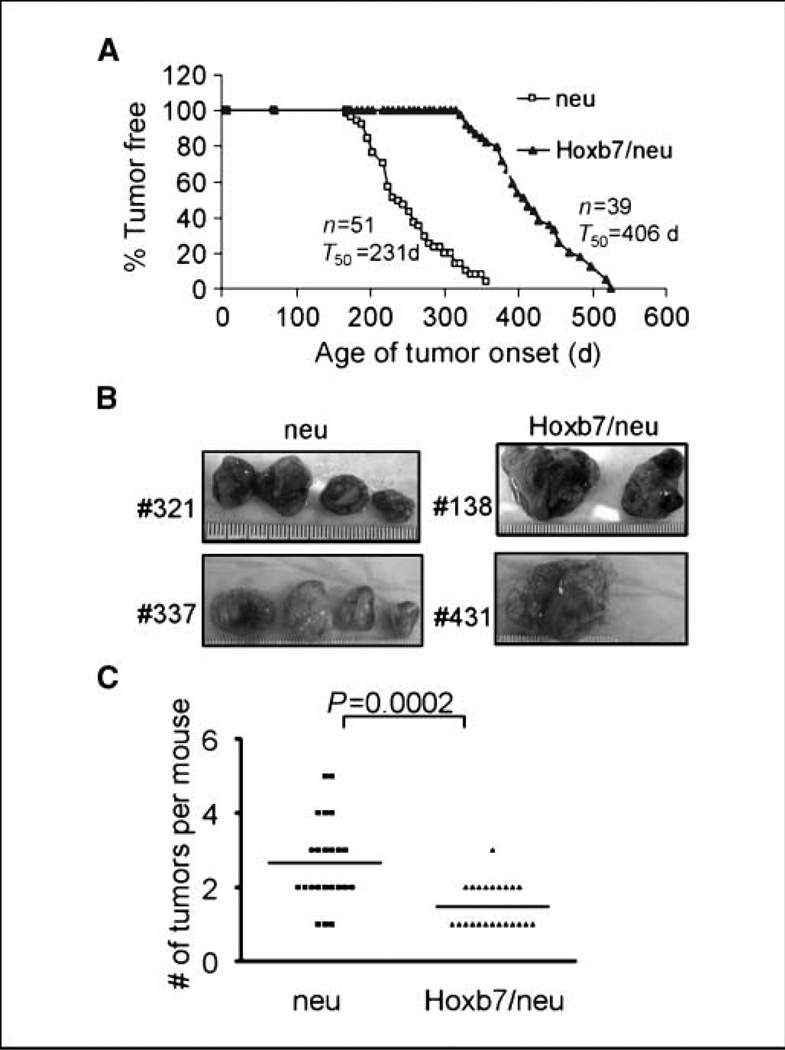
Interestingly, we found that overexpression of Hoxb7 significantly inhibited tumor onset and delayed the latency by ~6 months (231 days versus 406 days; Fig. 2A). A similar phenotype was observed in two other Hoxb7 transgenic lines when crossed with MMTV-HER-2/neu transgenic mice (Supplementary Fig. S4). These data suggested that overexpression of Hoxb7 exerted a repressive effect on tumor initiation by the HER-2/ neu oncogene. Consistent with this observation, upon sacrificing mice at 10 weeks after first palpation of the tumor, we found significantly reduced tumor multiplicity (2.6 ± 0.2 versus 1.5 ± 0.1 tumors per mouse; P = 0.0002) in mice overexpressing both Hoxb7 and HER-2/neu, compared with those expressing the single HER-2/ neu transgene (Fig. 2B and C).
Figure 2.
Overexpression of Hoxb7 inhibits HER-2/neu–induced tumor onset. A, kinetics of tumor formation in the HER-2/neu and Hoxb7/HER-2/neu transgenic mice. The number of tumor-free days for each group of animals is shown. T50 represents the time point when 50% of mice in that group developed tumors. B, tumors harvested from two representative HER-2/neu and Hoxb7/ HER-2/neu transgenic mice showing both size and multiplicity 10 wk after first detection of a palpable mass. C, mammary tumor multiplicity was significantly different (P = 0.002) in the HER-2/neu versus Hoxb7xHER-2/neu mice.
Overexpression of Hoxb7 modulates mammary gland development and rescues MMTV-HER-2/neu–caused defects in ductal elongation
It is possible that the inhibition of tumor onset is attributable to defects in mammary gland development in mice carrying both transgenes. Most steps of mouse mammary gland maturation occur postnatally. This provides an excellent system to study the function of oncogenes during mammary gland development and during tumorigenesis, which occurs as a consequence of a derailed developmental program. Normally, the epithelial ductal tree begins to grow rapidly at around 3 weeks of age upon the release of ovarian hormones. This rapid growth is inhibited when ducts approach the edge of the fat pad at around 10 to 12 weeks of age. During pregnancy, the hormones, progesterone, and prolactin, stimulate secondary branching and formation of lobular alveolar structures in preparation for the production of milk for suckling pups upon parturition. Beginning at day 3 postweaning, involution occurs and milk-producing epithelial cells die through apoptosis (15). To examine the effects of Hoxb7 and neu overexpression in mammary gland development, we performed whole-mount staining analysis in the mammary glands of transgenic mice at different stages of mammary gland development (virgin mice at 8 and 13 weeks old; pregnancy day 10 and involution day 3).
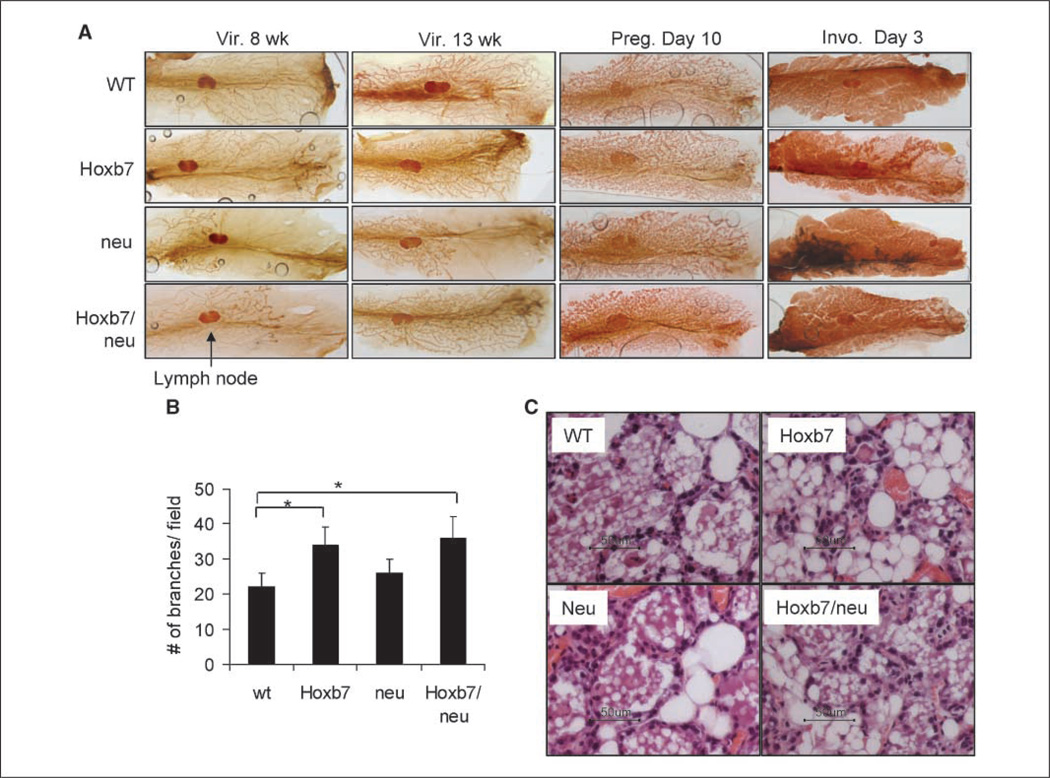
At 8 weeks, the ductal tree in the wt mouse mammary gland filled the fat pad almost completely. The mouse ductal tree of the Hoxb7 transgenic mouse seemed very similar to that in the wt mouse, except that there was a slightly greater level of branching. In the HER-2/neu transgenic mouse, ductal tree development was inhibited, confirming published findings (22, 23). The ductal tree extended only up to the lymph node, which is located in the center of mammary gland. Interestingly enough, in the double transgenic mice, the phenotype in HER-2/neu transgenic mice was partially rescued by Hoxb7, in that the mammary glands in the double transgenic mice grew beyond the lymph node, appearing more like the wt gland. At 13 weeks, very similar phenotypes were observed between wt, HER-2/neu, Hoxb7, and dual transgenic mice. Again, overexpression of Hoxb7 increased side branching and completely rescued the HER-2/neu phenotype (Fig. 3A and B). During pregnancy, there were no significant phenotypic differences in the mammary gland among these mice. At the involution stage, overexpression of Hoxb7 dramatically accelerated the involution process (Fig. 3A and C). At involution day 3, the alveolar structure was still intact in the wt mammary gland and some of epithelial cells begin to mobilize into the luminal spaces of alveoli. On the other hand, in the mammary gland of MMTV-Hoxb7 transgenic mice, most of the alveolar structures had already collapsed into clusters of epithelial cells and more adipocytes seem to be refilling the spaces. HER-2/neu is known to delay this process (24). As seen in Fig. 3C, at this time point, the alveolar structures in the HER-2/ neu mice were largely intact and filled with milk. The phenotype of the mammary gland of the double transgenic mice was very similar to that in the wt mice. In a nutshell, Hoxb7 and HER-2/neu seemed to play opposing roles in mammary gland development. These data are consistent with our finding that Hoxb7 delayed HER-2/neu– induced mammary tumor onset.
Figure 3.
Effects of Hoxb7 and neu overexpression on mammary gland development. A, inguinal mammary fat pads were excised from virgin mice at ages of 8 and 13 wk, pregnant mice at day 10, and involuting mice at day 3. The whole-mount staining analyses were performed as described previously (15). Vir., virgin; Preg., pregnant; Invo., involution. B, analysis of branching of mammary gland whole mounts of 13-week-old virgin mice under the dissecting microscope. All side structures, including secondary/tertiary ductal branches, were counted in three fields per gland (n = 4 animals per group), and the average numbers are presented in the figure. *, P < 0.05. C, H&E-stained section of representative mammary glands at day 3 of involution. Scale bar, 50 µm.
The apparent opposing effects of Hoxb7 and HER-2/neu on mammary gland development prompted us to examine whether Hoxb7 directly down-regulates the expression level of neu in mammary epithelium. Immunochemical analysis and Western blot analysis confirmed that the expression level of neu was not reduced in the mammary epithelium and tumor cells from double transgenic mouse. On the contrary, the expression level of neu was slightly higher in some of tumor cell lines derived from the double transgenic mouse tumors (data not shown and Supplementary Fig. S5). As expected, Hoxb7 expression was detected only in the MMTV-Hoxb7 × MMTV-HER-2/neu transgenic mammary tumor cells (Supplementary Fig. S6). Thus, the inhibition of tumor onset in the MMTV-Hoxb7 × MMTV-HER-2/neu mice was not caused by suppression of expression of the neu oncogene.
Overexpression of Hoxb7 promotes tumor progression and pulmonary metastasis
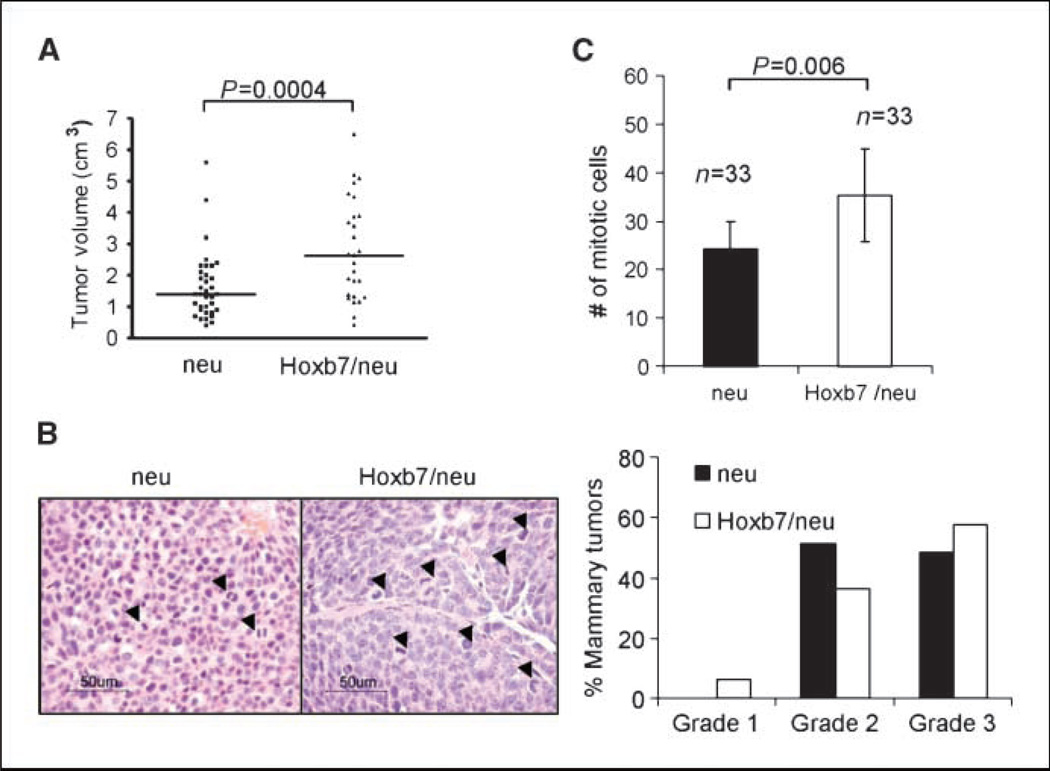
Despite the delay in tumor onset in double transgenic mice, comparison of the size of the first tumor on each animal at a fixed time after detection showed that the tumors in the double transgenic mice were significantly larger than those in the MMTV-neu transgenic mice (1.6 ± 0.2 versus 2.81 ± 0.3 cm in size, P = 0.0004; Fig. 2B and 4A). By histopathology, 57.6% of double transgenic tumors were poorly differentiated with a high histologic grade, <10% tubule formation, prominent nuclear pleomorphism, and evidence of high mitotic index (Fig. 4B). Similarly, in MMTV-HER-2/neu transgenic mice, 48.5% of the tumors were of high grade. Although there was no significant difference in the tumor grade distribution between MMTV-HER-2/neu and double transgenic tumors (Fig. 4B), the MMTV-Hoxb7 × MMTV-HER-2/neu tumors showed a significantly higher mitotic index compared with the MMTV-HER-2/neu transgenic tumors(35.2 ± 9.7 versus 24.2 ± 5.6 mitotic cells in 10 randomly selected fields, P = 0.006; Fig. 4C).
Figure 4.
Overexpression of Hoxb7 promotes tumor growth. A, mammary tumor volume was significantly different (P = 0.004) in the HER-2/neu and Hoxb7/HER-2/neu transgenic mouse. The tumors were harvested at 10 wk after the first detection of a palpable mass. Tumor volume was calculated using the formula: V (cm3) = [(length) / 2 × (width)2]. In each group, tumor volumes of only the earliest detectable tumors were compared. B, the grade distribution of tumors harvested from animals at 70 d after the initial tumor palpation. H&E-stained sections of tumors harvested from HER-2/neu and Hoxb7/HER-2/neu animals. Both tumors are poorly differentiated carcinomas. The nuclear size in the double transgenic tumors showed large variations. Tumors were graded according to the modified Elston and Ellis histologic grading system. Arrows indicate the mitotic cells. C, the number of mitotic cells counted on the H&E-stained tumor sections. Thirty-three randomly selected tumors from each group were analyzed. Mitotic cells were counted in 10 random high-power fields (×400) in each tumor sample. The Y axis represents the total mitotic cell number of 10 counted fields for each sample.
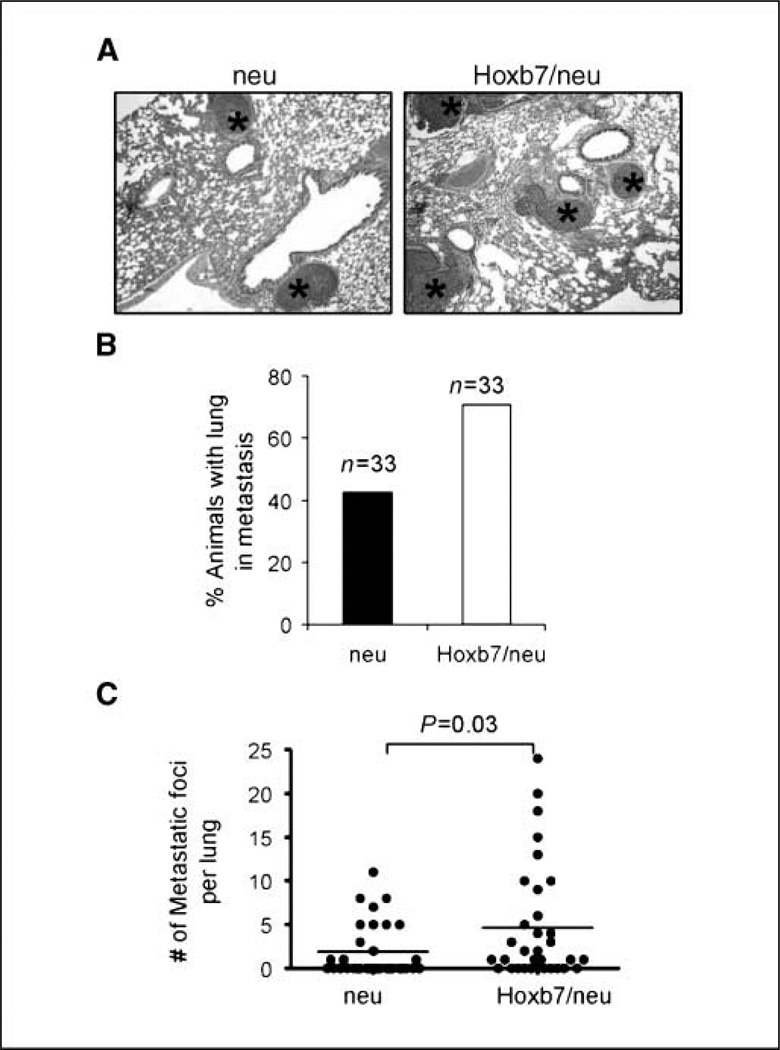
Furthermore, upon microscopic examination of multiple organs, we saw that 42.4% of MMTV-HER-2/neu transgenic mice harbored micrometastasis in the lung. In contrast, a comparatively higher number (66.7%) of double transgenic mice harbored micrometastasis in the lung (Fig. 5A and B). The average number of micrometastases per lung section in the double transgenic mice was more than twice that in the HER-2/neu transgenic mice (4.7 ± 1.1 versus 1.9 ± 0.5 lung foci per mouse; Fig. 5C). These data suggested that Hoxb7 promoted the formation of pulmonary metastatic lesions. No metastases were found in other organs in either MMTV-HER-2/neu or double transgenic mice.
Figure 5.
Hoxb7 transgene increases the frequency of pulmonary metastasis in the double transgenic mice. A, H&E-stained sections of lung from HER-2/neu and Hoxb7/HER-2/neu animals at 10 wk after the initial tumor palpation. Asterisks indicate lung metastasis. B, bar graph of number of animals harboring lung metastasis. C, comparison of the number of micrometastatic foci in the lung per animal in the two groups showed significant differences (P = 0.03 by the unpaired Student’s t test).
Effects of Hoxb7 overexpression on angiogenesis, apoptosis, and proliferation in primary tumor cells
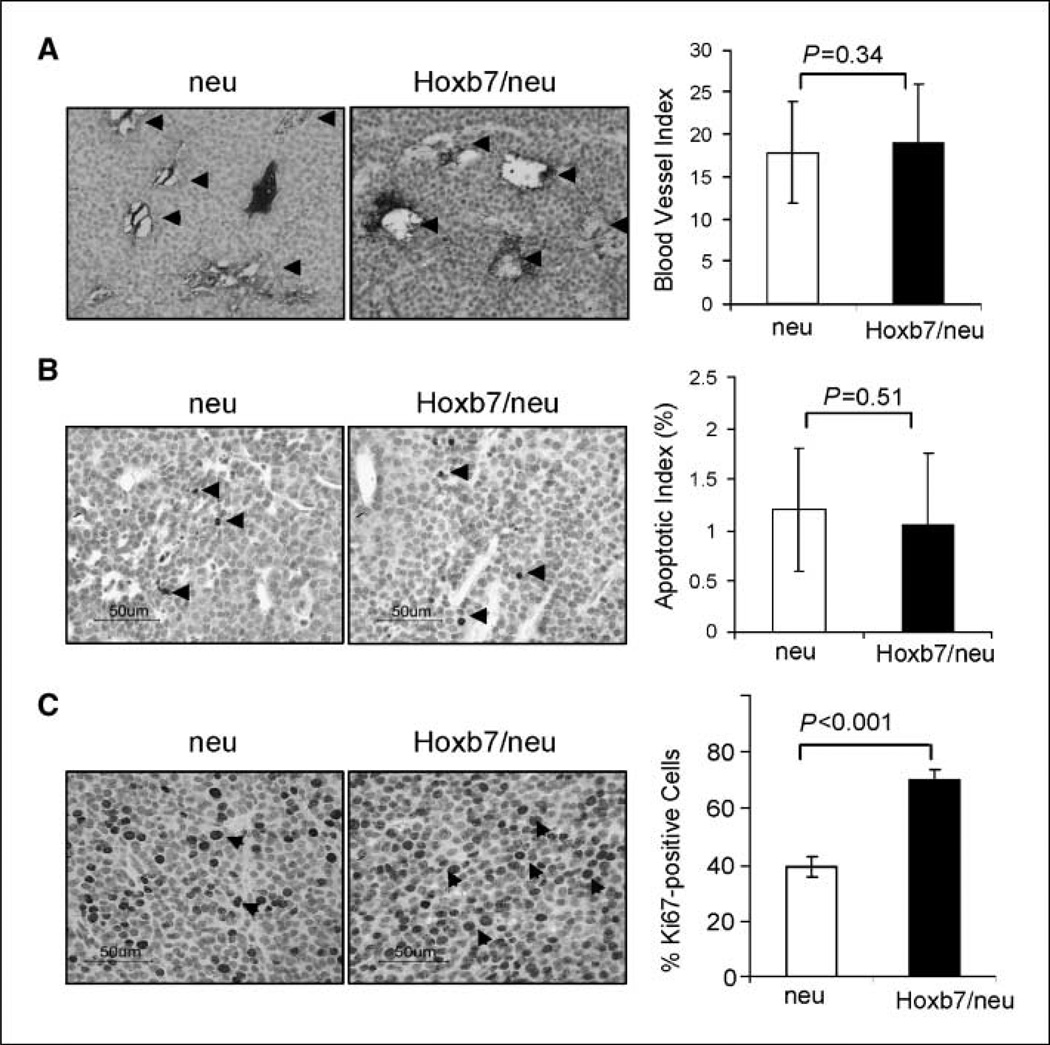
To gain an understanding of how Hoxb7 promoted tumor progression and metastasis, we examined the angiogenesis, cell death, and cell proliferation rate in the primary tumors. Overexpression of HOXB7 in breast cancer cell line SKBR3 cells was shown to promote angiogenesis and proliferation by up-regulating bFGF and a variety of proangiogenic factors (8,10, 11). We examined the expression levels of bFGF in the primary tumors. The expression levels of bFGF were found to be augmented in double transgenic mice tumors compared with that in MMTV-HER-2/neu tumors (Supplementary Fig. S7). Angiogenesis were then assessed by anti-CD34 immunochemical staining of tumors from transgenic mice (Fig. 6A). Immunohistology showed that there was no significant difference in vascularization between MMTV-HER-2/neu and MMTV-Hoxb7 × MMTV-HER-2/neu mice tumors. Similarly, no significant difference in apoptotic death rate between these two types of tumors was found by immunochemical staining assay with an antibody against active caspase-3 (Fig. 6B). In contrast, we found that the tumors from the double transgenic mice had a much higher rate of proliferation compared with tumors arising in MMTV-HER-2/neu transgenic mice (40.7 ± 3.8% versus 64.8 ± 3.6%; P < 0.001; Fig. 6C). Therefore, promotion of cell proliferation was most likely a major mechanism of action of Hoxb7 in the late stages of tumor progression.
Figure 6.
Analysis of angiogenesis, apoptosis, and proliferation in MMTV-neu and Hoxb7/neu tumors. The immunochemical staining analysis was performed as described in Materials and Methods. A, angiogenesis was assessed by counting blood vessels after immunostaining with an anti-CD34 antibody. A total of 33 pairs of tumors were analyzed. The average blood vessel index was presented by counting the number of blood vessels at 200× magnification. The arrows indicate representative blood vessels in both MMTV-neu and Hoxb7/neu tumors. B, apoptotic rate was analyzed by immunostaining analysis with antibody against active caspase-3. The arrows indicate positively staining cells. One thousand cells per tumor were counted at 400× magnification, and the average percentage of positive cells was presented. C, proliferation rate was assessed by immunochemical staining analysis. Fifteen pairs of randomly selected HER-2/neu and Hoxb7/HER-2/neu transgenic tumors were immunohistochemically examined using the anti-Ki67 antibody. One thousand tumor cells per tumor were counted, and the percentage of Ki67-positive cells was calculated. Arrows indicate some of the positive cells.
Discussion
In this study, we have established and characterized a mouse model to study the role of Hoxb7 in mammary tumorigenesis. To our knowledge, we have, for the first time, shown that a deregulated HOX gene plays a critical role in the formation and progression of solid tumors in a genetically engineered mouse model. An unpredicted and intriguing finding was that over-expression of Hoxb7 inhibited MMTV-HER-2/neu–induced tumor onset but, once tumors were formed, promoted tumor progression and metastasis. Our results further supported previous studies that overexpression of HOXB7 in human breast cancer cells increased cell proliferation rate and promoted tumor progression (11). In line with these findings, our clinical data also showed that HER-2– positive tumors with high levels of HOXB7 showed a higher rate of metastasis and a poorer survival outcome.
One of the surprising findings in this study was that over-expression of Hoxb7 in the dual transgenic mice delayed HER-2/ neu–induced tumor onset. At present, we can only speculate on the mechanisms underlying this phenomenon. Inhibition of tumorigenesis by overexpression of other oncogenes was reported recently in a few transgenic mouse models and cell culture systems (20–22, 25–27). There is growing recognition that overexpression of oncogenes can lead to premature growth arrest as a protection against tumor development (28). For example, overexpression of Ras or HER-2/neu in primary cultured cells induced premature senescence and differentiation (21, 22), whereas overexpression of oncogenic MYC triggered apoptosis (29). Only the cells which escape the oncogene-induced failsafe mechanism could eventually be transformed to form tumors. Recent studies have suggested that these escaping cells are most likely stem cells or precursor cells, which may truly be the targets of oncogenic transformation (20). In our double transgenic MMTV-Hoxb7 × MMTV-HER-2/neu mice, we speculate that overexpression of Hoxb7 in primary mammary epithelium triggered premature cellular differentiation, senescence, and/or apoptosis to eliminate large numbers of MMTV-HER-2/neu target cells and thereby protected the gland against MMTV-neu–induced tumorigenesis. In line with this proposed differentiation/senescence function of Hoxb7, the MMTV-Hoxb7 mice display an accelerated involution process, during which most of differentiated epithelial cells die through apoptosis but some of epithelial cells survive (30). This surviving epithelial cell population, named “parity-induced mammary epithelial cell population,” serve as alveolar progenitors in subsequent pregnancies and are likely to be the cellular targets of transformation in MMTV-HER-2/neu transgenic mice (30, 31). Whether overexpression of Hoxb7 specifically eliminated this cell population warrants further investigation. If so, it could provide a rational explanation of why Hoxb7 expression inhibited HER-2–induced tumor onset.
Another possibility is that Hoxb7 may directly or indirectly interfere with HER-2/neu signaling pathways. This hypothesis is supported by the fact that overexpression of Hoxb7 rescued the MMTV-HER-2/neu caused defects in ductal elongation. It is known that overexpression of neu impedes ductal growth accompanying puberty (22, 23). This phenotype seems to be contradictory to the role of neu in promoting cellular proliferation during tumorigenesis. Recently, it was proposed that in the pubertal MMTV transgenic mice neu too has dual but opposing effects: it promotes ERα-dependent proliferation in the hyperplastic tissue but causes premature differentiation with a concomitant decrease in ERα-dependent proliferation during normal ductal morphogenesis (22). Hoxb7 is normally expressed in virgin mammary gland, but its function in ductal morphogenesis is as yet unknown (32). Rescue of the MMTV-HER-2/neu caused phenotype by Hoxb7 during mammary gland development suggested that Hoxb7 may partially block HER-2/neu signaling pathways at this stage. To compensate for this effect, only the cells with relatively high levels of neu expression escaped the suppressive restriction imposed by overexpression of Hoxb7 and became transformed. Consistent with this notion, we observed that the expression levels of neu are slightly higher in double transgenic mouse tumors compared with MMTV-HER-2/neu tumors.
Upon detection, we observed that the double transgenic mouse tumors grew faster and were more aggressive. Examination of angio-genesis, cell proliferation, and apoptosis in these tumors revealed that the cell proliferation rate was significantly higher in double transgenic mice. However, overexpression of Hoxb7 had no dramatic effects on angiogenesis and cell death. It has been reported that overexpression of HOXB7 in human breast cancer cells promoted proliferation and angiogenesis in mouse xenografts by up-regulating bFGF and other proangiogenic factors (10, 11). In this study, as well, the expression levels of bFGF were relatively higher in double transgenic mice. However, contrary to previous reports, similar immunochemical staining did not reveal increased vascularization in Hoxb7/HER-2/neu tumors. These differences are most likely attributable to the different model systems used in these studies.
To our knowledge, this is the first report that dysregulation of HOX gene expression in a genetically engineered animal model dramatically modulated the process of tumor onset and progression. Despite numerous reports of misexpressed HOX genes in a variety of cancers, the loss or gain of HOX gene expression in genetically engineered animals is generally insufficient to cause tumor formation (6, 7). This has led to an argument whether dysregulated HOX gene expression is the cause or consequence of cancer (6). Our results clearly show that HOX genes played a critical role in the multistep process of tumor formation. In addition, we found that the function of Hoxb7 in carcinogenesis seems to be dependent on the physiologic setting. Because factors governing these steps in neoplastic transformation in vivo are largely unknown, it is hard to predict whether other HOX genes will behave in a similar manner or whether HOXB7 will play a dual role in carcinogenesis in other organs as well. Two opposing functions of HOXB7 are supported by other studies in different cellular contexts. On the one hand, it has been reported that HOXB7 exhibited transforming ability in NIH3T3 fibroblasts, a cell line prone to transformation (33). On the other hand, HOXB7 over-expression in hematopoietic stem cells promoted myeloid differentiation (34) and, in multipotent mesenchymal cells, promoted differentiation to smooth muscle cells (35). In yet another study, HOXB7 overexpression in immortalized normal ovarian surface epithelium cells failed to promote anchorage-independent growth, although increased proliferation and reduced contact inhibition were observed (9). These data suggest that elevated HOXB7 expression levels may be associated with higher proliferative activity in tumors, rather than representing a step in the transformation process. These results are consistent with our data that over-expression of Hoxb7 alone was insufficient to cause tumor formation in the mouse mammary gland. However, the possibility that HOXB7 functions in cell transformation by acting in cooperation with other oncoproteins cannot be excluded.
In summary, overexpression of Hoxb7 in the mammary gland of transgenic mice was insufficient to cause tumor formation but dramatically affected HER-2/neu–induced tumorigenesis. It inhibited HER-2/neu–induced tumor onset while it promoted tumor growth and metastasis. Further studies aimed at understanding the mechanisms underlying this unusual phenotype have the potential to lead to identification of a novel target for breast cancer diagnosis and treatment.
Supplementary Material
Acknowledgments
Grant support: Department of Defense DAMD-17-02-1-0426 (H. Chen), Department of Defense Center of Excellence W81XWH-04-1-0595, and National Cancer Institute/Specialized Programs of Research Excellence P50 CA88843 (S. Sukumar). This research was supported, in part, by Intramural Research Program of NIH, National Cancer Institute, Center for Cancer Research, and funded in part with federal funds from National Cancer Institute, NIH, under contract number NO1-CO-12400.
We thank Todd Armstrong and Elizabeth Jaffee for graciously providing the MMTV-HER-2/neu mice.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Krumlauf R. Hox genes in vertebrate development. Cell. 1994;78:191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- 2.Kongsuwan K, Webb E, Housiaux P, Adams JM. Expression of multiple homeobox genes within diverse mammalian haemopoietic lineages. EMBO J. 1988;7:2131–2138. doi: 10.1002/j.1460-2075.1988.tb03052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cillo C. HOX genes in human cancers. Invasion Metastasis. 1994;14:38–49. [PubMed] [Google Scholar]
- 4.Cillo C, Cantile M, Faiella A, Boncinelli E. Homeobox genes in normal and malignant cells. J Cell Physiol. 2001;188:161–169. doi: 10.1002/jcp.1115. [DOI] [PubMed] [Google Scholar]
- 5.Cillo C, Faiella A, Cantile M, Boncinelli E. Homeobox genes and cancer. Exp Cell Res. 1999;248:1–9. doi: 10.1006/excr.1999.4451. [DOI] [PubMed] [Google Scholar]
- 6.Abate-Shen C. Deregulated homeobox gene expression in cancer: cause or consequence? Nat Rev Cancer. 2002;2:777–785. doi: 10.1038/nrc907. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Sukumar S. Role of homeobox genes in normal mammary gland development and breast tumorigenesis. J Mammary Gland Biol Neoplasia. 2003;8:159–175. doi: 10.1023/a:1025996707117. [DOI] [PubMed] [Google Scholar]
- 8.Care A, Silvani A, Meccia E, et al. HOXB7 constitutively activates basic fibroblast growth factor in melanomas. Mol Cell Biol. 1996;16:4842–4851. doi: 10.1128/mcb.16.9.4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naora H, Yang YQ, Montz FJ, Seidman JD, Kurman RJ, Roden RB. A serologically identified tumor antigen encoded by a homeobox gene promotes growth of ovarian epithelial cells. Proc Natl Acad Sci U S A. 2001;98:4060–4065. doi: 10.1073/pnas.071594398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Care A, Felicetti F, Meccia E, et al. HOXB7: a key factor for tumor-associated angiogenic switch. Cancer Res. 2001;61:6532–6539. [PubMed] [Google Scholar]
- 11.Care A, Silvani A, Meccia E, Mattia G, Peschle C, Colombo MP. Transduction of the SkBr3 breast carcinoma cell line with the HOXB7 gene induces bFGF expression, increases cell proliferation and reduces growth factor dependence. Oncogene. 1998;16:3285–3289. doi: 10.1038/sj.onc.1201875. [DOI] [PubMed] [Google Scholar]
- 12.Wu X, Chen H, Parker B, et al. HOXB7, a homeodomain protein, is overexpressed in breast cancer and confers epithelial-mesenchymal transition. Cancer Res. 2006;66:9527–9534. doi: 10.1158/0008-5472.CAN-05-4470. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Klijn JG, Zhang Y, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365:671–679. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- 14.Gong Y, Yan K, Lin F, et al. Determination of oestrogen-receptor status and ERBB2 status of breast carcinoma: a gene-expression profiling study. Lancet Oncol. 2007;8:203–211. doi: 10.1016/S1470-2045(07)70042-6. [DOI] [PubMed] [Google Scholar]
- 15.Cardiff RD, Wagner U, Hennighausen L. Mammary cancer in humans and mice: a tutorial for comparative pathology. The CD-ROM. J Mammary Gland Biol Neoplasia. 2000;5:243–244. doi: 10.1023/a:1026451607575. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, Rubin E, Zhang H, et al. Identification of transcriptional targets of HOXA5. J Biol Chem. 2005;280:19373–19380. doi: 10.1074/jbc.M413528200. [DOI] [PubMed] [Google Scholar]
- 17.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer I. The value of histologic grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 18.Kominsky SL, Argani P, Korz D, et al. Loss of the tight junction protein claudin-7 correlates with histo-logical grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast. Oncogene. 2003;22:2021–2033. doi: 10.1038/sj.onc.1206199. [DOI] [PubMed] [Google Scholar]
- 19.Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci U S A. 1992;89:10578–10582. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarkisian CJ, Keister BA, Stairs DB, Boxer RB, Moody SE, Chodosh LA. Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat Cell Biol. 2007;9:493–505. doi: 10.1038/ncb1567. [DOI] [PubMed] [Google Scholar]
- 21.Trost TM, Lausch EU, Fees SA, et al. Premature senescence is a primary fail-safe mechanism of ERBB2-driven tumorigenesis in breast carcinoma cells. Cancer Res. 2005;65:840–849. [PubMed] [Google Scholar]
- 22.Shyamala G, Chou YC, Cardiff RD, Vargis E. Effect of c-neu/ErbB2 expression levels on estrogen receptor α -dependent proliferation in mammary epithelial cells: implications for breast cancer biology. Cancer Res. 2006;66:10391–10398. doi: 10.1158/0008-5472.CAN-06-0321. [DOI] [PubMed] [Google Scholar]
- 23.Mukherjee S, Louie SG, Campbell M, Esserman L, Shyamala G. Ductal growth is impeded in mammary glands of C-neu transgenic mice. Oncogene. 2000;19:5982–5987. doi: 10.1038/sj.onc.1203964. [DOI] [PubMed] [Google Scholar]
- 24.Lazar H, Baltzer A, Gimmi C, Marti A, Jaggi R. Over-expression of erbB-2/neu is paralleled by inhibition of mouse-mammary-epithelial-cell differentiation and developmental apoptosis. Int J Cancer. 2000;85:578–583. [PubMed] [Google Scholar]
- 25.Siegel PM, Shu W, Cardiff RD, Muller WJ, Massague J. Transforming growth factor β signaling impairs Neu-induced mammary tumorigenesis while promoting pulmonary metastasis. Proc Natl Acad Sci U S A. 2003;100:8430–8435. doi: 10.1073/pnas.0932636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kordon EC, McKnight RA, Jhappan C, Hennighausen L, Merlino G, Smith GH. Ectopic TGF β1 expression in the secretory mammary epithelium induces early senescence of the epithelial stem cell population. Dev Biol. 1995;168:47–61. doi: 10.1006/dbio.1995.1060. [DOI] [PubMed] [Google Scholar]
- 27.Braig M, Lee S, Loddenkemper C, et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–665. doi: 10.1038/nature03841. [DOI] [PubMed] [Google Scholar]
- 28.Sharpless NE, DePinho RA. Cancer: crime and punishment. Nature. 2005;436:636–637. doi: 10.1038/436636a. [DOI] [PubMed] [Google Scholar]
- 29.Pelengaris S, Khan M, Evan GI. Suppression of Mycinduced apoptosis in β cells exposes multiple oncogenic properties of Myc and triggers carcinogenic progression. Cell. 2002;109:321–334. doi: 10.1016/s0092-8674(02)00738-9. [DOI] [PubMed] [Google Scholar]
- 30.Wagner KU, Boulanger CA, Henry MD, Sgagias M, Hennighausen L, Smith GH. An adjunct mammary epithelial cell population in parous females: its role in functional adaptation and tissue renewal. Development. 2002;129:1377–1386. doi: 10.1242/dev.129.6.1377. [DOI] [PubMed] [Google Scholar]
- 31.Henry MD, Triplett AA, Oh KB, Smith GH, Wagner KU. Parity-induced mammary epithelial cells facilitate tumorigenesis in MMTV-neu transgenic mice. Oncogene. 2004;23:6980–6985. doi: 10.1038/sj.onc.1207827. [DOI] [PubMed] [Google Scholar]
- 32.Srebrow A, Friedmann Y, Ravanpay A, Daniel CW, Bissell MJ. Expression of Hoxa-1 and Hoxb-7 is regulated by extracellular matrix-dependent signals in mammary epithelial cells. J Cell Biochem. 1998;69:377–391. doi: 10.1002/(sici)1097-4644(19980615)69:4<377::aid-jcb1>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 33.Maulbecker CC, Gruss P. The oncogenic potential of deregulated homeobox genes. Cell Growth Differ. 1993;4:431–441. [PubMed] [Google Scholar]
- 34.Care A, Valtieri M, Mattia G, et al. Enforced expression of HOXB7 promotes hematopoietic stem cell proliferation and myeloid-restricted progenitor differentiation. Oncogene. 1999;18:1993–2001. doi: 10.1038/sj.onc.1202498. [DOI] [PubMed] [Google Scholar]
- 35.Bostrom K, Tintut Y, Kao SC, Stanford WP, Demer LL. HOXB7 overexpression promotes differentiation of C3H10T1/2 cells to smooth muscle cells. J Cell Biochem. 2000;78:210–221. doi: 10.1002/(sici)1097-4644(20000801)78:2<210::aid-jcb4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.