Abstract
The prostate gland is the most common site of cancer and the second leading cause of cancer death in American men. Recent emerging molecular biological technologies help us to know that epigenetic alterations such as DNA methylation within the regulatory (promoter) regions of genes are associated with transcriptional silencing in cancer. Promoter hypermethylation of critical pathway genes could be potential biomarkers and therapeutic targets for prostate cancer. In this chapter, we updated current information on methylated genes associated with the development and progression of prostate cancer. Over 40 genes have been investigated for methylation in promoter region in prostate cancer. These methylated genes are involved in critical pathways, such as DNA repair, metabolism, and invasion/metastasis. The role of hypermethylated genes in regulation of critical pathways in prostate cancer is discussed. These findings may provide new information of the pathogenesis, the exciting potential to be predictive and to provide personalized treatment of prostate cancer. Indeed, some epigenetic alterations in prostate tumors are being translated into clinical practice for therapeutic use.
Keywords: Prostate cancer, DNA methylation, Epigenetic variation, Biomarker
1. Introduction
Prostate cancer is the most common type of cancer and the second leading cause of cancer mortality in the American men. One man in six will develop prostate cancer during his lifetime, and one man in 34 will die of the disease (1). In 2010, it is estimated that 217,730 new cases will be diagnosed in the United States, and 32,050 men will die from the disease (2). The low mortality rate and gradual decrease of incidence rates, from 2000 to 2006, suggest that public awareness of early detection and advanced treatments of prostate cancer has begun to affect prostate cancer outcomes. However, the probability of developing prostate cancer sharply increases with age, e.g., ~30-fold increase among men over 40 years of age, compared to men under 40 years old. The aging of the current population means that the disease will become an even greater public health problem in the future.
There are substantial individual differences in the risk or progression of prostate cancer. In some patients with prostate cancer, the disease progresses relatively slow. In these cases, patients often die with prostate cancer rather than from prostate cancer. However, some cases grow aggressively and metastasize through the bloodstream and lymphatic system to other parts of the body. Currently, there are two important clinical challenges. The first challenge is the early detection of prostate cancer. Digital rectal examination (DRE) and serum prostate-specific antigen (PSA) are two main diagnostic tools. There is a considerable overlap in PSA levels between patients with prostate cancer and patients with benign prostatic hyperplasia (BPH). Approximately 25% of patients with prostate cancer show no elevation of serum PSA and must be diagnosed by other methods (3). Therefore, the identification of biomarkers that can facilitate the diagnosis of prostate cancer at the early stages could improve the current standard of treatments. The second challenge is to determine which of prostate cancer’s clinical forms a patient is presenting with, i.e., aggressive vs. indolent. This is critically important information given the significant morbidity associated with treatment interventions and could eventually help distinguish men who need intensive treatment from those who may be better served by watchful waiting. Currently, the level of PSA, clinical stage, and the grade of tumor (Gleason score) are used to estimate prognosis and determine treatment modalities. To overcome limitations of PSA and DRE, new biomarkers are demanded to improve the outcome of prostate cancer.
2. Role of DNA Methylation in the Promoter Regions in Prostate Cancer
Development and progression of prostate cancer are results of the accumulation of genetic and epigenetic alterations. Although genetic changes are involved in the inactivation of genes with important anticancer functions (e.g., tumor suppressor and DNA repair genes), DNA methylation in a promoter region is an important epigenetic mechanism for the downregulation (silencing) of expression of these genes. DNA methylation in the promoter region of tumor suppressor genes appears to occur at early stages of carcinogenesis and occurs with various frequencies. Therefore, epigenetic changes have the potential to be a new generation of biomarkers. Several types of epigenetic changes have been reported for prostate cancer including DNA hypermethylation, loss of imprinting, and altered histone modification patterns.
CpG islands are CpG-rich areas of 200 bp to several kilobases in length, usually located near the promoters of highly expressed genes, and are the sites of common methylation in human tumors (4), including the prostate. A common molecular feature associated with tumorigenesis is hypermethylation of cytosines 5′ to guanosines (CpG) within the regulatory (promoter) region of suppressor gene genomic DNA (5–8). 5-methyl cytosine is unstable and mutates to thymine and methylated CpG sites degrade to TpG/CpA. In tumors, many CpG islands exhibit aberrant hypermethylation, resulting in gene silencing (Fig. 1). Many tumor suppressor genes have been found to be silenced by promoter hypermethylation in tumors.
Fig. 1.
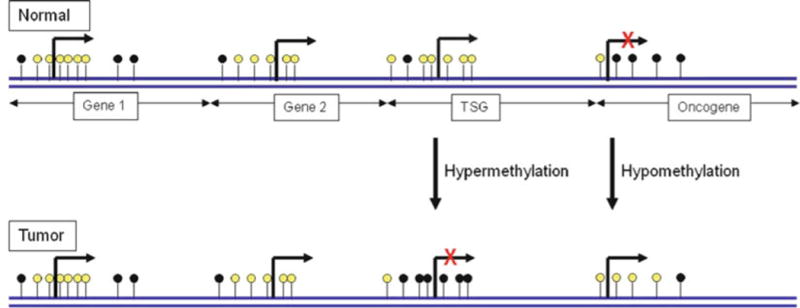
Role of DNA methylation in cancer: unmethylated and methylated CpG sites are indicated by white and black circles, respectively. This figure shows a representative region of genomic DNA in normal and tumor cell. The promoter regions in gene1, gene2, and tumor suppressor gene are rarely methylated in normal cells and, therefore, expressed. Cytosines 5′ to guanosines (CpG) islands in promoter region of tumor suppressor gene are methylated, and it results in gene silencing. Conversely, hypomethylation in the promoter region of oncogene in tumor reactivates transcription.
It is firmly established that an increase of methylation across the promoter region affects transcription of genes. However, how methylated genes are downregulated is not completely known. Furthermore, the extent of methylation in the CpG islands required for gene silencing is not clear except for a short list of genes (9–18). Yet, regardless of mechanism, the observation of methylated promoter regions in silenced tumor suppressor genes in prostate tumor tissues suggests that DNA methylation may indicate a significant association with carcinogenesis and progression of prostate cancer.
3. Hypermethylated Genes in Prostate Tumor
The majority of previous publications in epigenetic research in prostate cancer focused on DNA hypermethylation. Indeed, a gene silencing by DNA hypermethylation in the promoter region is a more common event than a gene silencing by DNA mutations in carcinogenesis. Numerous studies on various hypermethylated genes in different cancers suggest that this is a key part of the carcinogenesis and progression of cancer.
Currently, over 40 genes have been investigated for their frequencies of hypermethylation and for their potential role in prostate cancer (Table 1). Most data in Table 1 were obtained from prostate tumor tissues. The functions of tumor suppressor genes in prostate cancer fall into four major categories: tumor suppressor genes, tumor cell invasion/metastasis, metabolism, and DNA repair. Defected function of these genes by promoter hypermethylation can contribute to carcinogenesis and progression of prostate cancer.
Table 1.
Frequencies of methylated genes in prostate tumor and biosamples
| Gene | Common name | Function | Frequency | References |
|---|---|---|---|---|
| ALDHla2 | Aldehyde dehydrogenase 1 family, member A2 | Tumor suppressor (synthesis of RA) | 100% (7/7)a | (208) |
|
| ||||
| ALDHla3 | Aldehyde dehydrogenase 1 family, member A3 | Tumor suppressor (synthesis of RA) | 21% (5/24) | (48) |
|
| ||||
| AFC | Adenomatous polyposis coli | Tumor suppressor | 12% (2/17)b | (194) |
| 90% (66/73) | (30) | |||
| 14% (11/76)c | (89) | |||
| 92% (36/39)c | (165) | |||
| 57% (21/37) | (92) | |||
| 27% (27/101) | (29) | |||
| 100% (118/118) | (25) | |||
| 41% (182/447) | (216) | |||
| 79% (48/61) | (203) | |||
| 65% (117/179) | (121) | |||
| 3.0d | (51) | |||
| 83% (44/53)c | (85) | |||
| 73% (131/179) | (121) | |||
| 27% (21/79) | (50) | |||
| 82% (59/72) | (203) | |||
| 64% (109/170) | (191) | |||
| 83% (65/78) | (88) | |||
| 51% (48/95)b | (36) | |||
| 51% (58/113)b | (183) | |||
| 51% (18/35) | (120) | |||
| 48% (25/52)b | (31) | |||
| 78% (88/113) | (35) | |||
|
| ||||
| AR | Androgen receptor | Steroid hormonal response | 29% (2/7)a | (148) |
| 13% (2/15) | (145) | |||
| 8% (3/38) | (146) | |||
| 25% (6/24) | (147) | |||
| 15% (16/109) | (56) | |||
| 39% (30/76)c | (132) | |||
|
| ||||
| CAV1 | Caveolin-1 | Tumor suppressor | 91% (20/22) | (20) |
| 100% (4/4) | (21) | |||
| 0% (0/8) | (22) | |||
|
| ||||
| CCNA1 | Cyclin A1 | Tumor suppressor | 79% (19/24) | (48) |
|
| ||||
| CCND2 | Cyclin D2 | Tumor suppressor | 25% (21/83) | (50) |
| 32% (32/101) | (45) | |||
| 99% (117/118) | (49) | |||
| 1.78d | (51) | |||
|
| ||||
| CD44 | CD44 molecule | Tumor invasion/metastasis (lipid-raft-associated) | 78% (31/40) | (219) |
| 33% (30/90) | (22) | |||
| 3% (1/30)c,e | (28) | |||
| 68% (27/40) | (218) | |||
| 32% (36/111) | (177) | |||
| 72% (58/81) | (94) | |||
| 0% (0/18)c | (86) | |||
| 20% (2/8) | (256) | |||
| 22% (39/179) | (121) | |||
|
| ||||
| CDH1 | E-cadherin | Tumor invasion/metastasis (lipid-raft-associated) | 31% (29/95)b | (36) |
| 0% (0/30)c,e | (28) | |||
| 54% (19/35) | (221) | |||
| 70% (14/20)a | (222) | |||
| 27% (27/101) | (29) | |||
| 0% (0/111) | (177) | |||
| 0% (0/73) | (30) | |||
| 4% (5/114) | (35) | |||
| 61% (49/81) | (94) | |||
| 30% (6/20) | (159) | |||
| 77% (40/52)b | (31) | |||
| 24% (22/90) | (22) | |||
| 69% (70/101) | (45) | |||
| 21% (38/179) | (121) | |||
|
| ||||
| CDH13 | H-cadherin | Tumor invasion/metastasis (lipid-raft-associated) | 45% (68/151) | (225) |
| 31% (31/101) | (29) | |||
| 54% (96/179) | (121) | |||
|
| ||||
| CDKN2A (p16INK4a) | Cyclin-dependent kinase inhibitor 2A | Tumor suppressor | 73% (8/11) | (27) |
| 3% (3/101) | (29) | |||
| 6% (4/73) | (30) | |||
| 77% (91/118) | (25) | |||
| 66% (21/32) | (26) | |||
| 13% (3/24) | (34) | |||
| 70% (21/30) | (32) | |||
| 4% (5/113) | (35) | |||
| 37% (19/52)b | (31) | |||
| 15% (8/53) | (37) | |||
| 12% (11/95)b | (36) | |||
| 10% (3/30)c | (28) | |||
| 60% (3/5)a | (33) | |||
|
| ||||
| CRBP1 | Cellular retinol-binding protein 1 | Steroid hormonal response (control of retinoids) | 81% (96/118) | (25) |
| 47% (17/36) | (188) | |||
| 34% (34/101) | (189) | |||
|
| ||||
| P14ARF | Cyclin-dependent kinase inhibitor 2A | Tumor suppressor | 4% (2/53) | (37) |
| 6% (6/95)b | (36) | |||
| 37% (19/52)b | (31) | |||
| 4% (5/118) | (25) | |||
| 0% (0/73) | (30) | |||
| 3% (1/32) | (42) | |||
| 6% (1/16) | (26) | |||
| 22% (2/9) | (27) | |||
|
| ||||
| DAPK | Death-associated protein kinase | Tumor suppressor | 36% (39/109) | (56) |
| 1% (1/101) | (29) | |||
| 0% (0/73) | (30) | |||
| 28% (27/95)b | (36) | |||
| 10.9–18.7f | (57) | |||
|
| ||||
| EDNRB | Endothelin receptor type B | Steroid hormonal response (cell adhesion) | 49% (36/73) | (30) |
| 72% (58/81) | (94) | |||
| 70% (23/35) | (193) | |||
| 100% (80/80) | (87) | |||
| 50% (9/18)b | (86) | |||
| 83% (40/48) | (195) | |||
| 66% (8/12)b | (194) | |||
|
| ||||
| EPHA7 | EPH receptor A7 | Steroid hormonal response (cell differentiation, apoptosis) | 42% (20/48) | (197) |
|
| ||||
| Esr1 | Estrogen receptor alpha | Steroid hormonal response | 90% (28/31) | (157) |
| 19% (14/73) | (30) | |||
| 95% (36/38) | (146) | |||
| 41% (64/156) | (156) | |||
|
| ||||
| Esr2 | Estrogen receptor beta | Steroid hormonal response | 83% (19/23) | (160) |
| 65% (13/20) | (159) | |||
| 79% (30/38) | (146) | |||
|
| ||||
| FHIT | Fragile histidine triad gene | Tumor suppressor | 15% (15/101) | (29) |
| 65% (15/23) | (67) | |||
| > 106 | (57) | |||
|
| ||||
| FyN | SRC family tyrosine kinase | Tumor invasion/metastasis (cell differentiation) | 67% (12/18) | (242) |
|
| ||||
| GSTP1 | Glutathione S transferase P1 | Steroid hormonal response (metabolism) | 58% (7/12) | (175) |
| 81% (68/84)c | (179) | |||
| 39% (31/80)b | (179) | |||
| 26% (20/76) | (89) | |||
| 86% (37/43) | (164) | |||
| 85% (89/105) | (176) | |||
| 36% (36/101) | (29) | |||
| 88% (96/109) | (56) | |||
| 84% (99/118) | (177) | |||
| 100% (18/18) | (178) | |||
| 95% (69/73) | (30) | |||
| 87% (32/37) | (92) | |||
| 79% (22/28)b | (170) | |||
| 71% (43/61) | (169) | |||
| 95% (112/118) | (25) | |||
| 75% (24/32) | (42) | |||
| 72% (58/81) | (94) | |||
| 79% (89/113) | (35) | |||
| 48% (25/52)b | (31) | |||
| 83% (79/95)b | (36) | |||
| 42% (71/168)c | (87) | |||
| 28% (5/18)c | (86) | |||
| 93% (74/80) | (87) | |||
| 100% (20/20) | (168) | |||
| 91% (52/57) | (171) | |||
| 75% (24/32) | (172) | |||
| 44% (4/9)g | (180) | |||
| 90% (18/20) | (181) | |||
| 94% (16/17) | (173) | |||
| 42% (71/168)c | (182) | |||
| 91% (63/69) | (174) | |||
|
| ||||
| HIC1 | Hypermethylated in cancer 1 | Tumor suppressor | 99% (108/109) | (56) |
| 67% (52/78) | (75) | |||
| 100% (73/73) | (30) | |||
|
| ||||
| LPL | Lipoprotein lipase | Tumor suppressor (metabolism of lipids) | 38% (21/56) | (67) |
|
| ||||
| MDR1/ABCB1 | Multidrug resistance 1, ATP-binding cassette, subfamily B (MDR/TAP), member 1 | Steroid hormonal response | 48% (36/76) | (89) |
| 83% (15/18)c | (86) | |||
| 55% (97/177) | (190) | |||
| 88% (64/73) | (30) | |||
| 100% (35/35) | (120) | |||
| 51% (91/179) | (121) | |||
|
| ||||
| MGMT | O6-methylguanine DNA methyltransferase | DNA repair | 26% (14/53) | (37) |
| 34% (21/62) | (251) | |||
| 2% (2/109) | (56) | |||
| 19% (22/118) | (25) | |||
| 25% (8/32) | (42) | |||
| 76% (28/37) | (92) | |||
| 0% (0/101) | (29) | |||
| l% (1/73) | (30) | |||
| 19% (10/52)b | (31) | |||
| 15% (14/95)b | (36) | |||
|
| ||||
| NEP | Neuroepithelial tyrosine kinase | Tumor cell invasion/metastasis | 17% (3/18)c | (86) |
| 14% (3/21) | (243) | |||
| 73% (16/22) | (245) | |||
|
| ||||
| NKX3.1 | Tumor suppress (defense for oxidative damage) | 83% (33/40) | (128) | |
|
| ||||
| NKX2.5 | Tumor suppress (defense for oxidative damage) | 30% (6/20) | (130) | |
|
| ||||
| PITX2 | Paired-like homeodomain 2 | Tumor suppress | 3.4d | (81) |
| 2.99d | (82) | |||
| 100% (17/17) | (83) | |||
|
| ||||
| PTGS2 | Prostaglandin-endoperoxide synthase 2 | Tumor suppressor | 88% (64/73) | (30) |
| 11% (8/76) | (89) | |||
| 71% (38/53) | (85) | |||
| 68% (54/80) | (87) | |||
| 65% (51/78) | (88) | |||
| 0% (0/18)c | (86) | |||
|
| ||||
| RARβ | Retinoic acid receptor beta | Steroid hormonal response | 79% (11/14) | (162) |
| 71% (25/35) | (120) | |||
| 91% (39/43) | (164) | |||
| 79% (33/42)c | (165) | |||
| 53% (54/101) | (29) | |||
| 78% (85/109) | (56) | |||
| 84% (42/50) | (163) | |||
| 70% (79/113) | (35) | |||
| 35% (18/52)b | (31) | |||
| 40% (32/81) | (94) | |||
| 39% (7/18)c | (86) | |||
| 62% (59/95)b | (36) | |||
|
| ||||
| RASSF1A | Ras association domain family 1 | Tumor suppressor | 21% (16/76) | (89) |
| 71% (37/52) | (91) | |||
| 99% (117/118) | (25) | |||
| 53% (54/101) | (29) | |||
| 96% (70/73) | (30) | |||
| 84% (31/37) | (92) | |||
| 74% (97/131) | (93) | |||
| 73% (38/52)b | (31) | |||
| 49% (40/81) | (94) | |||
| 78% (88/113) | (35) | |||
| 78% (74/95)b | (36) | |||
| 17% (3/18)c | (86) | |||
| 50% (7/14) | (95) | |||
|
| ||||
| S100A2 | S100 calcium-binding protein A2 | Tumor cell invasion/metastasis | 94% (32/34) | (229) |
| 99% (117/118) | (25) | |||
|
| ||||
| S100A6 | S100 calcium-binding protein A6 | 52% (14/27) | (229) | |
|
| ||||
| SFN | 14-3-3σ | Tumor suppressor | 87% (45/52)c | (132) |
| 99% (121/122) | (134) | |||
|
| ||||
| SLC5A8 | Solute carrier family 5, member 8 | Tumor suppressor | 70% (7/10) | (110) |
|
| ||||
| SLC18A2 | Vesicular monoamine transporter 2 | Tumor suppressor | 88% (15/17) | (114) |
|
| ||||
| TIG1 | Tazarotene-induced gene 1 | Steroid hormonal response (chloroplast trigger factor) | 53% (26/50) | (163) |
| 55% (17/31) | (204) | |||
| 70% (43/61) | (203) | |||
| 10% (16/168)c | (182) | |||
| 96% (77/80) | (87) | |||
| 42% (75/179) | (121) | |||
|
| ||||
| TIMP-2 | Tissue inhibitor of metalloproteinase-2 | Tumor cell invasion/metastasis | 60% (25/42) | (234) |
|
| ||||
| TIMP-3 | Tissue inhibitor of metalloproteinase-3 | Tumor cell invasion/metastasis | 41% (37/91)b | (36) |
| 37% (19/52)b | (31) | |||
| 6% (7/109) | (56) | |||
| 97% (114/118) | (25) | |||
| 0% (0/73) | (30) | |||
|
| ||||
| TNFRSFlOC/DcR1 | TNF receptor superfamily, member 10c | Tumor suppressor | 65% (117/180) | (121) |
| 50% (25/50) | (117) | |||
| 78% (46/59) | (116) | |||
| 0% (0/35) | (120) | |||
|
| ||||
| TNFRSF1OD/DcR2 | TNF receptor superfamily, member 10D | Tumor suppressor | 38% (5/8) | (119) |
Cell culture
Urine samples
Serum DNA
Hazard ratio
Bone marrow
Methylation fold compared to normal cells
Ejaculates
3.1. Tumor Suppressors Genes
3.1.1. Caveolin-1 (CAV1)
Caveolin-1 (CAV1) is known as a tumor suppressor gene and involved in the vesicular transport, cholesterol balance, transformation, and tumorigenesis. Recent studies reported the dual function of CAV1 both as a tumor suppressor gene and metastasis-promoting gene (19, 20).
Cui et al. found that 91% (20/22) of cases showed differential hypermethylation in the prostate tumor tissues when compared with adjacent normal tissues (20). Increased DNA methylation of CAV1 was correlated with biochemical recurrence. Therefore, CAV1 plays a role as a tumor suppressor gene which is silenced by hypermethylation in carcinogenesis in prostate. A recent study supports that CAV1 is downregulated in prostate tumor due to hypermethylation in the promoter region of CAV1 (21). However, Woodson et al. did not observe CAV1 methylation in prostate tumor tissues (22). Karam et al. reported overexpression of CAV1 as an established feature of prostate cancer and aggressive PSA recurrence (23). Moreover, CAV1 is reported to upregulate fatty acid synthase (FASN), a tumor promoter, in the progression of prostate cancer (24). These data suggest that the methylation status of CAV1 may not be a reliable biomarker for prostate cancer.
3.1.2. Cyclin-Dependent Kinase Inhibitors
The tumor suppressor gene CDKN2(p16) is one of the cyclin-dependent kinase inhibitors (CDKIs). CDKN2A(p16INK4a) is a key protein in the signaling pathway, which can be damaged by a variety of genetic and epigenetic changes including hypermethylation in prostate tumors. Aberrant CDKI expression is observed in many tumor tissues including prostate (25–28). The reported frequencies of CDKN2A promoter methylation are inconsistent in prostate tumors, ranging from 0 to 77% (25–27, 29–36). Perhaps these inconsistent results are due to different detection methods and/or different targets of methylated loci. For example, Gu et al. identified DNA methylation at the Smal site for 21 of 30 samples and found only one sample had an altered methylation pattern at the Smal site downstream of exon 1 of the CDKN2A (32). Since Herman et al. first reported inactivation of CDKN/p16 by DNA methylation in prostate tumors (33), other researchers have investigated the role of hypermethylated CDKN2A in carcinogenesis and progression of prostate cancer (25–27, 29–35). Nguyen et al. observed methylation of p16INK4a only in exon 2. Although methylation at exon 2 may not be functional, this exon 2 methylation may be a potential biomarker for prostate tumor because of a high prevalence of methylation in tumor tissues (27). These results were confirmed by other groups, who reported that methylation occurred in the promoter region in 9%, 15% of tumors in exon 1 (26, 37), and 66% in exon 2 (26). Jeronimo et al. found that the p16INK4a gene was frequently methylated in tumor tissues (77%). However, the high frequency of methylation was also found in BPH (25). These data suggested that p16INK4a methylation may be a potential biomarker for an early detection of prostate cancer.
Another CDKI, the CDKN2A/p14ARF, generated from an alternative splicing process that replaces the first exon of p16INK4a, has been known as a growth suppressor. Therefore, epigenetic alterations of p14ARF may affect p16INK4a/RB1 pathways in the tumorigenesis and progression of prostate cancer. The p14ARF promoter has been methylated in various cancers, glioma (38), bladder (39), leukemia (40), head and neck (41), and prostate cancers (25–27, 30, 31, 36, 37, 42). Based upon eight independent studies, frequencies of p14ARF methylation in prostate cancer range from 0 to 37% (25–27, 30, 31, 36, 37, 42). With the exception of two studies (27, 31), most studies reported low methylation frequencies that ranged from 0 to 6%. The p16INK4a and p14ARF are frequently comethylated, which may deregulate the RB1 or p53 pathway (42). However, promoter methylation in p14ARF is rare in prostate tumors. Therefore, methylation in p16INK4a rather than p14ARF may be the predominant event in the INK4a/ARF loci in tumor tissues.
3.1.3. Cyclin A1 (CCNA1) and Cyclin D2 (CCND2)
The cell cycle is controlled by a family of cyclin-dependent kinases (CDKs). Cyclin A1 (CCNA1) activates two different CDKs and functions in both S phase and G2 (43, 44), while cyclin D2 (CCND2) is involved in the regulation of transition from G1 to S (45). Abnormal expression of CCND2 may disrupt the normal cell cycle, and therefore, it is considered as both an oncogene and tumor suppressor gene. Aaltomaa et al. reported that expressions of CCNA1 and CCND2 were interrelated in prostate cancer tissues (46, 47).
Shames et al. observed a higher frequency of hypermethylation of CCNA1 in both prostate tumors and benign tissues (48). However, Wegiel et al. reported that levels of CCNA1 protein and mRNA expression were significantly higher in prostate tumors than in adjacent benign tissues (47).
Aberrant expression of CCND2 by DNA methylation has been noted in prostate cancer (45, 49). The frequencies of methylation in CCND2 were significantly higher in prostate tumors (32%) than in normal tissues (6%) (45). Studies observed a positive correlation between the methylation in CCND2 and clinicopathological features such as Gleason score and preoperative serum PSA (45, 50). Moreover, methylation status of CCND2 was significantly associated with the risk for recurrence among prostate cancer patients who underwent a prostatectomy treatment (51). Henrique et al. further reported that CCND2 methylation levels were significantly higher in prostate tumors compared to tissues of high-grade prostatic intraepithelial neoplasia (HGPIN), BPH, or normal prostate, whereas mRNA expression levels followed the opposite trend (49). They found that high CCND2 methylation levels correlate with clinicopathological parameters of tumor aggressiveness. Altogether, CCND2 promoter methylation, but not cyclin A1 gene, may be a useful prostate cancer biomarker for the identification of the aggressive prostate cancer that may benefit from different therapeutic modalities.
3.1.4. Death-Associated Protein Kinase
Death-associated protein kinase (DAPK) is a serine/threonine kinase involved in apoptosis pathway (52). Overexpression of DAPK induces apoptosis, whereas loss of its function leads to protection against apoptosis (53). Therefore, DAPK may function as a suppressor of metastasis. A repressed expression of DAPK by hypermethylation in the promoter region has been shown for various human cancers (52, 54, 55). The methylation frequencies in prostate cancer range from 0% to 36% in four independent studies (29, 30, 36, 56). In addition, Mishra et al. observed that methylation level of DAPK in a prostate cancer cell line (LNCaP) is significantly higher than one in a normal cell line (RWPE1) through global methylation analysis (57). However, DAPK overexpression and repressed function in prostate tumors (58) suggest that DAPK activity may be damaged at a posttranslational level in cancer cells (59). Based on its unclear function and a persistently low frequency of methylation in both tumors and normal tissues, DAPK needs to be further tested for a potential biomarker for prostate cancer.
3.1.5. Fragile Histidine Triad
Fragile histidine triad (FHIT) is known as a tumor suppressor gene and frequently methylated in various cancers such as lung (60), leukemia (61), ovarian (62), skin (63), cervical (64), gastric (65), renal (66), and prostate cancers (29, 67). Previous studies indicate that FHIT is a proapoptotic factor (68). Guo et al. (69) reported that downregulation of FHIT protein in more than half of the prostate tumors is determined by immunohistochemistry. However, these results were not confirmed by another study (70). Although there are indications for a potential role of FHIT methylation in prostate cancer, previous studies show its limited value due to a persistently low frequency of methylation in tumors and normal tissues (29, 57, 67).
3.1.6. Hypermethylated in Cancer 1
The tumor suppressor hypermethylated in cancer 1 (HIC1) is a transcriptional repressor, which is epigenetically silenced in solid tumors (71– 73). Loss of heterozygosity (LOH) of the short arm of chromosome 17 (17p) is a frequent genetic alteration in human cancers. Moreover, frequent LOH or DNA methylation changes occur in a more telomeric region at 17p13.3. In the animal study, heterozygous HIC1+/− mice developed spontaneous malignant tumors of different types (74, 75). These results suggest that HIC1 may be involved in tumorigenesis. Three studies investigated methylation in the promoter region of HIC1 in prostate tumors. Results of three studies indicated that CpG island at the HIC1 was methylated in 89–100% of prostate tumors (30, 56, 76). However, the methylation status of HIC1 in prostate tumors parallels the respective normal tissue, although a high proportion of tumors are methylated. Therefore, DNA methylation sites in HIC1 gene are not good candidates as prognostic markers for progression or early detection of prostate cancer (30, 76).
3.1.7. Lipoprotein Lipase
Lipoprotein lipase gene (LPL) is common locus of the somatic deletions in prostate tumors. Gallucci et al. reported LPL deletion in 76% of prostate tumor determined by fluorescence in situ hybridization (FISH) (77). LPL deletion was associated with higher stages, biochemical/clinical progression, and Gleason grade. Only one published study evaluated methylation status in LPL using 56 prostate tumors and matching normal tissue pairs. Kim et al. found that 21 samples out of 56 primary cancers (38%) were methylated in the LPL promoter region, while methylation was not detected in any normal tissues. In addition, the methylation status in LPL was positively associated with the preoperative PSA levels (67). These data suggest that biallelic inactivation of LPL by gene deletion and hypermethylation may affect progression of prostate cancer.
3.1.8. Paired-Like Homeodomain Transcription Factor 2 (PITX2)
Paired-like homeodomain transcription factor 2 gene (PITX2) encodes a member of the RIEG/PITX homeobox family, which is in the bicoid class of homeodomain proteins. The protein acts as a transcription factor, and it is involved in the development of several major organs. PITX2 expression is induced by the Wnt pathway, and the protein mediates cell-type-specific proliferation by inducing growth-regulating genes (78). Methylation in PITX2 was reported as one of the best validated methylated genes for predicting distant recurrence outcome of breast cancer by Maier et al. (79). These results were validated by an independent cohort and confirmed by two additional studies. Harbeck et al. reported that PITX2 methylation can predict outcome in node-negative, tamoxifen-treated breast cancer (80). PITX2 promoter methylation is also a biomarker for disease recurrence, early distant metastasis, and poor overall survival in breast cancer patients (81).
Recently, two cohort studies (N = 605 (82); N = 476 (83)) showed prostate cancer patients with high PITX2 methylation had threefold higher chance of biochemical recurrence than patients with low PITX2 methylation. They also showed the prognostic capability of PITX2 methylation status in patient strata defined by the Gleason score. These results were supported by Vanaja et al. (84). Methylation profile of six genes including PITX2 was significantly associated with prediction of biochemical, local, and systemic recurrence of prostate cancer. Together, the data show the ability of PITX2 methylation status to provide prognostic information beyond the traditional Gleason score. Therefore, the prognostic potential of the PITX2 methylation may help to determine a personalized treatment.
3.1.9. Prostaglandin-Endoperoxide Synthase 2
Prostaglandin-endoperoxide synthase 2 (PTGS2) is a key regulator of inflammation and may play a role in prostate carcinogenesis. The two PTGS isoforms, PTGS1 and PTGS2, differ in their expression patterns. While PTGS1 is constitutively expressed in most tissues, PTGS2 is usually not expressed and is induced by inflammation, hypoxia, and Wnt signaling (85). An elevated expression of PTGS2 is frequently reported in different human cancer sites including prostate. PTGS2 over expression and enzymatic activation can enhance the level of antiapoptotic protein B-cell CLL/lymphoma 2 (BCL2) and matrix metalloproteinase (MMP) family. Antiapoptotic and proproliferative and inflammatory functions of PTGS2 support its role in tumorigenesis. However, other studies show that PTGS2 may not be expressed or downregulated in prostate tumor. Bastian et al. observed PTGS2 gene is silenced in prostate cancer by hypermethylation (86, 87). Range of methylation in PTGS2 promoter was 0–88% of prostate tumor (30, 86, 88–90).
Methylation at the PTGS2 gene was significantly different in prostate tumor and in BPH. These data indicated that methylation in PTGS2 could be a reliable biomarker which can distinguish tumor from nontumor tissues (88). Moreover, the CpG island hypermethylation at PTGS2 correlated with seminal vesicle infiltration, capsular penetration, pathologic T-stage, and recurrence (89). However, there was no PTSG2 methylation in hormone-refractory metastatic prostate cancer (87).
3.1.10. RAS Association Domain Family Protein 1 Isoform A
The RAS family of proto-oncogenes plays a key role in signal transduction pathways involved in cellular proliferation and survival, interacting with other regulatory circuits of cell growth and death. Overexpression of RAS may cause reduction of growth factor dependency, resistance to apoptosis, or other features of the tumor phenotype. However, RAS association domain family protein 1 isoform A (RASSF1A), a tumor suppressor gene, was known to be associated with the DNA repair proteins and with the apoptotic effect (91). Inactivation by methylation of RASSF1A may deregulate the DNA repair pathway and cell-cycle control in the tumor. Methylation in RASSF1A promoter gene was found in a large fraction of various tumors including prostate (92). In prostate tumors, RASSF1A promoter methylation is a common event, occurring in 21–99% of tumor tissues (25, 29– 31, 35, 36, 90– 96). RASSF1A promoter methylation is also positively associated with aggressiveness of prostate cancer (29, 92, 93). In addition, Aitchison et al. reported that there was over 50% of methylation in normal epithelial cells and benign prostatic tissues as well as prostatic intraepithelial neoplasms (96). These findings indicate that RASSF1A promoter methylation may be associated with early event of carcinogenesis and progression.
3.1.11. Solute Carrier Family 5A8 (SLC5A8)
Solute carrier family 5 (iodide transporter) (SLC5) is a solute-linked carrier gene family that contains 12 sodium-coupled transporters for several chemicals (97). SLC5A8 is downregulated by methylation, obesity, or chronic hypoxia, while it is up regulated by lactate, butylate, TNF (tumor necrosis factor)-α, or nitric oxide (NO) (98). The potential function of SLC5A8 protein in normal prostate tissues is likely to mediate concentrative uptake of butyrate and propionate, all of which are inhibitors of histone deacetylases (HDACs). SLC5A8 can also transport a variety of pharmacologically relevant monocarboxylates, e.g., various nonsteroidal anti-inflammatory drugs such as ibuprofen and ketoprofen (99) especially transport pyruvate into epithelial cells, and may explain a potential tumor suppressive role (100). SLC5A8 was identified as a differentially methylated gene by restriction landmark genome scanning which provides a global analysis of methylation events in colon cancer cell lines and lung tumor (101, 102). Since then, increasing evidence suggests that gene silencing of SLC5A8 may contribute to the carcinogenesis and progression of tumors. SLC5A8 promoter methylation and gene silencing were detected in lung, brain, thyroid, gastric, pancreatic, breast, and prostate tumors (100, 102– 112).
We previously reported hypermethylation of SLC5A8 in prostate (111) and pancreatic tumors (110), and its expression was restored by treatment with either 5-azacytidine or TSA in cancer cell lines (111). Although these results hint a potential role of HDACs on SLC5A8 expression, aberrant methylation represents the principal mechanism for inactivating SLC5A8 in prostate tumor.
3.1.12. Solute Carrier Family 18 (Vesicular Monoamine) Transporter 2
Solute carrier family 18 (vesicular monoamine) transporter 2 (SLC18A2) transports monoamines, such as dopamine, serotonin, and histamine, from the cytosol into vesicles for storage and/or exocytotic release during neurotransmission or autocrine/paracrine factor release (113). Although SLC18A2 is expressed in prostate tumors, biological function in normal and tumor prostate tissues is unknown. However, several of the monoamines that are substrates for SLC18A2-mediated transport have been shown to influence growth, proliferation, migration, or apoptosis of prostate cancer cells in vitro and in vivo. Kristiansen et al. reported that 50% of tumor tissues had silenced SLC18A2 expression, by performing microarray analyses (114). A recent study confirmed that SLC18A2 is frequently downregulated in tumor tissues by methylation, as compared with nonmalignant prostate tissue samples. Level of expression of SLC18A2 is also negatively associated with risk for biochemical recurrence after radical prostatectomy (115).
3.1.13. Tumor Necrosis Factor Receptor Superfamily, Member 10C and 10D (TNFRSF10C and 10D)
The TNF receptor superfamily member 10C is one of several TNF-related apoptosis-inducing ligand (TRAIL)-like decoy receptors. TNFRSF10C is located on 8p21.3, which is a common prostate cancer susceptibility region (116, 117). TNFRSF10C encodes for DCR1 and is involved in the inhibition of the apoptosis pathway. TNFRSF10C lacks the intracellular death domain and appears unable to induce apoptosis. The extracellular domains of TNFRSF10C compete with those of DR4 or DR5 for TRAIL binding. Thus, TNFRSF10C inhibits apoptosis induction through DR4 and DR5 (118). Previous studies reported that frequent loss of expression of TNFRSF10C by aberrant methylation of promoter regions in human tumor tissues (118, 119) and low expression of TNFRSF10C was associated with tumor recurrence (120). Hypermethylation of TNFRSF10C promoter region had been reported in prostate tumor tissues, with a range from 0 to 78% (117, 118, 121, 122). A recent German study reported that TNFRSF10D, which codes for DCR2, was also downregulated by methylation in tumors (120).
3.1.14. NK3 Homeobox 1 (NKX3.1) and NK2 Transcription Factor Related, Locus 5 (NKX2.5)
The NKX3.1 is located on 8p21, which is a common prostate cancer susceptibility region (123). This gene is an NK family homeodomain protein and a tumor suppressor gene that is downregulated in the early phases of prostate cancer. Like its cardiac homolog, NKX2.5, NKX3.1 acts synergistically with serum response factor (SRF) (124).
Loss of function of the NKX3.1 homeobox gene in the mouse prostate leads to deregulated expression of oxidative damage response genes and increased levels of 8-oxy-dG, correlated with the onset of PIN (125, 126). Downregulation of NKX3.1 was observed throughout prostate cancer progression (125, 127, 128). In addition, downregulation of NKX3.1 is frequently observed with overexpression of MYC, an oncogene, at the early stage of prostate cancer (125). Asatiani et al. found hypermethylation at CpG sites −921, −903, and −47 of NKX3.1 in tumors, as compared with adjacent normal cells (129). However, these data were not supported by another study. Lind et al. reported that downregulation of NKX3.1 expression might not be caused by DNA methylation, but other epigenetic mechanisms (130). Chung et al. reported that NKX2.5 promoter was significantly highly methylated in prostate tumor, as compared to normal tissues (131). These results were confirmed by another group (132). We expect that further methylation information at their promoters will be available.
3.1.15. Stratifin (SFN/14-3-3σ)
The p53-regulated gene 14-3-3σ is a putative tumor suppressor gene involved in cell-cycle regulation and apoptosis following DNA damage. In response to DNA damage, 14-3-3σ enforces a G2/M arrest by inhibiting the cyclin B1–cdc2 complex from entering the nucleus. This allows DNA repair before cell-cycle progression (133). 14-3-3σ undergoes frequent epigenetic silencing in several types of cancer, including prostate cancer, suggesting that the loss of 14-3-3σ expression may be causally involved in tumor progression (134). However, there were similar high frequency of 14-3-3σmethylation in both of prostate cancer and BPH (133, 135). Thus, promoter methylation at 14-3-3σ may not be a specific biomarker for prostate cancer.
3.2. Genes Involved in Metabolism
The specific causes of prostate cancer are not known, but multiple etiological factors, including genetics, hormones, diet, infection, and environmental exposures, are thought to play significant roles. Although the precise role of androgens and their receptors in carcinogenesis and progression of prostate cancer has not been fully studied, previous studies suggest that these processes are important (136, 137). The production of estrogens from androgens is mediated by the aromatase enzyme, the aberrant expression of which plays a critical role in the development of malignancy in a number of tissues (138). Differences in the activities of these enzymes are determined to a large extent by genetic and epigenetic changes in the genes encoding them.
3.2.1. Androgen Receptor
It had been known that androgens stimulate the growth of prostate cells through the androgen receptor (AR) (139). There are two well-known AR target genes, PSA and TMPRSS2–ETS fusion genes. The exact roles of PSA and TMPRSS2–ETS in prostate cancer are not fully defined yet. While silencing of AR expression leads to decrease growth and induce apoptosis in vitro (140–142), overexpression of AR also induces growth inhibition and apoptosis (143). In addition to prostatectomy and radiation therapy, androgen deprivation is one of the most effective treatments for prostate cancer. However, many advanced prostate cancers turn into a castrate-resistant cases. Prostate tumor cells in this stage grow aggressively without stimulation of androgens. Androgen receptor is one of the most frequently overexpressed proteins in the castrate-resistant cases (144). Jarrard et al. (145) reported a significant association between AR promoter methylation and its expression in vitro using prostate cancer cell lines.
Several groups found AR promoter methylation in 8–39% of the prostate tumor tissues (56, 133, 146–149). Frequencies of AR promoter methylation are higher in castrate-resistant cases than ones in primary prostate tumor tissues (146, 148). Until now, the biological significance of AR silencing by promoter methylation in castrate-resistant prostate cancer is not clear. Recently, Wang et al. reported that AR selectively upregulates M-phase cell-cycle genes in castrate-resistant cells, including ubiquitin-conjugating enzyme E2C (UBE2C), a gene that inactivates the M-phase checkpoint. They also found that epigenetic marks at the UBE2C enhancer are present in castrate-resistant cells and direct AR-enhancer binding and UBE2C activation (139). On the other hand, Schayek et al. found that progression to metastatic stage in a cellular model of prostate cancer is associated with methylation of AR, and AR suppresses the insulin-like growth factor-I receptor (IGF), therefore suggesting roles of IGF for stimulating AR signal in castrate-resistant prostate cancer (149).
3.2.2. Estrogen Receptors
Estrogens are effective against androgen-dependent prostate cancer, but paradoxically, estrogens might also be involved in the causation of this malignancy (150). The biological actions of estrogens are meditated by the estrogen receptor (ER) (151). There are two ERs which are highly homologous DNA-binding domains but different N-terminus and ligand-binding domains. Stimulation of ER a(Esr1) leads to aberrant proliferation, inflammation, and premalignant pathology, whereas activation of ERβ(Esr2) appears to have beneficial effects regarding cellular proliferation and a putative protective role against carcinogenesis (138).
Both ERs, Esr1 and Esr2, are downregulated in prostate tumor tissues (152, 153). Promoter methylation is the primary mechanism responsible for low expression of ERs (147, 154, 155). Esr1 expression is associated with a poor prognosis for hormonal therapy (156), and its hypermethylation is correlated with cancer progression (157). The range of Esr1 methylation in prostate cancer is diverse from 19 to 95% (31, 147, 157, 158). Esr2 may serve as a tumor suppressor gene because it protects against uncontrolled cell proliferation in normal prostate cells (155). However, high expression of Esr2 in prostate tumors is associated with increased risk for recurrence and distant metastasis (153, 159). Therefore, Esr2 may have multiple roles in carcinogenesis and progression. The frequency of Esr2 promoter methylation ranges from 65 to 83% in prostate tumors (147, 160, 161). The extent of ERs promoter methylation is significantly higher in prostate tumors than in the BPH samples (158, 161). In addition, the percentage of methylated CpG sites in Esr2 promoter increased progressively from 0.29% (normal) to 35% (grade 4/5 prostate cancer) (154).
3.2.3. Retinoic Acid Receptor β (RARβ)
Retinoic acid receptor β (RARβ) is known as a tumor suppressor gene by interacting with retinoic acid. Expression of retinoic acid receptor B (RARβ) is reported to be absent or downregulated in tumor tissues (162). The RARβ2 promoter is aberrantly methylated in many cancers, including prostate cancer (163). Several groups reported that frequencies of methylation of the RARβ2 promoter range from 40% to 84% of primary prostate cancers but rarely in normal prostate tissues or BPH samples (29, 35, 56, 95, 121, 163–166). Moderate or high frequencies of RARβ promoter methylation were also observed in urine or blood samples, respectively (31, 36, 87). Therefore, RARβ2 gene methylation may be an ideal biomarker candidate for early detection of prostate cancer (56, 163).
3.2.4. Glutathione S Transferase P1
Glutathione S transferase P1 (GSTP1) is involved in the detoxifying process and elimination of potentially genotoxic foreign compounds by conjugating glutathione into toxic chemicals. These processes protect prostate cells from DNA adducts and carcinogenesis (167). Thus, defective GSTP1 activity may increase DNA mutations and, therefore, may increase the prostate cancer risk (168). Because of its consistently frequent hypermethylation in the promoter region in prostate cancer, GSTP1 is perhaps one of the most studied genes in prostate cancer.
Lee et al. first reported a high frequency of GSTP1 hypermethylation in prostate tumor tissues (169). Since then, numerous studies confirmed similar results consistently. Methylation of the GSTP1 promoter region occurs in 26–100% of tumor tissues (25, 29–31, 35, 42, 56, 88, 90, 93, 95, 169–180). However, this methylation is rarely detected in normal prostate or BPH tissues. GSTP1 methylation was also detected consistently with high frequency in urine, blood, and ejaculates of prostate cancer patients, while either low or no methylation was detected in the samples from healthy controls (31, 36, 87, 181–183). Different frequencies of GSTP1 promoter hypermethylation between tumor and normal prostate tissues make an ideal biomarker for prostate cancer. To increase the accuracy of detection, some investigators used multiple gene panel approaches, had commonly chosen GSTP1, and studied its promoter hypermethylation as a biomarker for prostate cancer incidence, progress, and recurrence or survival (31, 36, 165, 184).
3.2.5. Cellular Retinol-Binding Protein 1
Effects of retinoids on prostate gland or prostate cell lines implicate retinoids in the regulation of prostate growth and suppression of prostate cancer development (185). Retinoids exert their effects through a variety of binding proteins including cellular retinol-binding protein (CRBP), retinol-binding proteins (RBP), cellular retinoic-acid-binding protein (CRABP), and two classes of nuclear proteins, i.e., retinoic acid receptors (RARs) and retinoic acid X receptors (RXRs) (186). CRBP1 is postulated to promote apoptosis via its upregulation of all trans-retinoic acid (ATRA) synthesis. Therefore, loss of CRBP1 could disrupt a retinoic-acid-mediated apoptosis pathway and hence support prostatic tumor progression (187). Low expression of CRBP1 by promoter methylation has been associated with the malignant tumor tissues including prostate (188, 189). CRBP1 promoter hypermethylation was selectively found in prostate cancer tissue, rare in BPHs or normal prostate tissues (25, 189, 190). Low expression and hypermethylation in CRBP1 occur frequently in prostate tumors. However, data indicated that CRBP1 hypermethylation is not an early event in tumorigenesis (189).
3.2.6. Multidrug Resistance 1 (MDR1/ABCB1)
Multidrug resistance 1 (MDR1) is a transmembrane calcium-dependent efflux pump to detoxify xenobiotics or induce multidrug resistance with GSTs. It is reported to be inactivated in prostate cancer, and some reports showed significantly high hypermethylation at MDR1 promoter compared to BPH (30, 87, 90, 122, 191). A recent global methylation study showed 6.2- and 13.7-fold higher methylation at MDR1 in AR-positive (LNCaP) and AR-negative prostate cancer cells (DU145 and PC3), respectively, compared to normal prostate epithelial cell lines (RWPE1) (57). However, Cho et al. showed no significant differences in frequency of MDR1 methylation among normal (N = 20), PIN (N = 25), and prostate cancer tissues (N = 35), while the prevalence of MDR1 methylation was as high as 100% (121). Recent multigene methylation analyses showed that the frequency of methylation in MDR1 gene in prostate cancer samples was 55.3 and 11.6% in BPH. Multigene methylation models, which contain MDR1 and GSTs, may serve as a good biomarker for prostate cancer (192).
3.2.7. Endothelin B Receptor Gene (EDNRB)
Endothelin B receptor interacts with endothelins to regulate several critical biological processes and may induce cell death by apoptosis and inhibit tumor progression (193). Several studies reported that the EDNRB promoter is hypermethylated in a high proportion of prostate tumors and that much less frequency of methylation was found in normal tissues (30, 87, 194, 195). However, other studies found that EDNRB methylation frequencies in prostate tumors and paired normal were same, although a high proportion of tumors are methylated (88, 95, 196). Because a high methylation is present in normal and tumor tissues, methylation in EDNRB cannot be considered as a specific biomarker for prostate cancer.
3.2.8. EPH Receptor A7 (EPHA7)
Ephrins and EPHS are involved in embryonic development and play a key role for the differentiation of the nervous and vascular systems (197, 198). Their signaling pathway networks with the Wnt signaling pathway during embryogenesis, tissue regeneration, and carcinogenesis (199). Recent expression microarray data, which were profiling androgen-dependent and castrate-resistant cells, revealed that EPHA7 is downregulated in castrate-resistant cells (200). Silencing of EPHA7 is reactivated by 5-aza treatment (198). These data are supported by a significant correlation between methylation and loss of expression of EPHA7 (201). A recent report showed higher frequency of methylation of EPHA7 promoter region in prostate tumor tissues than hyperplasias (42% vs. 19%) (198). A role of EPHA7 methylation in progression of prostate cancer was confirmed by a positive association between hypermethylation and Gleason scores (198).
3.2.9. Tazarotene-Induced Gene 1
Tazarotene-induced gene 1 (TIG1) is frequently silenced in prostate tumors (202). This gene, also known as retinoid-acid-receptor-responsive 1 gene, was first identified as an RA-responsive gene (203). Several researchers reported that TIG1 was methylated frequently in prostate tumors, but was not or barely low methylated in normal tissues or BPH (88, 122, 164, 183, 204, 205). Zhang et al. further found that the methylation of TIG1 and RARβ was positively correlated. Therefore, it is possible that the methylation of the retinoid response gene TIG1 occurred in response to the methylation and inactivation of RARβ. In addition, concordant hypermethylation of retinoid signaling genes, e.g., RARβ or TIG1 (164), was observed.
Ellinger et al. analyzed the diagnostic and prognostic possibilities of methylation analysis in serum DNA of prostate cancer patients. They found hypermethylation in TIG1 was more frequent in prostate cancer patients (10%) than in BPH (0%) and healthy individuals (0%) (88). Although the levels of hypermethylation frequency for specific genes are usually lower in serum or urine DNA than those in prostate tissues (Table 1), use of non-invasive biosamples may be worth it for the specific diagnosis of prostate cancer (87).
3.2.10. Aldehyde Dehydrogenase 1A2 and 1A3
Aldehyde dehydrogenases (ALDHs) are a group of NAD(P)+-dependent enzymes involved in metabolism of wide variety of aliphatic and aromatic aldehydes (206). ALDH1A2, known as retinaldehyde dehydrogenases (RALDHs), and 1A3 are embryonically lethal in gene knockout mice and involved in retinaldehyde oxidation into retinoic acid (RA), a compound with prodifferentiation properties. Most prostate cancer patients show a decreased prostatic RA concentration, and altered retinoid metabolism has been noted in prostate cancer (207, 208). Kim et al. reported ALDH1A2 promoter region was hypermethylated in primary prostate tumors, as compared with normal prostate specimens (209). Their results are supported by Touma et al., who observed a lower expression of ALDH1A2 in all prostate tumor FFPE sections relative to normal prostate tissue on the same sections. Therefore, ALDH1A2 is suggested as a tentative tumor suppressor gene in prostate cancer, and its alteration is suspected as an early event in prostate cancer. ALDH1A3 was reported to be androgen responsive (210), and upregulation of ALDH1A3 can increase the oxidation of retinal to RA. Shames et al. reported hypermethylation in the promoter region of ALDH1A3 in prostate tumor (48). Recently, disulfiram, an inhibitor of ALDHs and demethylation agent, showed inhibition of prostate cancer cell growth (211). Thus, promoter methylation at ALDH1A2 or 1A3 is a suspected biomarker for prostate cancer diagnosis or prevention.
3.3. Tumor Cell Invasion/Metastasis
Metastasis is an extremely complicated process, which occurs through a series of sequential steps that include the invasion, transport, adhesion at a distant site, and outgrowth into a secondary organ. Although metastases are the cause of 90% of human cancer mortality, little is known about the genetic and biochemical determinants of metastasis.
3.3.1. Adenomatous Polyposis Coli
The methylated adenomatous polyposis coli (APC) gene causes familial adenomatous polyposis, which is an inherited disorder characterized by extensive colon polyps and the development of colorectal cancer in early adulthood. The APC is involved in the Wnt signal transduction pathway (212). The APC complex is known to function as a gatekeeper in the cell, preventing the transcription of gene products that promote cell proliferation and survival rather than differentiation and apoptosis (213). Hypermethylation of APC implies silencing of this gatekeeper, making the cell vulnerable to further epigenetic and genetic changes and, thus, progression toward the development of invasive cancer.
APC promoter methylation is common in various human tumors, especially colon (214). Most studies found a prevalence of 14–100% in prostate cancer tissues but only 5–6% in noncancerous tissues (25, 29–31, 35, 36, 50, 51, 86, 89, 90, 93, 121, 122, 166, 184, 192, 204, 215, 216). Recent studies found that methylation in APC is associated with progression of prostate cancer (50, 51, 217). In two small cohorts of prostate cancer patients, a threefold statistically significantly increased HR for promoter methylation in APC has been reported among the patients who experienced PSA recurrence, metastasis, or death (50, 51). Richiardi et al. found that hypermethylation in the promoter of the APC gene is involved in prostate cancer progression using large survival analysis of two independent series of unselected prostate cancer patients (217). Rogers et al. reported somewhat low methylation frequency of APC in urine collected after DRE; however, overall, 100% of patients with biopsy-proven prostate cancer had at least one gene methylation among APC, GSTP1, and EDNRB in urine vs. 60% of those without evidence of prostate cancer on biopsy (195). A recent multiplex urine assay study for prostate cancer diagnosis (184) showed that the sensitivities of APC (52%) in the urine sediments were similar to those seen by other investigators, who demonstrated a similar sensitivity for APC (36).
3.3.2. CD44 (CD44)
CD44 is a transmembrane glycoprotein that is involved in signal transduction and cell–cell and cell–matrix interactions by serving as a receptor. It codes a lipid raft protein like CAV1 or E-cadherin. Lipid rafts are also involved in angiogenesis and local invasion (19). The CD44 expression in prostate tumor tissues is lower than ones in adjacent normal tissues. This low expression is correlated with CD44 promoter methylation (22, 178). Gao et al. reported that decreased CD44 expression is associated with Gleason score and the distant metastatic progression of prostate cancer (218). Therefore, CD44 is considered as a metastasis suppressor gene. Furthermore, CD44 expression and its promoter methylation may correlate with not only tumorigenesis but also progression of prostate cancer (219). However, there are inconsistent results for CD44 promoter methylation in many reports (22, 28, 87, 95, 122, 178, 219, 220).
3.3.3. E-Cadherin (CDH1)
The E and one of the key proteins in the maintenance of cell differentiation and the normal architecture of epithelial tissues (221). DNA methylation-induced CDH1 silencing was observed in prostate tumor and was associated with tumorigenesis, metastasis, and poor patient outcome (29). Treatment with the demethylating agent 5-aza restored E-cadherin expression in the E-cadherin-negative prostate cancer cell lines (222). The prevalence of methylation varies from 0 to 77% (22, 28–31, 35, 36, 45, 95, 122, 160, 178, 222, 223). The reason for the discrepancy among these studies may come from technical issues, e.g., different CpG targets, detection methods, and samples, but also tumor status issues. Li et al. reported that the overall methylation frequencies of E-cadherin promoter were high in advanced stage samples (70%) and low in early stage (33%) prostate tumors (222). In addition, a recent study reported that methylated and unmethylated E-cadherin gene expression is dominant in primary prostate cancer and bone metastasis, respectively (223). These data suggested that CDH1 methylation might be a useful biomarker to assess progression of prostate cancer (222).
3.3.4. H-Cadherin (CDH13)
H-cadherin (CDH13) belongs to the cadherin family of cell surface glycoproteins responsible for selective cell recognition and adhesion (224). Like CDH1, previous reports suggested a role for CDH13 in cancer invasion and metastasis in human cancers (29, 225, 226). Low expression by CDH13 methylation has frequently been observed in various cancers (225), including prostate cancer (29, 45, 226). CDH13 was known as a tumor suppressor gene because low expression of CDH13 resulted in significant inhibition of tumor growth (227). However, data from animal study suggested that CDH13 is not involved in the metastasis (228). Although the molecular and biological mechanisms underlying the functions of CDH13 are unknown, several groups reported CDH13 promoter methylation in prostate tumors (29, 226). However, Cho et al. reported that the frequency of CDH13 promoter methylation in prostate cancer was not different from that in BPH tissues (53.6 and 53.3%, respectively) (122).
3.3.5. S100 Calcium-Binding Protein A2 (S100A2) and A6 (S100A6)
Although most S100 proteins are commonly upregulated in tumors and this is often associated with tumor progression, S100A2 has been documented as a tumor suppressor in some cancers but as an oncogene in others (229). In the case of prostate cancer, Rehman et al. reported that S100A2 is downregulated (230). S100A2 methylation was seen in 94% of prostate tumor and 100% of cases of metastatic cancer. However, S100A2 methylation was also seen in 75% of cases of nonmalignant tissues and in 100% of cases of BPH (25). One interesting fact was age-related increase in S100A2 methylation levels. This age-related methylation of S100A2 might be zone dependent because it was observed in a transition zone lesion, but not in a lesion from the peripheral zone (25).
S100A6 is coexpressed with S100A2 in prostate tissue. S100A6 methylation was absent in nonmalignant tissues and 100% in BPH tissues, whereas methylation was seen in 52% of prostate tumors. Loss of S100A6 proteins is frequent in prostatic tumors (230).
3.3.6. Tissue Inhibitor of Metalloproteinase-2 and -3
MMPs are proteolytic enzymes that degrade the extracellular matrix and the basement membrane. High expressions of this enzyme have been associated with tumor growth, invasion, and tumor-induced angiogenesis (231). These pathways are controlled by the balance between the levels of the MMPs and tissue inhibitors of metalloproteinases (TIMPs) (232). Thus, TIMPs are called angiogenesis inhibitors.
TIMP-2 is one of the frequently investigated members of this family because of its involvement in cancer progression and metastasis in a variety of human cancers (233, 234). Pulukuri et al. observed that 25 (60%) of 42 prostate tumors were methylated in comparison with 5 (16%) of 32 normal prostate samples (235). These findings further supported that majority of the prostate cancer tissues have weak or no expression of TIMP-2 when compared with BPH or normal prostate tissues (235). However, these results were not confirmed by a previous study (236). Ross et al. found that TIMP-2 was expressed in a majority of prostate tumors and correlated with clinical stages and recurrence. TIMP-2 expression appears to have a tumor-promoting role in prostate cancer and warrants further investigation (236). This was in contrary to the Pulukuri’s study which indicated antitumor effects.
The roles of TIMP-3 in cancer progression were investigated by several groups. High expression of TIMP-3 reduces metastasis, induces apoptosis, increases drug sensitivity, and inhibits tumor growth (237–239). A low expression by promoter methylation of TIMP-3 has been reported to be associated with poor outcomes (240). A recent global methylation study showed 12.08- and 22.3fold higher methylation at TIMP-3 in AR-positive (LNCaP) and AR-negative cells (DU145 or PC3), respectively, compared to normal prostate epithelial cell lines (RWPE1) (57). The promoter region of TIMP-3 was found to be methylated in 97% of prostate tumors (25). However, other studies reported low (0%) and 6% frequencies of TIMP-3 methylation (30, 56), while additional two studies found TIMP-3 promoter methylation in 37 and 41% of urine sediments from prostate cancer patients (31, 36). As a diagnostic biomarker in urine DNA, value of TIMP-3 may be limited due to low frequency of methylation in normal samples.
3.3.7. SRC Family Tyrosine Kinase (FYN)
The SRC family of kinases (SFKs) is the largest family of nonreceptor protein tyrosine kinases and is responsible for signal transduction during differentiation, adhesion, and migration. Aberrant SRC/SFK activity has been widely implicated in cancer development. Several lines of evidence indicate a role for SFKs in the development of prostate cancer, e.g., SFK overexpression in prostate cancer cell lines and tissues (241).
Posadas et al. reported overexpression of FYN, a member of SFK, in prostate cancer cell lines and tissues than in normal tissues (242). Sorensen et al. reported frequent aberrant methylation in the FYN promoter region in both prostate cancer cell lines and primary prostate tumors. In addition, methylation-induced silencing was confirmed by Western blot and RT-PCR results (243). Methylation at FYN promoter should be further investigated to be evaluated as a biomarker of prostate cancer.
3.3.8. Neutral Endopeptidase 24.11
Neutral endopeptidase 24.11 (NEP), one of cell surface peptidases, is expressed in prostate. This protein inactivates growth factors needed in the growth of castrate-resistant prostate cancer (244). Therefore, loss of NEP activates protein kinase B (Akt), which may accelerate prostate tumor growth (245). Several investigators reported hypermethylation in NEP promoter in prostate tumor tissues (87, 244, 246). Usmani et al. observed that methylation of the NEP promoter was present only in castrate-resistant prostate cancer cell lines not in androgen-dependent prostate cancer cell lines. Reactivation of NEP by demethylating agent 5-aza-2′-deoxycytidine shows that hypermethylation of NEP is associated with a loss of NEP expression in prostate tumor (244). Further studies are needed to elucidate the impact of NEP promoter methylation on the progression to castrate-resistant prostate cancer.
3.4. DNA Repair Genes
Although the specific causes of prostate cancer are not known, androgens, estrogens abnormalities, inflammation, and DNA repair capacity have been implicated. DNA is constantly damaged by endogenous oxygen free radicals and exogenous chemicals. DNA mutations are estimated to spontaneously occur 20,000–40,000 times everyday (247, 248). The DNA repair process is important to the survival of cell; therefore, different repair pathways are available to reverse the different types of DNA damage. In fact, over 250 DNA repair enzymes participate in this process (249, 250). Defects in these DNA repair pathways may increase persistent mutations in daughter cell generations, genomic instability, and ultimately prostate cancer risk.
3.4.1. Methylguanine-Methyltransferase
DNA repair genes can be classified into several distinct pathways, including the direct reversal (DR) pathway. The only known enzyme in the DR pathway is methylguanine-methyltransferase (MGMT). MGMT transfers the alkyl group at the O6 position of guanine to a cysteine residue within its active site, leading to the direct restoration of the natural chemical composition of DNA without the need for genomic reconstruction. Therefore, defective MGMT activity is associated with an increased mutation rate (251). Reports regarding MGMT methylation in prostate tumor tissues have been inconsistent.
While three studies reported a low frequency of MGMT promoter hypermethylation (0–2%) in prostate tumor tissues (29, 30, 56), others observed higher prevalence of hypermethylation (19–76%) (25, 31, 36, 37, 42, 93, 252). Two other groups reported 15 and 19% MGMT hypermethylation frequencies in urine sediment samples from prostate cancer patients, respectively (31, 36). These data suggest that MGMT promoter methylation can be a potential biomarker for early detection and surveillance of prostate cancer. However, larger studies will be necessary to resolve these inconsistent results.
4. Conclusions
Although a few large-scale genome-wide analyses of epigenetic variations are currently ongoing, most published studies are small scale with a retrospective design. Therefore, meta-analysis or large studies should be performed to obtain the complete extent and pattern of differential DNA methylation in the promoter region in the critical genes. Since epigenetic changes are involved in carcinogenesis and progression of prostate cancer, information of these epigenetic changes may provide clues for better diagnostic, prognostic, and predictive modalities than existing ones. The ultimate goals of these epigenetic studies are to improve patients’ outcomes and enhance quality of life.
A number of clinical trials and therapies are targeting methylated genes. Unlike DNA somatic mutations, DNA methylations are reversible. Thus, hypermethylated tumor suppressor genes can be reactivated with drugs. Several demethylating agents such as 5-azacytidine (Vidaza) and 5-aza-2′-deoxycytidine (decitabine) have been approved as treatments for the myelodysplastic syndrome (MDS) and leukemia (253–255). Some MDS patients treated with 5-azacytidine showed a significant survival benefit (256). However, a major limitation of these therapies is their nonspecific target approach, which may induce unintended side effects. Therefore, not only tumor suppressor genes but also silenced oncogenes can be reactivated. Future studies should focus on developing drugs that can target specific genes.
References
- 1.Crawford ED. Epidemiology of prostate cancer. Urology. 2003;62:3–12. doi: 10.1016/j.urology.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.EMGT. 2009 http://www.egtm.eu/tumour_markers_in_prostate_cancer.htm,.
- 4.Baylin SB, Herman JG. DNA hypermethylation in tumorigenesis: epigenetics joins genetics. Trends Genet. 2000;16:168–174. doi: 10.1016/s0168-9525(99)01971-x. [DOI] [PubMed] [Google Scholar]
- 5.Smiraglia DJ, Plass C. The study of aberrant methylation in cancer via restriction landmark genomic scanning. Oncogene. 2002;21:5414–5426. doi: 10.1038/sj.onc.1205608. [DOI] [PubMed] [Google Scholar]
- 6.Rush LJ, Dai Z, Smiraglia DJ, Gao X, Wright FA, Fruhwald M, Costello JF, Held WA, Yu L, Krahe R, Kolitz JE, Bloomfield CD, Caligiuri MA, Plass C. Novel methylation targets in de novo acute myeloid leukemia with prevalence of chromosome 11 loci. Blood. 2001;97:3226–3233. doi: 10.1182/blood.v97.10.3226. [DOI] [PubMed] [Google Scholar]
- 7.Costello JF, Fruhwald MC, Smiraglia DJ, Rush LJ, Robertson GP, Gao X, Wright FA, Feramisco JD, Peltomaki P, Lang JC, Schuller DE, Yu L, Bloomfield CD, Caligiuri MA, Yates A, Nishikawa R, Su Huang H, Petrelli NJ, Zhang X, O’Dorisio MS, Held WA, Cavenee WK, Plass C. Aberrant CpG-island methylation has non-random and tumour-type-specific patterns. Nat Genet. 2000;24:132–138. doi: 10.1038/72785. [DOI] [PubMed] [Google Scholar]
- 8.Baylin SB, Herman JG, Graff JR, Vertino PM, Issa JP. Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res. 1998;72:141–196. [PubMed] [Google Scholar]
- 9.Di Croce L, Raker VA, Corsaro M, Fazi F, Fanelli M, Faretta M, Fuks F, Lo Coco F, Kouzarides T, Nervi C, Minucci S, Pelicci PG. Methyltransferase recruitment and DNA hypermethylation of target promoters by an oncogenic transcription factor. Science. 2002;295:1079–1082. doi: 10.1126/science.1065173. [DOI] [PubMed] [Google Scholar]
- 10.Yan PS, Shi H, Rahmatpanah F, Hsiau TH, Hsiau AH, Leu YW, Liu JC, Huang TH. Differential distribution of DNA methylation within the RASSF1A CpG island in breast cancer. Cancer Res. 2003;63:6178–6186. [PubMed] [Google Scholar]
- 11.Graff JR, Herman JG, Myohanen S, Baylin SB, Vertino PM. Mapping patterns of CpG island methylation in normal and neoplastic cells implicates both upstream and downstream regions in de novo methylation. J Biol Chem. 1997;272:22322–22329. doi: 10.1074/jbc.272.35.22322. [DOI] [PubMed] [Google Scholar]
- 12.Esteller M. Epigenetic lesions causing genetic lesions in human cancer: promoter hypermethylation of DNA repair genes. Eur J Cancer. 2000;36:2294–2300. doi: 10.1016/s0959-8049(00)00303-8. [DOI] [PubMed] [Google Scholar]
- 13.Bachman KE, Herman JG, Corn PG, Merlo A, Costello JF, Cavenee WK, Baylin SB, Graff JR. Methylation-associated silencing of the tissue inhibitor of metalloproteinase-3 gene suggest a suppressor role in kidney, brain, and other human cancers. Cancer Res. 1999;59:798–802. [PubMed] [Google Scholar]
- 14.Toyota M, Ohe-Toyota M, Ahuja N, Issa JPJ. Distinct genetic profiles in colorectal tumors with or without the CpG island methylator phenotype. PNAS. 2000;97:710–715. doi: 10.1073/pnas.97.2.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stirzaker C, Millar DS, Paul CL, Warnecke PM, Harrison J, Vincent PC, Frommer M, Clark SJ. Extensive DNA methylation spanning the Rb promoter in retinoblastoma tumors. Cancer Res. 1997;57:2229–2237. [PubMed] [Google Scholar]
- 16.Deng G, Chen A, Hong J, Chae HS, Kim YS. Methylation of CpG in a small region of the hMLH1 promoter invariably correlates with the absence of gene expression. Cancer Res. 1999;59:2029–2033. [PubMed] [Google Scholar]
- 17.Gonzalgo ML, Bender CM, You EH, Glendening JM, Flores JF, Walker GJ, Hayward NK, Jones PA, Fountain JW. Low frequency of p16/CDKN2A methylation in sporadic melanoma: comparative approaches for methylation analysis of primary tumors. Cancer Res. 1997;57:5336–5347. [PubMed] [Google Scholar]
- 18.Gonzalez-Zulueta M, Bender CM, Yang AS, Nguyen T, Beart RW, Van Tornout JM, Jones PA. Methylation of the 5′ CpG island of the p16/CDKN2 tumor suppressor gene in normal and transformed human tissues correlates with gene silencing. Cancer Res. 1995;55:4531–4535. [PubMed] [Google Scholar]
- 19.Patra SK, Bettuzzi S. Epigenetic DNA-methylation regulation of genes coding for lipid raft-associated components: a role for raft proteins in cell transformation and cancer progression (review) Oncol Rep. 2007;17:1279–1290. [PubMed] [Google Scholar]
- 20.Cui J, Rohr LR, Swanson G, Speights VO, Maxwell T, Brothman AR. Hypermethylation of the caveolin-1 gene promoter in prostate cancer. Prostate. 2001;46:249–256. doi: 10.1002/1097-0045(20010215)46:3<249::aid-pros1030>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 21.Bachmann N, Haeusler J, Luedeke M, Kuefer R, Perner S, Assum G, Paiss T, Hoegel J, Vogel W, Maier C. Expression changes of CAV1 and EZH2, located on 7q31 approximately q36, are rarely related to genomic alterations in primary prostate carcinoma. Cancer Genet Cytogenet. 2008;182:103–110. doi: 10.1016/j.cancergencyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Woodson K, Hanson J, Tangrea J. A survey of gene-specific methylation in human prostate cancer among black and white men. Cancer Lett. 2004;205:181–188. doi: 10.1016/j.canlet.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 23.Karam JA, Lotan Y, Roehrborn CG, Ashfaq R, Karakiewicz PI, Shariat SF. Caveolin-1 overexpression is associated with aggressive prostate cancer recurrence. Prostate. 2007;67:614–622. doi: 10.1002/pros.20557. [DOI] [PubMed] [Google Scholar]
- 24.Di Vizio D, Sotgia F, Williams TM, Hassan GS, Capozza F, Frank PG, Pestell RG, Loda M, Freeman MR, Lisanti MP. Caveolin-1 is required for the upregulation of fatty acid synthase (FASN), a tumor promoter, during prostate cancer progression. Cancer Biol Ther. 2007;6:1263–1268. doi: 10.4161/cbt.6.8.4447. [DOI] [PubMed] [Google Scholar]
- 25.Jeronimo C, Henrique R, Hoque MO, Mambo E, Ribeiro FR, Varzim G, Oliveira J, Teixeira MR, Lopes C, Sidransky D. A quantitative promoter methylation profile of prostate cancer. Clin Cancer Res. 2004;10:8472–8478. doi: 10.1158/1078-0432.CCR-04-0894. [DOI] [PubMed] [Google Scholar]
- 26.Konishi N, Nakamura M, Kishi M, Nishimine M, Ishida E, Shimada K. Heterogeneous methylation and deletion patterns of the INK4a/ARF locus within prostate carcinomas. Am J Pathol. 2002;160:1207–1214. doi: 10.1016/S0002-9440(10)62547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen TT, Nguyen CT, Gonzales FA, Nichols PW, Yu MC, Jones PA. Analysis of cyclin-dependent kinase inhibitor expression and methylation patterns in human prostate cancers. Prostate. 2000;43:233–242. doi: 10.1002/(sici)1097-0045(20000515)43:3<233::aid-pros10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 28.Schwarzenbach H, Chun FK, Isbarn H, Huland H, Pantel K. Genomic profiling of cell-free DNA in blood and bone marrow of prostate cancer patients. J Cancer Res Clin Oncol. 2010 doi: 10.1007/s00432-010-0941-5. [DOI] [PubMed] [Google Scholar]
- 29.Maruyama R, Toyooka S, Toyooka KO, Virmani AK, Zochbauer-Muller S, Farinas AJ, Minna JD, McConnell J, Frenkel EP, Gazdar AF. Aberrant promoter methylation profile of prostate cancers and its relationship to clinicopathological features. Clin Cancer Res. 2002;8:514–519. [PubMed] [Google Scholar]
- 30.Yegnasubramanian S, Kowalski J, Gonzalgo ML, Zahurak M, Piantadosi S, Walsh PC, Bova GS, De Marzo AM, Isaacs WB, Nelson WG. Hypermethylation of CpG islands in primary and metastatic human prostate cancer. Cancer Res. 2004;64:1975–1986. doi: 10.1158/0008-5472.can-03-3972. [DOI] [PubMed] [Google Scholar]
- 31.Hoque MO, Topaloglu O, Begum S, Henrique R, Rosenbaum E, Van Criekinge W, Westra WH, Sidransky D. Quantitative methylation-specific polymerase chain reaction gene patterns in urine sediment distinguish prostate cancer patients from control subjects. J Clin Oncol. 2005;23:6569–6575. doi: 10.1200/JCO.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 32.Gu K, Mes-Masson AM, Gauthier J, Saad F. Analysis of the p16 tumor suppressor gene in early-stage prostate cancer. Mol Carcinog. 1998;21:164–170. [PubMed] [Google Scholar]
- 33.Herman JG, Merlo A, Mao L, Lapidus RG, Issa JP, Davidson NE, Sidransky D, Baylin SB. Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res. 1995;55:4525–4530. [PubMed] [Google Scholar]
- 34.Jarrard DF, Bova GS, Ewing CM, Pin SS, Nguyen SH, Baylin SB, Cairns P, Sidransky D, Herman JG, Isaacs WB. Deletional, mutational, and methylation analyses of CDKN2 (p16/MTS1) in primary and metastatic prostate cancer. Genes Chromosomes Cancer. 1997;19:90–96. [PubMed] [Google Scholar]
- 35.Florl AR, Steinhoff C, Muller M, Seifert HH, Hader C, Engers R, Ackermann R, Schulz WA. Coordinate hypermethylation at specific genes in prostate carcinoma precedes LINE-1 hypomethylation. Br J Cancer. 2004;91:985–994. doi: 10.1038/sj.bjc.6602030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roupret M, Hupertan V, Yates DR, Catto JW, Rehman I, Meuth M, Ricci S, Lacave R, Cancel-Tassin G, de la Taille A, Rozet F, Cathelineau X, Vallancien G, Hamdy FC, Cussenot O. Molecular detection of localized prostate cancer using quantitative methylation-specific PCR on urinary cells obtained following prostate massage. Clin Cancer Res. 2007;13:1720–1725. doi: 10.1158/1078-0432.CCR-06-2467. [DOI] [PubMed] [Google Scholar]
- 37.Higuchi T, Nakamura M, Shimada K, Ishida E, Hirao K, Konishi N. HRK inactivation associated with promoter methylation and LOH in prostate cancer. Prostate. 2008;68:105–113. doi: 10.1002/pros.20600. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura M, Watanabe T, Klangby U, Asker C, Wiman K, Yonekawa Y, Kleihues P, Ohgaki H. p14ARF deletion and methylation in genetic pathways to glio-blastomas. Brain Pathol. 2001;11:159–168. doi: 10.1111/j.1750-3639.2001.tb00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin HH, Ke HL, Huang SP, Wu WJ, Chen YK, Chang LL. Increase sensitivity in detecting superficial, low grade bladder cancer by combination analysis of hypermethylation of E-cadherin, p16, p14, RASSF1A genes in urine. Urol Oncol. 2009 doi: 10.1016/j.urolonc.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Chim CS, Chan WW, Kwong YL. Epigenetic dysregulation of the DAP kinase/p14/HDM2/p53/Apaf-1 apoptosis pathway in acute leukaemias. J Clin Pathol. 2008;61:844–847. doi: 10.1136/jcp.2007.047324. [DOI] [PubMed] [Google Scholar]
- 41.Calmon MF, Colombo J, Carvalho F, Souza FP, Filho JF, Fukuyama EE, Camargo AA, Caballero OL, Tajara EH, Cordeiro JA, Rahal P. Methylation profile of genes CDKN2A (p14 and p16), DAPK1, CDH1, and ADAM23 in head and neck cancer. Cancer Genet Cytogenet. 2007;173:31–37. doi: 10.1016/j.cancergencyto.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 42.Konishi N, Nakamura M, Kishi M, Nishimine M, Ishida E, Shimada K. DNA hypermethylation status of multiple genes in prostate adenocarcinomas. Jpn J Cancer Res. 2002;93:767–773. doi: 10.1111/j.1349-7006.2002.tb01318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yam CH, Fung TK, Poon RY. Cyclin A in cell cycle control and cancer. Cell Mol Life Sci. 2002;59:1317–1326. doi: 10.1007/s00018-002-8510-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang N, Eijsink JJ, Lendvai A, Volders HH, Klip H, Buikema HJ, van Hemel BM, Schuuring E, van der Zee AG, Wisman GB. Methylation markers for CCNA1 and C13ORF18 are strongly associated with high-grade cervical intraepithelial neoplasia and cervical cancer in cervical scrapings. Cancer Epidemiol Biomarkers Prev. 2009;18:3000–3007. doi: 10.1158/1055-9965.EPI-09-0405. [DOI] [PubMed] [Google Scholar]
- 45.Padar A, Sathyanarayana UG, Suzuki M, Maruyama R, Hsieh JT, Frenkel EP, Minna JD, Gazdar AF. Inactivation of cyclin D2 gene in prostate cancers by aberrant promoter methylation. Clin Cancer Res. 2003;9:4730–4734. [PubMed] [Google Scholar]
- 46.Aaltomaa S, Eskelinen M, Lipponen P. Expression of cyclin A and D proteins in prostate cancer and their relation to clinicopathological variables and patient survival. Prostate. 1999;38:175–182. doi: 10.1002/(sici)1097-0045(19990215)38:3<175::aid-pros1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 47.Wegiel B, Bjartell A, Tuomela J, Dizeyi N, Tinzl M, Helczynski L, Nilsson E, Otterbein LE, Harkonen P, Persson JL. Multiple cellular mechanisms related to cyclin A1 in prostate cancer invasion and metastasis. J Natl Cancer Inst. 2008;100:1022–1036. doi: 10.1093/jnci/djn214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shames DS, Girard L, Gao B, Sato M, Lewis CM, Shivapurkar N, Jiang A, Perou CM, Kim YH, Pollack JR, Fong KM, Lam CL, Wong M, Shyr Y, Nanda R, Olopade OI, Gerald W, Euhus DM, Shay JW, Gazdar AF, Minna JD. A genome-wide screen for promoter methylation in lung cancer identifies novel methylation markers for multiple malignancies. PLoS Med. 2006;3:e486. doi: 10.1371/journal.pmed.0030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henrique R, Costa VL, Cerveira N, Carvalho AL, Hoque MO, Ribeiro FR, Oliveira J, Teixeira MR, Sidransky D, Jeronimo C. Hypermethylation of Cyclin D2 is associated with loss of mRNA expression and tumor development in prostate cancer. J Mol Med. 2006;84:911–918. doi: 10.1007/s00109-006-0099-4. [DOI] [PubMed] [Google Scholar]
- 50.Henrique R, Ribeiro FR, Fonseca D, Hoque MO, Carvalho AL, Costa VL, Pinto M, Oliveira J, Teixeira MR, Sidransky D, Jeronimo C. High promoter methylation levels of APC predict poor prognosis in sextant biopsies from prostate cancer patients. Clin Cancer Res. 2007;13:6122–6129. doi: 10.1158/1078-0432.CCR-07-1042. [DOI] [PubMed] [Google Scholar]
- 51.Rosenbaum E, Hoque MO, Cohen Y, Zahurak M, Eisenberger MA, Epstein JI, Partin AW, Sidransky D. Promoter hypermethylation as an independent prognostic factor for relapse in patients with prostate cancer following radical prostatectomy. Clin Cancer Res. 2005;11:8321–8325. doi: 10.1158/1078-0432.CCR-05-1183. [DOI] [PubMed] [Google Scholar]
- 52.Mittag F, Kuester D, Vieth M, Peters B, Stolte B, Roessner A, Schneider-Stock R. DAPK promotor methylation is an early event in colorectal carcinogenesis. Cancer Lett. 2006;240:69–75. doi: 10.1016/j.canlet.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 53.Cohen O, Feinstein E, Kimchi A. DAP-kinase is a Ca2+/calmodulin-dependent, cytoskeletal-associated protein kinase, with cell death-inducing functions that depend on its catalytic activity. EMBO J. 1997;16:998–1008. doi: 10.1093/emboj/16.5.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chan MW, Chan LW, Tang NL, Tong JH, Lo KW, Lee TL, Cheung HY, Wong WS, Chan PS, Lai FM, To KF. Hypermethylation of multiple genes in tumor tissues and voided urine in urinary bladder cancer patients. Clin Cancer Res. 2002;8:464–470. [PubMed] [Google Scholar]
- 55.Simpson DJ, Clayton RN, Farrell WE. Preferential loss of Death Associated Protein kinase expression in invasive pituitary tumours is associated with either CpG island methylation or homozygous deletion. Oncogene. 2002;21:1217–1224. doi: 10.1038/sj.onc.1205195. [DOI] [PubMed] [Google Scholar]
- 56.Yamanaka M, Watanabe M, Yamada Y, Takagi A, Murata T, Takahashi H, Suzuki H, Ito H, Tsukino H, Katoh T, Sugimura Y, Shiraishi T. Altered methylation of multiple genes in carcinogenesis of the prostate. Int J Cancer. 2003;106:382–387. doi: 10.1002/ijc.11227. [DOI] [PubMed] [Google Scholar]
- 57.Mishra DK, Chen Z, Wu Y, Sarkissyan M, Koeffler HP, Vadgama JV. Global methylation pattern of genes in androgen-sensitive and androgen-independent prostate cancer cells. Mol Cancer Ther. 2010;9:33–45. doi: 10.1158/1535-7163.MCT-09-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carvalho JR, Filipe L, Costa VL, Ribeiro FR, Martins AT, Teixeira MR, Jeronimo C, Henrique R. Detailed analysis of expression and promoter methylation status of apoptosis-related genes in prostate cancer. Apoptosis. 2010;15:956–965. doi: 10.1007/s10495-010-0508-6. [DOI] [PubMed] [Google Scholar]
- 59.Michie AM, McCaig AM, Nakagawa R, Vukovic M. Death-associated protein kinase (DAPK) and signal transduction: regulation in cancer. FEBS J. 2010;277:74–80. doi: 10.1111/j.1742-4658.2009.07414.x. [DOI] [PubMed] [Google Scholar]
- 60.Verri C, Roz L, Conte D, Liloglou T, Livio A, Vesin A, Fabbri A, Andriani F, Brambilla C, Tavecchio L, Calarco G, Calabro E, Mancini A, Tosi D, Bossi P, Field JK, Brambilla E, Sozzi G. Fragile histidine triad gene inactivation in lung cancer: the European Early Lung Cancer project. Am J Respir Crit Care Med. 2009;179:396–401. doi: 10.1164/rccm.200807-1153OC. [DOI] [PubMed] [Google Scholar]
- 61.Paulsson K, An Q, Moorman AV, Parker H, Molloy G, Davies T, Griffiths M, Ross FM, Irving J, Harrison CJ, Young BD, Strefford JC. Methylation of tumour suppressor gene promoters in the presence and absence of transcriptional silencing in high hyperdiploid acute lymphoblastic leukaemia. Br J Haematol. 2009;144:838–847. doi: 10.1111/j.1365-2141.2008.07523.x. [DOI] [PubMed] [Google Scholar]
- 62.Hong FZ, Wang B, Li HM, Liew CT. Hypermethylation of fragile histidine triad gene and 3p14 allelic deletion in ovarian carcinomas. Zhonghua Bing Li Xue Za Zhi. 2005;34:257–261. [PubMed] [Google Scholar]
- 63.Goldberg M, Rummelt C, Laerm A, Helmbold P, Holbach LM, Ballhausen WG. Epigenetic silencing contributes to frequent loss of the fragile histidine triad tumour suppressor in basal cell carcinomas. Br J Dermatol. 2006;155:1154–1158. doi: 10.1111/j.1365-2133.2006.07433.x. [DOI] [PubMed] [Google Scholar]
- 64.Neyaz MK, Kumar RS, Hussain S, Naqvi SH, Kohaar I, Thakur N, Kashyap V, Das BC, Husain SA, Bharadwaj M. Effect of aberrant promoter methylation of FHIT and RASSF1A genes on susceptibility to cervical cancer in a North Indian population. Biomarkers. 2008;13:597–606. doi: 10.1080/13547500802078859. [DOI] [PubMed] [Google Scholar]
- 65.Leal MF, Lima EM, Silva PN, Assumpcao PP, Calcagno DQ, Payao SL, Burbano RR, Smith MA. Promoter hypermethylation of CDH1, FHIT, MTAP and PLAGL1 in gastric adenocarcinoma in individuals from Northern Brazil. World J Gastroenterol. 2007;13:2568–2574. doi: 10.3748/wjg.v13.i18.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kvasha S, Gordiyuk V, Kondratov A, Ugryn D, Zgonnyk YM, Rynditch AV, Vozianov AF. Hypermethylation of the 5′ CpG island of the FHIT gene in clear cell renal carcinomas. Cancer Lett. 2008;265:250–257. doi: 10.1016/j.canlet.2008.02.036. [DOI] [PubMed] [Google Scholar]
- 67.Kim JW, Cheng Y, Liu W, Li T, Yegnasubramanian S, Zheng SL, Xu J, Isaacs WB, Chang BL. Genetic and epigenetic inactivation of LPL gene in human prostate cancer. Int J Cancer. 2009;124:734–738. doi: 10.1002/ijc.23972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sard L, Accornero P, Tornielli S, Delia D, Bunone G, Campiglio M, Colombo MP, Gramegna M, Croce CM, Pierotti MA, Sozzi G. The tumor-suppressor gene FHIT is involved in the regulation of apoptosis and in cell cycle control. Proc Natl Acad Sci USA. 1999;96:8489–8492. doi: 10.1073/pnas.96.15.8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guo Z, Johansson SL, Rhim Js, Vishwanatha JK. Fragile histidine triad gene expression in primary prostate cancer and in an in vitro model. Prostate. 2000;43:101–110. doi: 10.1002/(sici)1097-0045(20000501)43:2<101::aid-pros4>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 70.Latil A, Bieche I, Fournier G, Cussenot O, Pesche S, Lidereau R. Molecular analysis of the FHIT gene in human prostate cancer. Oncogene. 1998;16:1863–1868. doi: 10.1038/sj.onc.1201703. [DOI] [PubMed] [Google Scholar]
- 71.Waha A, Koch A, Hartmann W, Mack H, Schramm J, Sorensen N, Berthold F, Wiestler OD, Pietsch T. Analysis of HIC-1 methylation and transcription in human ependymomas. Int J Cancer. 2004;110:542–549. doi: 10.1002/ijc.20165. [DOI] [PubMed] [Google Scholar]
- 72.Tam KF, Liu VW, Liu SS, Tsang PC, Cheung AN, Yip AM, Ngan HY. Methylation profile in benign, borderline and malignant ovarian tumors. J Cancer Res Clin Oncol. 2007;133:331–341. doi: 10.1007/s00432-006-0178-5. [DOI] [PubMed] [Google Scholar]
- 73.Chopin V, Leprince D. Chromosome arm 17p13.3: could HIC1 be the one? Med Sci (Paris) 2006;22:54–61. doi: 10.1051/medsci/200622154. [DOI] [PubMed] [Google Scholar]
- 74.Chen WY, Zeng X, Carter MG, Morrell CN, Chiu Yen RW, Esteller M, Watkins DN, Herman JG, Mankowski JL, Baylin SB. Heterozygous disruption of Hic1 predisposes mice to a gender-dependent spectrum of malignant tumors. Nat Genet. 2003;33:197–202. doi: 10.1038/ng1077. [DOI] [PubMed] [Google Scholar]
- 75.Chen W, Cooper TK, Zahnow CA, Overholtzer M, Zhao Z, Ladanyi M, Karp JE, Gokgoz N, Wunder JS, Andrulis IL, Levine AJ, Mankowski JL, Baylin SB. Epigenetic and genetic loss of Hic1 function accentuates the role of p53 in tumorigenesis. Cancer Cell. 2004;6:387–398. doi: 10.1016/j.ccr.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 76.Kekeeva TV, Popova OP, Shegai PV, Alekseev B, Adnreeva I, Zaletaev DV, Nemtsova MV. Abberant methylation of p16, HIC1, N33 and GSTP1 genes in tumor epitelium and tumor-associated stromal cells of prostate cancer. Mol Biol (Mosk) 2007;41:79–85. [PubMed] [Google Scholar]
- 77.Gallucci M, Merola R, Leonardo C, De Carli P, Farsetti A, Sentinelli S, Sperduti I, Mottolese M, Carlini P, Vico E, Simone G, Cianciulli A. Genetic profile identification in clinically localized prostate carcinoma. Urol Oncol. 2009;27:502–508. doi: 10.1016/j.urolonc.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 78.Kioussi C, Briata P, Baek SH, Rose DW, Hamblet NS, Herman T, Ohgi KA, Lin C, Gleiberman A, Wang J, Brault V, Ruiz-Lozano P, Nguyen HD, Kemler R, Glass CK, Wynshaw-Boris A, Rosenfeld MG. Identification of a Wnt/Dvl/beta-Catenin – > Pitx2 pathway mediating cell-type-specific proliferation during development. Cell. 2002;111:673–685. doi: 10.1016/s0092-8674(02)01084-x. [DOI] [PubMed] [Google Scholar]
- 79.Maier S, Nimmrich I, Koenig T, Eppenberger-Castori S, Bohlmann I, Paradiso A, Spyratos F, Thomssen C, Mueller V, Nahrig J, Schittulli F, Kates R, Lesche R, Schwope I, Kluth A, Marx A, Martens JW, Foekens JA, Schmitt M, Harbeck N. DNA-methylation of the homeodomain transcription factor PITX2 reliably predicts risk of distant disease recurrence in tamoxifen-treated, node-negative breast cancer patients-Technical and clinical validation in a multi-centre setting in collaboration with the European Organisation for Research and Treatment of Cancer (EORTC) PathoBiology group. Eur J Cancer. 2007;43:1679–1686. doi: 10.1016/j.ejca.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 80.Harbeck N, Nimmrich I, Hartmann A, Ross JS, Cufer T, Grutzmann R, Kristiansen G, Paradiso A, Hartmann O, Margossian A, Martens J, Schwope I, Lukas A, Muller V, Milde-Langosch K, Nahrig J, Foekens J, Maier S, Schmitt M, Lesche R. Multicenter study using paraffin-embedded tumor tissue testing PITX2 DNA methylation as a marker for outcome prediction in tamoxifen-treated, node-negative breast cancer patients. J Clin Oncol. 2008;26:5036–5042. doi: 10.1200/JCO.2007.14.1697. [DOI] [PubMed] [Google Scholar]
- 81.Nimmrich I, Sieuwerts AM, Meijer-van Gelder ME, Schwope I, Bolt-de Vries J, Harbeck N, Koenig T, Hartmann O, Kluth A, Dietrich D, Magdolen V, Portengen H, Look MP, Klijn JG, Lesche R, Schmitt M, Maier S, Foekens JA, Martens JW. DNA hypermethylation of PITX2 is a marker of poor prognosis in untreated lymph node-negative hormone receptor-positive breast cancer patients. Breast Cancer Res Treat. 2008;111:429–437. doi: 10.1007/s10549-007-9800-8. [DOI] [PubMed] [Google Scholar]
- 82.Weiss G, Cottrell S, Distler J, Schatz P, Kristiansen G, Ittmann M, Haefliger C, Lesche R, Hartmann A, Corman J, Wheeler T. DNA methylation of the PITX2 gene promoter region is a strong independent prognostic marker of biochemical recurrence in patients with prostate cancer after radical prostatectomy. J Urol. 2009;181:1678–1685. doi: 10.1016/j.juro.2008.11.120. [DOI] [PubMed] [Google Scholar]
- 83.Banez LL, Sun L, van Leenders GJ, Wheeler TM, Bangma CH, Freedland SJ, Ittmann MM, Lark AL, Madden JF, Hartman A, Weiss G, Castanos-Velez E. Multicenter clinical validation of PITX2 methylation as a prostate specific antigen recurrence predictor in patients with post-radical prostatectomy prostate cancer. J Urol. 2010;184:149–156. doi: 10.1016/j.juro.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 84.Vanaja DK, Ehrich M, Van den Boom D, Cheville JC, Karnes RJ, Tindall DJ, Cantor CR, Young CY. Hypermethylation of genes for diagnosis and risk stratification of prostate cancer. Cancer Invest. 2009;27:549–560. doi: 10.1080/07357900802620794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373–2380. doi: 10.1002/ijc.23173. [DOI] [PubMed] [Google Scholar]
- 86.Bastian PJ, Ellinger J, Wellmann A, Wernert N, Heukamp LC, Muller SC, von Ruecker A. Diagnostic and prognostic information in prostate cancer with the help of a small set of hypermethylated gene loci. Clin Cancer Res. 2005;11:4097–4106. doi: 10.1158/1078-0432.CCR-04-1832. [DOI] [PubMed] [Google Scholar]
- 87.Bastian PJ, Palapattu GS, Yegnasubra-manian S, Rogers CG, Lin X, Mangold LA, Trock B, Eisenberger MA, Partin AW, Nelson WG. CpG island hypermethylation profile in the serum of men with clinically localized and hormone refractory metastatic prostate cancer. J Urol. 2008;179:529–534. doi: 10.1016/j.juro.2007.09.038. discussion 534–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ellinger J, Bastian PJ, Jurgan T, Biermann K, Kahl P, Heukamp LC, Wernert N, Muller SC, von Ruecker A. CpG island hypermethylation at multiple gene sites in diagnosis and prognosis of prostate cancer. Urology. 2008;71:161–167. doi: 10.1016/j.urology.2007.09.056. [DOI] [PubMed] [Google Scholar]
- 89.Bastian PJ, Ellinger J, Heukamp LC, Kahl P, Muller SC, von Rucker A. Prognostic value of CpG island hypermethylation at PTGS2, RAR-beta, EDNRB, and other gene loci in patients undergoing radical prostatectomy. Eur Urol. 2007;51:665–674. doi: 10.1016/j.eururo.2006.08.008. discussion 674. [DOI] [PubMed] [Google Scholar]
- 90.Okegawa T, Nutahara K, Higashihara E. Association of circulating tumor cells with tumor-related methylated DNA in patients with hormone-refractory prostate cancer. Int J Urol. 2010;17:466–475. doi: 10.1111/j.1442-2042.2010.02502.x. [DOI] [PubMed] [Google Scholar]
- 91.Kuzmin I, Gillespie JW, Protopopov A, Geil L, Dreijerink K, Yang Y, Vocke CD, Duh FM, Zabarovsky E, Minna JD, Rhim JS, Emmert-Buck MR, Linehan WM, Lerman MI. The RASSF1A tumor suppressor gene is inactivated in prostate tumors and suppresses growth of prostate carcinoma cells. Cancer Res. 2002;62:3498–3502. [PubMed] [Google Scholar]
- 92.Liu L, Yoon JH, Dammann R, Pfeifer GP. Frequent hypermethylation of the RASSF1A gene in prostate cancer. Oncogene. 2002;21:6835–6840. doi: 10.1038/sj.onc.1205814. [DOI] [PubMed] [Google Scholar]
- 93.Kang GH, Lee S, Lee HJ, Hwang KS. Aberrant CpG island hypermethylation of multiple genes in prostate cancer and prostatic intraepithelial neoplasia. J Pathol. 2004;202:233–240. doi: 10.1002/path.1503. [DOI] [PubMed] [Google Scholar]
- 94.Kawamoto K, Okino ST, Place RF, Urakami S, Hirata H, Kikuno N, Kawakami T, Tanaka Y, Pookot D, Chen Z, Majid S, Enokida H, Nakagawa M, Dahiya R. Epigenetic modifications of RASSF1A gene through chromatin remodeling in prostate cancer. Clin Cancer Res. 2007;13:2541–2548. doi: 10.1158/1078-0432.CCR-06-2225. [DOI] [PubMed] [Google Scholar]
- 95.Singal R, Ferdinand L, Reis IM, Schlesselman JJ. Methylation of multiple genes in prostate cancer and the relationship with clinicopathological features of disease. Oncol Rep. 2004;12:631–637. [PubMed] [Google Scholar]
- 96.Aitchison A, Warren A, Neal D, Rabbitts P. RASSF1A promoter methylation is frequently detected in both pre-malignant and non-malignant microdissected prostatic epithelial tissues. Prostate. 2007;67:638–644. doi: 10.1002/pros.20475. [DOI] [PubMed] [Google Scholar]
- 97.Srinivas SR, Gopal E, Zhuang L, Itagaki S, Martin PM, Fei YJ, Ganapathy V, Prasad PD. Cloning and functional identification of slc5a12 as a sodium-coupled low-affinity transporter for monocarboxylates (SMCT2) Biochem J. 2005;392:655–664. doi: 10.1042/BJ20050927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kennedy KM, Dewhirst MW. Tumor metabolism of lactate: the influence and therapeutic potential for MCT and CD147 regulation. Future Oncol. 2010;6:127–148. doi: 10.2217/fon.09.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ganapathy V, Thangaraju M, Gopal E, Martin PM, Itagaki S, Miyauchi S, Prasad PD. Sodium-coupled monocarboxylate transporters in normal tissues and in cancer. AAPS J. 2008;10:193–199. doi: 10.1208/s12248-008-9022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schagdarsurengin U, Gimm O, Dralle H, Hoang-Vu C, Dammann R. CpG island methylation of tumor-related promoters occurs preferentially in undifferentiated carcinoma. Thyroid. 2006;16:633–642. doi: 10.1089/thy.2006.16.633. [DOI] [PubMed] [Google Scholar]
- 101.Li H, Myeroff L, Smiraglia D, Romero MF, Pretlow TP, Kasturi L, Lutterbaugh J, Rerko RM, Casey G, Issa JP, Willis J, Willson JK, Plass C, Markowitz SD. SLC5A8, a sodium transporter, is a tumor suppressor gene silenced by methylation in human colon aberrant crypt foci and cancers. Proc Natl Acad Sci USA. 2003;100:8412–8417. doi: 10.1073/pnas.1430846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Park J, Brena RM, Gruidl M, Zhou J, Huang T, Plass C, Tockman MS. CpG island hypermethylation profiling of lung cancer using restriction landmark genomic scanning (RLGS) analysis. Cancer Biomarkers. 2005;1:193–200. doi: 10.3233/cbm-2005-12-307. [DOI] [PubMed] [Google Scholar]
- 103.Thangaraju M, Gopal E, Martin PM, Ananth S, Smith SB, Prasad PD, Sterneck E, Ganapathy V. SLC5A8 triggers tumor cell apoptosis through pyruvate-dependent inhibition of histone deacetylases. Cancer Res. 2006;66:11560–11564. doi: 10.1158/0008-5472.CAN-06-1950. [DOI] [PubMed] [Google Scholar]
- 104.Dong SM, Lee EJ, Jeon ES, Park CK, Kim KM. Progressive methylation during the serrated neoplasia pathway of the colorectum. Mod Pathol. 2005;18:170–178. doi: 10.1038/modpathol.3800261. [DOI] [PubMed] [Google Scholar]
- 105.Ganapathy V, Gopal E, Miyauchi S, Prasad PD. Biological functions of SLC5A8, a candidate tumour suppressor. Biochem Soc Trans. 2005;33:237–240. doi: 10.1042/BST0330237. [DOI] [PubMed] [Google Scholar]
- 106.Hong C, Maunakea A, Jun P, Bollen AW, Hodgson JG, Goldenberg DD, Weiss WA, Costello JF. Shared epigenetic mechanisms in human and mouse gliomas inactivate expression of the growth suppressor SLC5A8. Cancer Res. 2005;65:3617–3623. doi: 10.1158/0008-5472.CAN-05-0048. [DOI] [PubMed] [Google Scholar]
- 107.Porra V, Ferraro-Peyret C, Durand C, Selmi-Ruby S, Giroud H, Berger-Dutrieux N, Decaussin M, Peix JL, Bournaud C, Orgiazzi J, Borson-Chazot F, Dante R, Rousset B. Silencing of the tumor suppressor gene SLC5A8 is associated with BRAF mutations in classical papillary thyroid carcinomas. J Clin Endocrinol Metab. 2005;90:3028–3035. doi: 10.1210/jc.2004-1394. [DOI] [PubMed] [Google Scholar]
- 108.Ueno M, Toyota M, Akino K, Suzuki H, Kusano M, Satoh A, Mita H, Sasaki Y, Nojima M, Yanagihara K, Hinoda Y, Tokino T, Imai K. Aberrant methylation and histone deacetylation associated with silencing of SLC5A8 in gastric cancer. Tumour Biol. 2004;25:134–140. doi: 10.1159/000079145. [DOI] [PubMed] [Google Scholar]
- 109.Hu S, Liu D, Tufano RP, Carson KA, Rosenbaum E, Cohen Y, Holt EH, Kiseljak-Vassiliades K, Rhoden KJ, Tolaney S, Condouris S, Tallini G, Westra WH, Umbricht CB, Zeiger MA, Califano JA, Vasko V, Xing M. Association of aberrant methylation of tumor suppressor genes with tumor aggressiveness and BRAF mutation in papillary thyroid cancer. Int J Cancer. 2006;119:2322–2329. doi: 10.1002/ijc.22110. [DOI] [PubMed] [Google Scholar]
- 110.Park JY, Helm JF, Zheng W, Ly QP, Hodul PJ, Centeno BA, Malafa MP. Silencing of the candidate tumor suppressor gene solute carrier family 5 member 8 (SLC5A8) in human pancreatic cancer. Pancreas. 2008;36:e32–39. doi: 10.1097/MPA.0b013e3181630ffe. [DOI] [PubMed] [Google Scholar]
- 111.Park JY, Zheng W, Kim D, Cheng JQ, Kumar N, Ahmad N, Pow-Sang J. Candidate tumor suppressor gene SLC5A8 is frequently down-regulated by promoter hypermethylation in prostate tumor. Cancer Detect Prev. 2007;31:359–365. doi: 10.1016/j.cdp.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 112.Pinheiro C, Reis RM, Ricardo S, Longatto-Filho A, Schmitt F, Baltazar F. Expression of monocarboxylate transporters 1, 2, and 4 in human tumours and their association with CD147 and CD44. J Biomed Biotechnol. 2010;2010:427694. doi: 10.1155/2010/427694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Weihe E, Eiden LE. Chemical neuroanatomy of the vesicular amine transporters. FASEB J. 2000;14:2435–2449. doi: 10.1096/fj.00-0202rev. [DOI] [PubMed] [Google Scholar]
- 114.Kristiansen G, Pilarsky C, Wissmann C, Kaiser S, Bruemmendorf T, Roepcke S, Dahl E, Hinzmann B, Specht T, Pervan J, Stephan C, Loening S, Dietel M, Rosenthal A. Expression profiling of microdissected matched prostate cancer samples reveals CD166/MEMD and CD24 as new prognostic markers for patient survival. J Pathol. 2005;205:359–376. doi: 10.1002/path.1676. [DOI] [PubMed] [Google Scholar]
- 115.Sorensen KD, Wild PJ, Mortezavi A, Adolf K, Torring N, Heeboll S, Ulhoi BP, Ottosen P, Sulser T, Hermanns T, Moch H, Borre M, Orntoft TF, Dyrskjot L. Genetic and epigenetic SLC18A2 silencing in prostate cancer is an independent adverse predictor of biochemical recurrence after radical prostatectomy. Clin Cancer Res. 2009;15:1400–1410. doi: 10.1158/1078-0432.CCR-08-2268. [DOI] [PubMed] [Google Scholar]
- 116.Chang BL, Liu W, Sun J, Dimitrov L, Li T, Turner AR, Zheng SL, Isaacs WB, Xu J. Integration of somatic deletion analysis of prostate cancers and germline linkage analysis of prostate cancer families reveals two small consensus regions for prostate cancer genes at 8p. Cancer Res. 2007;67:4098–4103. doi: 10.1158/0008-5472.CAN-06-4570. [DOI] [PubMed] [Google Scholar]
- 117.Cheng Y, Kim JW, Liu W, Dunn TA, Luo J, Loza MJ, Kim ST, Zheng SL, Xu J, Isaacs WB, Chang BL. Genetic and epigenetic inactivation of TNFRSF10C in human prostate cancer. Prostate. 2009;69:327–335. doi: 10.1002/pros.20882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shivapurkar N, Toyooka S, Toyooka KO, Reddy J, Miyajima K, Suzuki M, Shigematsu H, Takahashi T, Parikh G, Pass HI, Chaudhary PM, Gazdar AF. Aberrant methylation of trail decoy receptor genes is frequent in multiple tumor types. Int J Cancer. 2004;109:786–792. doi: 10.1002/ijc.20041. [DOI] [PubMed] [Google Scholar]
- 119.van Noesel MM, van Bezouw S, Salomons GS, Voute PA, Pieters R, Baylin SB, Herman JG, Versteeg R. Tumor-specific down-regulation of the tumor necrosis factor-related apoptosis-inducing ligand decoy receptors DcR1 and DcR2 is associated with dense promoter hypermethylation. Cancer Res. 2002;62:2157–2161. [PubMed] [Google Scholar]
- 120.Hornstein M, Hoffmann MJ, Alexa A, Yamanaka M, Muller M, Jung V, Rahnenfuhrer J, Schulz WA. Protein phosphatase and TRAIL receptor genes as new candidate tumor genes on chromosome 8p in prostate cancer. Cancer Genomics Proteomics. 2008;5:123–136. [PubMed] [Google Scholar]
- 121.Cho NY, Kim JH, Moon KC, Kang GH. Genomic hypomethylation and CpG island hypermethylation in prostatic intraepithelial neoplasm. Virchows Arch. 2009;454:17–23. doi: 10.1007/s00428-008-0706-6. [DOI] [PubMed] [Google Scholar]
- 122.Cho NY, Kim BH, Choi M, Yoo EJ, Moon KC, Cho YM, Kim D, Kang GH. Hypermethylation of CpG island loci and hypomethylation of LINE-1 and Alu repeats in prostate adenocarcinoma and their relationship to clinicopathological features. J Pathol. 2007;211:269–277. doi: 10.1002/path.2106. [DOI] [PubMed] [Google Scholar]
- 123.Barnabas N, Xu L, Savera A, Hou Z, Barrack ER. Chromosome 8 markers of metastatic prostate cancer in African American men: Gain of the MIR151 gene and loss of the NKX3-1 gene. Prostate. 2010 doi: 10.1002/pros.21302. [DOI] [PubMed] [Google Scholar]
- 124.Ju JH, Maeng JS, Zemedkun M, Ahronovitz N, Mack JW, Ferretti JA, Gelmann EP, Gruschus JM. Physical and functional interactions between the prostate suppressor homeoprotein NKX3.1 and serum response factor. J Mol Biol. 2006;360:989–999. doi: 10.1016/j.jmb.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 125.Shen MM, Abate-Shen C. Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev. 2010;24:1967–2000. doi: 10.1101/gad.1965810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ouyang X, DeWeese TL, Nelson WG, Abate-Shen C. Loss-of-function of Nkx3.1 promotes increased oxidative damage in prostate carcinogenesis. Cancer Res. 2005;65:6773–6779. doi: 10.1158/0008-5472.CAN-05-1948. [DOI] [PubMed] [Google Scholar]
- 127.Bowen C, Bubendorf L, Voeller HJ, Slack R, Willi N, Sauter G, Gasser TC, Koivisto P, Lack EE, Kononen J, Kallioniemi OP, Gelmann EP. Loss of NKX3.1 expression in human prostate cancers correlates with tumor progression. Cancer Res. 2000;60:6111–6115. [PubMed] [Google Scholar]
- 128.Gurel B, Ali TZ, Montgomery EA, Begum S, Hicks J, Goggins M, Eberhart CG, Clark DP, Bieberich CJ, Epstein JI, De Marzo AM. NKX3.1 as a marker of prostatic origin in metastatic tumors. Am J Surg Pathol. 2010;34:1097–1105. doi: 10.1097/PAS.0b013e3181e6cbf3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Asatiani E, Huang WX, Wang A, Rodriguez Ortner E, Cavalli LR, Haddad BR, Gelmann EP. Deletion, methylation, and expression of the NKX3.1 suppressor gene in primary human prostate cancer. Cancer Res. 2005;65:1164–1173. doi: 10.1158/0008-5472.CAN-04-2688. [DOI] [PubMed] [Google Scholar]
- 130.Lind GE, Skotheim RI, Fraga MF, Abeler VM, Henrique R, Saatcioglu F, Esteller M, Teixeira MR, Lothe RA. The loss of NKX3.1 expression in testicular-and prostate-cancers is not caused by promoter hypermethylation. Mol Cancer. 2005;4:8. doi: 10.1186/1476-4598-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chung W, Kwabi-Addo B, Ittmann M, Jelinek J, Shen L, Yu Y, Issa JP. Identification of novel tumor markers in prostate, colon and breast cancer by unbiased methylation profiling. PLoS One. 2008;3:e2079. doi: 10.1371/journal.pone.0002079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kwabi-Addo B, Wang S, Chung W, Jelinek J, Patierno SR, Wang BD, Andrawis R, Lee NH, Apprey V, Issa JP, Ittmann M. Identification of differentially methylated genes in normal prostate tissues from African American and Caucasian men. Clin Cancer Res. 2010;16:3539–3547. doi: 10.1158/1078-0432.CCR-09-3342. [DOI] [PubMed] [Google Scholar]
- 133.Reibenwein J, Pils D, Horak P, Tomicek B, Goldner G, Worel N, Elandt K, Krainer M. Promoter hypermethylation of GSTP1, AR, and 14-3-3sigma in serum of prostate cancer patients and its clinical relevance. Prostate. 2007;67:427–432. doi: 10.1002/pros.20533. [DOI] [PubMed] [Google Scholar]
- 134.Lodygin D, Hermeking H. The role of epigenetic inactivation of 14-3-3sigma in human cancer. Cell Res. 2005;15:237–246. doi: 10.1038/sj.cr.7290292. [DOI] [PubMed] [Google Scholar]
- 135.Henrique R, Jeronimo C, Hoque MO, Carvalho AL, Oliveira J, Teixeira MR, Lopes C, Sidransky D. Frequent 14-3-3 sigma promoter methylation in benign and malignant prostate lesions. DNA Cell Biol. 2005;24:264–269. doi: 10.1089/dna.2005.24.264. [DOI] [PubMed] [Google Scholar]
- 136.Henderson BE, Ross RK, Pike MC, Casagrande JT. Endogenous hormones as a major factor in human cancer. Cancer Res. 1982;42:3232–3239. [PubMed] [Google Scholar]
- 137.Henderson BE, Ross RK, Pike MC. Toward the primary prevention of cancer. Science. 1991;254:1131–1138. doi: 10.1126/science.1957166. [DOI] [PubMed] [Google Scholar]
- 138.Ellem SJ, Risbridger GP. Aromatase and regulating the estrogen: androgen ratio in the prostate gland. J Steroid Biochem Mol Biol. 2010;118:246–251. doi: 10.1016/j.jsbmb.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 139.Wang Q, Li W, Zhang Y, Yuan X, Xu K, Yu J, Chen Z, Beroukhim R, Wang H, Lupien M, Wu T, Regan MM, Meyer CA, Carroll JS, Manrai AK, Janne OA, Balk SP, Mehra R, Han B, Chinnaiyan AM, Rubin MA, True L, Fiorentino M, Fiore C, Loda M, Kantoff PW, Liu XS, Brown M. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell. 2009;138:245, 256. doi: 10.1016/j.cell.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Eder IE, Culig Z, Ramoner R, Thurnher M, Putz T, Nessler-Menardi C, Tiefenthaler M, Bartsch G, Klocker H. Inhibition of LncaP prostate cancer cells by means of androgen receptor anti-sense oligonucleotides. Cancer Gene Ther. 2000;7:997–1007. doi: 10.1038/sj.cgt.7700202. [DOI] [PubMed] [Google Scholar]
- 141.Mitchell SH, Zhu W, Young CY. Resveratrol inhibits the expression and function of the androgen receptor in LNCaP prostate cancer cells. Cancer Res. 1999;59:5892–5895. [PubMed] [Google Scholar]
- 142.Tong Q, Zeng F, Lin C, Zhao J, Lu G. Growth inhibiting effects of anti-sense eukaryotic expression vector of proliferating cell nuclear antigen gene on human bladder cancer cells. Chin Med J (Engl) 2003;116:1203–1206. [PubMed] [Google Scholar]
- 143.Heisler LE, Evangelou A, Lew AM, Trachtenberg J, Elsholtz HP, Brown TJ. Androgen-dependent cell cycle arrest and apoptotic death in PC-3 prostatic cell cultures expressing a full-length human androgen receptor. Mol Cell Endocrinol. 1997;126:59–73. doi: 10.1016/s0303-7207(96)03970-6. [DOI] [PubMed] [Google Scholar]
- 144.Grossmann ME, Huang H, Tindall DJ. Androgen receptor signaling in androgen-refractory prostate cancer. J Natl Cancer Inst. 2001;93:1687–1697. doi: 10.1093/jnci/93.22.1687. [DOI] [PubMed] [Google Scholar]
- 145.Jarrard DF, Kinoshita H, Shi Y, Sandefur C, Hoff D, Meisner LF, Chang C, Herman JG, Isaacs WB, Nassif N. Methylation of the androgen receptor promoter CpG island is associated with loss of androgen receptor expression in prostate cancer cells. Cancer Res. 1998;58:5310–5314. [PubMed] [Google Scholar]
- 146.Kinoshita H, Shi Y, Sandefur C, Meisner LF, Chang C, Choon A, Reznikoff CR, Bova GS, Friedl A, Jarrard DF. Methylation of the androgen receptor minimal promoter silences transcription in human prostate cancer. Cancer Res. 2000;60:3623–3630. [PubMed] [Google Scholar]
- 147.Sasaki M, Tanaka Y, Perinchery G, Dharia A, Kotcherguina I, Fujimoto S, Dahiya R. Methylation and inactivation of estrogen, progesterone, and androgen receptors in prostate cancer. J Natl Cancer Inst. 2002;94:384–390. doi: 10.1093/jnci/94.5.384. [DOI] [PubMed] [Google Scholar]
- 148.Nakayama T, Watanabe M, Suzuki H, Toyota M, Sekita N, Hirokawa Y, Mizokami A, Ito H, Yatani R, Shiraishi T. Epigenetic regulation of androgen receptor gene expression in human prostate cancers. Lab Invest. 2000;80:1789–1796. doi: 10.1038/labinvest.3780190. [DOI] [PubMed] [Google Scholar]
- 149.Schayek H, Bentov I, Sun S, Plymate SR, Werner H. Progression to metastatic stage in a cellular model of prostate cancer is associated with methylation of the androgen receptor gene and transcriptional suppression of the insulin-like growth factor-I receptor gene. Exp Cell Res. 2010;316:1479–1488. doi: 10.1016/j.yexcr.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bosland MC. The role of estrogens in prostate carcinogenesis: a rationale for chemo-prevention. Rev Urol. 2005;7(Suppl 3):S4–S10. [PMC free article] [PubMed] [Google Scholar]
- 151.Li LC, Okino ST, Dahiya R. DNA methylation in prostate cancer. Biochim Biophys Acta. 2004;1704:87–102. doi: 10.1016/j.bbcan.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 152.Hobisch A, Hittmair A, Daxenbichler G, Wille S, Radmayr C, Hobisch-Hagen P, Bartsch G, Klocker H, Culig Z. Metastatic lesions from prostate cancer do not express oestrogen and progesterone receptors. J Pathol. 1997;182:356–361. doi: 10.1002/(SICI)1096-9896(199707)182:3<356::AID-PATH863>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 153.Horvath LG, Henshall SM, Lee CS, Head DR, Quinn DI, Makela S, Delprado W, Golovsky D, Brenner PC, O’Neill G, Kooner R, Stricker PD, Grygiel JJ, Gustafsson JA, Sutherland RL. Frequent loss of estrogen receptor-beta expression in prostate cancer. Cancer Res. 2001;61:5331–5335. [PubMed] [Google Scholar]
- 154.Zhu X, Leav I, Leung YK, Wu M, Liu Q, Gao Y, McNeal JE, Ho SM. Dynamic regulation of estrogen receptor-beta expression by DNA methylation during prostate cancer development and metastasis. Am J Pathol. 2004;164:2003–2012. doi: 10.1016/s0002-9440(10)63760-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang X, Leung YK, Ho SM. AP-2 regulates the transcription of estrogen receptor (ER)-beta by acting through a methylation hotspot of the 0N promoter in prostate cancer cells. Oncogene. 2007;26:7346–7354. doi: 10.1038/sj.onc.1210537. [DOI] [PubMed] [Google Scholar]
- 156.Konishi N, Nakaoka S, Hiasa Y, Kitahori Y, Ohshima M, Samma S, Okajima E. Immunohistochemical evaluation of estrogen receptor status in benign prostatic hypertrophy and in prostate carcinoma and the relationship to efficacy of endocrine therapy. Oncology. 1993;50:259–263. doi: 10.1159/000227191. [DOI] [PubMed] [Google Scholar]
- 157.Moriyama-Gonda N, Shiina H, Terashima M, Satoh K, Igawa M. Rationale and clinical implication of combined chemotherapy with cisplatin and oestrogen in prostate cancer: primary evidence based on methylation analysis of oestrogen receptor-alpha. BJU Int. 2008;101:485–491. doi: 10.1111/j.1464-410X.2007.07256.x. [DOI] [PubMed] [Google Scholar]
- 158.Li LC, Chui R, Nakajima K, Oh BR, Au HC, Dahiya R. Frequent methylation of estrogen receptor in prostate cancer: correlation with tumor progression. Cancer Res. 2000;60:702–706. [PubMed] [Google Scholar]
- 159.Leav I, Lau KM, Adams JY, McNeal JE, Taplin ME, Wang J, Singh H, Ho SM. Comparative studies of the estrogen receptors beta and alpha and the androgen receptor in normal human prostate glands, dysplasia, and in primary and metastatic carcinoma. Am J Pathol. 2001;159:79–92. doi: 10.1016/s0002-9440(10)61676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yao Q, He XS, Zhang JM, He J. Promotor hypermethylation of E-cadherin, p16 and estrogen receptor in prostate carcinoma. Zhonghua Nan Ke Xue. 2006;12:28–31. [PubMed] [Google Scholar]
- 161.Nojima D, Li LC, Dharia A, Perinchery G, Ribeiro-Filho L, Yen TS, Dahiya R. CpG hypermethylation of the promoter region inactivates the estrogen receptor-beta gene in patients with prostate carcinoma. Cancer. 2001;92:2076–2083. doi: 10.1002/1097-0142(20011015)92:8<2076::aid-cncr1548>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 162.Hayashi K, Yokozaki H, Naka K, Yasui W, Lotan R, Tahara E. Overexpression of retinoic acid receptor beta induces growth arrest and apoptosis in oral cancer cell lines. Jpn J Cancer Res. 2001;92:42–50. doi: 10.1111/j.1349-7006.2001.tb01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nakayama T, Watanabe M, Yamanaka M, Hirokawa Y, Suzuki H, Ito H, Yatani R, Shiraishi T. The role of epigenetic modifications in retinoic acid receptor beta2 gene expression in human prostate cancers. Lab Invest. 2001;81:1049–1057. doi: 10.1038/labinvest.3780316. [DOI] [PubMed] [Google Scholar]
- 164.Zhang J, Liu L, Pfeifer GP. Methylation of the retinoid response gene TIG1 in prostate cancer correlates with methylation of the retinoic acid receptor beta gene. Oncogene. 2004;23:2241–2249. doi: 10.1038/sj.onc.1207328. [DOI] [PubMed] [Google Scholar]
- 165.Zon G, Barker MA, Kaur P, Groshen S, Jones LW, Imam SA, Boyd VL. Formamide as a denaturant for bisulfite conversion of genomic DNA: Bisulfite sequencing of the GSTPi and RARb eta2 genes of 43 formalin-fixed paraffin-embedded prostate cancer specimens. Anal Biochem. 2009;392:117–125. doi: 10.1016/j.ab.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 166.Roupret M, Hupertan V, Catto JW, Yates DR, Rehman I, Proctor LM, Phillips J, Meuth M, Cussenot O, Hamdy FC. Promoter hypermethylation in circulating blood cells identifies prostate cancer progression. Int J Cancer. 2008;122:952–956. doi: 10.1002/ijc.23196. [DOI] [PubMed] [Google Scholar]
- 167.Henrique R, Jeronimo C. Molecular detection of prostate cancer: a role for GSTP1 hypermethylation. Eur Urol. 2004;46:660–669. doi: 10.1016/j.eururo.2004.06.014. discussion 669. [DOI] [PubMed] [Google Scholar]
- 168.Nelson CP, Kidd LC, Sauvageot J, Isaacs WB, De Marzo AM, Groopman JD, Nelson WG, Kensler TW. Protection against 2-hydroxyamino-1-methyl-6-phenylimidazo(4,5-b)pyridine cytotoxicity and DNA adduct formation in human prostate by glutathione S-transferase P1. Cancer Res. 2001;61:103–109. [PubMed] [Google Scholar]
- 169.Lee WH, Morton RA, Epstein JI, Brooks JD, Campbell PA, Bova GS, Hsieh WS, Isaacs WB, Nelson WG. Cytidine methylation of regulatory sequences near the pi-class glutathione S-transferase gene accompanies human prostatic carcinogenesis. Proc Natl Acad Sci USA. 1994;91:11733–11737. doi: 10.1073/pnas.91.24.11733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Harden SV, Guo Z, Epstein JI, Sidransky D. Quantitative GSTP1 methylation clearly distinguishes benign prostatic tissue and limited prostate adenocarcinoma. J Urol. 2003;169:1138–1142. doi: 10.1097/01.ju.0000049627.90307.4d. [DOI] [PubMed] [Google Scholar]
- 171.Cairns P, Esteller M, Herman JG, Schoenberg M, Jeronimo C, Sanchez-Cespedes M, Chow NH, Grasso M, Wu L, Westra WB, Sidransky D. Molecular detection of prostate cancer in urine by GSTP1 hypermethylation. Clin Cancer Res. 2001;7:2727–2730. [PubMed] [Google Scholar]
- 172.Lee WH, Isaacs WB, Bova GS, Nelson WG. CG island methylation changes near the GSTP1 gene in prostatic carcinoma cells detected using the polymerase chain reaction: a new prostate cancer bio-marker. Cancer Epidemiol Biomarkers Prev. 1997;6:443–450. [PubMed] [Google Scholar]
- 173.Santourlidis S, Florl A, Ackermann R, Wirtz HC, Schulz WA. High frequency of alterations in DNA methylation in adenocarcinoma of the prostate. Prostate. 1999;39:166–174. doi: 10.1002/(sici)1097-0045(19990515)39:3<166::aid-pros4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 174.Goessl C, Krause H, Muller M, Heicappell R, Schrader M, Sachsinger J, Miller K. Fluorescent methylation-specific polymerase chain reaction for DNA-based detection of prostate cancer in bodily fluids. Cancer Res. 2000;60:5941–5945. [PubMed] [Google Scholar]
- 175.Jeronimo C, Usadel H, Henrique R, Oliveira J, Lopes C, Nelson WG, Sidransky D. Quantitation of GSTP1 methylation in non-neoplastic prostatic tissue and organ-confined prostate adenocarcinoma. J Natl Cancer Inst. 2001;93:1747–1752. doi: 10.1093/jnci/93.22.1747. [DOI] [PubMed] [Google Scholar]
- 176.Gonzalgo ML, Pavlovich CP, Lee SM, Nelson WG. Prostate cancer detection by GSTP1 methylation analysis of postbiopsy urine specimens. Clin Cancer Res. 2003;9:2673–2677. [PubMed] [Google Scholar]
- 177.Jeronimo C, Varzim G, Henrique R, Oliveira J, Bento MJ, Silva C, Lopes C, Sidransky D. I105V polymorphism and promoter methylation of the GSTP1 gene in prostate adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2002;11:445–450. [PubMed] [Google Scholar]
- 178.Woodson K, Hayes R, Wideroff L, Villaruz L, Tangrea J. Hypermethylation of GSTP1, CD44, and E-cadherin genes in prostate cancer among US Blacks and Whites. Prostate. 2003;55:199–205. doi: 10.1002/pros.10236. [DOI] [PubMed] [Google Scholar]
- 179.Kollermann J, Muller M, Goessl C, Krause H, Helpap B, Pantel K, Miller K. Methylation-specific PCR for DNA-based detection of occult tumor cells in lymph nodes of prostate cancer patients. Eur Urol. 2003;44:533–538. doi: 10.1016/s0302-2838(03)00361-0. [DOI] [PubMed] [Google Scholar]
- 180.Payne SR, Serth J, Schostak M, Kamradt J, Strauss A, Thelen P, Model F, Day JK, Liebenberg V, Morotti A, Yamamura S, Lograsso J, Sledziewski A, Semjonow A. DNA methylation biomarkers of prostate cancer: confirmation of candidates and evidence urine is the most sensitive body fluid for non-invasive detection. Prostate. 2009;69:1257–1269. doi: 10.1002/pros.20967. [DOI] [PubMed] [Google Scholar]
- 181.Suh CI, Shanafelt T, May DJ, Shroyer KR, Bobak JB, Crawford ED, Miller GJ, Markham N, Glode LM. Comparison of telomerase activity and GSTP1 promoter methylation in ejaculate as potential screening tests for prostate cancer. Mol Cell Probes. 2000;14:211–217. doi: 10.1006/mcpr.2000.0307. [DOI] [PubMed] [Google Scholar]
- 182.Goessl C, Muller M, Heicappell R, Krause H, Miller K. DNA-based detection of prostate cancer in blood, urine, and ejaculates. Ann N Y Acad Sci. 2001;945:51–58. doi: 10.1111/j.1749-6632.2001.tb03863.x. [DOI] [PubMed] [Google Scholar]
- 183.Ellinger J, Haan K, Heukamp LC, Kahl P, Buttner R, Muller SC, von Ruecker A, Bastian PJ. CpG island hypermethylation in cell-free serum DNA identifies patients with localized prostate cancer. Prostate. 2008;68:42–49. doi: 10.1002/pros.20651. [DOI] [PubMed] [Google Scholar]
- 184.Vener T, Derecho C, Baden J, Wang H, Rajpurohit Y, Skelton J, Mehrotra J, Varde S, Chowdary D, Stallings W, Leibovich B, Robin H, Pelzer A, Schafer G, Auprich M, Mannweiler S, Amersdorfer P, Mazumder A. Development of a multiplexed urine assay for prostate cancer diagnosis. Clin Chem. 2008;54:874–882. doi: 10.1373/clinchem.2007.094912. [DOI] [PubMed] [Google Scholar]
- 185.Pasquali D, Rossi V, Bellastella G, Bellastella A, Sinisi AA. Natural and synthetic retinoids in prostate cancer. Curr Pharm Des. 2006;12:1923–1929. doi: 10.2174/138161206776873554. [DOI] [PubMed] [Google Scholar]
- 186.Bushue N, Wan YJ. Retinoid pathway and cancer therapeutics. Adv Drug Deliv Rev. 2010 doi: 10.1016/j.addr.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Murphy TM, Perry AS, Lawler M. The emergence of DNA methylation as a key modulator of aberrant cell death in prostate cancer. Endocr Relat Cancer. 2008;15:11–25. doi: 10.1677/ERC-07-0208. [DOI] [PubMed] [Google Scholar]
- 188.Esteller M, Guo M, Moreno V, Peinado MA, Capella G, Galm O, Baylin SB, Herman JG. Hypermethylation-associated Inactivation of the Cellular Retinol-Binding-Protein 1 Gene in Human Cancer. Cancer Res. 2002;62:5902–5905. [PubMed] [Google Scholar]
- 189.Jeronimo C, Henrique R, Oliveira J, Lobo F, Pais I, Teixeira MR, Lopes C. Aberrant cellular retinol binding protein 1 (CRBP1) gene expression and promoter methylation in prostate cancer. J Clin Pathol. 2004;57:872–876. doi: 10.1136/jcp.2003.014555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Suzuki M, Shigematsu H, Shivapurkar N, Reddy J, Miyajima K, Takahashi T, Gazdar AF, Frenkel EP. Methylation of apoptosis related genes in the pathogenesis and prognosis of prostate cancer. Cancer Lett. 2006;242:222–230. doi: 10.1016/j.canlet.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 191.Enokida H, Shiina H, Igawa M, Ogishima T, Kawakami T, Bassett WW, Anast JW, Li LC, Urakami S, Terashima M, Verma M, Kawahara M, Nakagawa M, Kane CJ, Carroll PR, Dahiya R. CpG hypermethylation of MDR1 gene contributes to the pathogenesis and progression of human prostate cancer. Cancer Res. 2004;64:5956–5962. doi: 10.1158/0008-5472.CAN-04-0081. [DOI] [PubMed] [Google Scholar]
- 192.Enokida H, Shiina H, Urakami S, Igawa M, Ogishima T, Li LC, Kawahara M, Nakagawa M, Kane CJ, Carroll PR, Dahiya R. Multigene methylation analysis for detection and staging of prostate cancer. Clin Cancer Res. 2005;11:6582–6588. doi: 10.1158/1078-0432.CCR-05-0658. [DOI] [PubMed] [Google Scholar]
- 193.Knight LJ, Burrage J, Bujac SR, Haggerty C, Graham A, Gibson NJ, Ellison G, Growcott JW, Brooks AN, Hughes AM, Xinarianos G, Nikolaidis G, Field JK, Liloglou T. Epigenetic silencing of the endothelin-B receptor gene in non-small cell lung cancer. Int J Oncol. 2009;34:465–471. [PubMed] [Google Scholar]
- 194.Nelson JB, Lee WH, Nguyen SH, Jarrard DF, Brooks JD, Magnuson SR, Opgenorth TJ, Nelson WG, Bova GS. Methylation of the 5′ CpG island of the endothelin B receptor gene is common in human prostate cancer. Cancer Res. 1997;57:35–37. [PubMed] [Google Scholar]
- 195.Rogers CG, Gonzalgo ML, Yan G, Bastian PJ, Chan DY, Nelson WG, Pavlovich CP. High concordance of gene methylation in post-digital rectal examination and post-biopsy urine samples for prostate cancer detection. J Urol. 2006;176:2280–2284. doi: 10.1016/j.juro.2006.07.047. [DOI] [PubMed] [Google Scholar]
- 196.Jeronimo C, Henrique R, Campos PF, Oliveira J, Caballero OL, Lopes C, Sidransky D. Endothelin B receptor gene hypermethylation in prostate adenocarcinoma. J Clin Pathol. 2003;56:52–55. doi: 10.1136/jcp.56.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Adams RH. Vascular patterning by Eph receptor tyrosine kinases and ephrins. Semin Cell Dev Biol. 2002;13:55–60. doi: 10.1006/scdb.2001.0289. [DOI] [PubMed] [Google Scholar]
- 198.Guan M, Xu C, Zhang F, Ye C. Aberrant methylation of EphA7 in human prostate cancer and its relation to clinicopathologic features. Int J Cancer. 2009;124:88–94. doi: 10.1002/ijc.23890. [DOI] [PubMed] [Google Scholar]
- 199.Katoh M. Comparative integromics on Eph family. Int J Oncol. 2006;28:1243–1247. [PubMed] [Google Scholar]
- 200.Oudes AJ, Roach JC, Walashek LS, Eichner LJ, True LD, Vessella RL, Liu AY. Application of Affymetrix array and Massively Parallel Signature Sequencing for identification of genes involved in prostate cancer progression. BMC Cancer. 2005;5:86. doi: 10.1186/1471-2407-5-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wang J, Kataoka H, Suzuki M, Sato N, Nakamura R, Tao H, Maruyama K, Isogaki J, Kanaoka S, Ihara M, Tanaka M, Kanamori M, Nakamura T, Shinmura K, Sugimura H. Downregulation of EphA7 by hypermethylation in colorectal cancer. Oncogene. 2005;24:5637–5647. doi: 10.1038/sj.onc.1208720. [DOI] [PubMed] [Google Scholar]
- 202.Youssef EM, Chen XQ, Higuchi E, Kondo Y, Garcia-Manero G, Lotan R, Issa JP. Hypermethylation and silencing of the putative tumor suppressor Tazarotene-induced gene 1 in human cancers. Cancer Res. 2004;64:2411–2417. doi: 10.1158/0008-5472.can-03-0164. [DOI] [PubMed] [Google Scholar]
- 203.Lotan R. Is TIG1 a new tumor suppressor in prostate cancer? J Natl Cancer Inst. 2002;94:469–470. doi: 10.1093/jnci/94.7.469. [DOI] [PubMed] [Google Scholar]
- 204.Tokumaru Y, Harden SV, Sun DI, Yamashita K, Epstein JI, Sidransky D. Optimal use of a panel of methylation markers with GSTP1 hypermethylation in the diagnosis of prostate adenocarcinoma. Clin Cancer Res. 2004;10:5518–5522. doi: 10.1158/1078-0432.CCR-04-0108. [DOI] [PubMed] [Google Scholar]
- 205.Tokumaru Y, Sun DI, Nomoto S, Yamashita K, Sidransky D. Re: Is TIG1 a new tumor suppressor in prostate cancer? J Natl Cancer Inst. 2003;95:919–920. doi: 10.1093/jnci/95.12.919. [DOI] [PubMed] [Google Scholar]
- 206.Vasiliou V, Pappa A, Estey T. Role of human aldehyde dehydrogenases in endobiotic and xenobiotic metabolism. Drug Metab Rev. 2004;36:279–299. doi: 10.1081/dmr-120034001. [DOI] [PubMed] [Google Scholar]
- 207.Pasquali D, Thaller C, Eichele G. Abnormal level of retinoic acid in prostate cancer tissues. J Clin Endocrinol Metab. 1996;81:2186–2191. doi: 10.1210/jcem.81.6.8964849. [DOI] [PubMed] [Google Scholar]
- 208.Touma SE, Perner S, Rubin MA, Nanus DM, Gudas LJ. Retinoid metabolism and ALDH1A2(RALDH2) expression are altered in the transgenic adenocarcinoma mouse prostate model. Biochem Pharmacol. 2009;78:1127–1138. doi: 10.1016/j.bcp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kim H, Lapointe J, Kaygusuz G, Ong DE, Li C, van de Rijn M, Brooks JD, Pollack JR. The retinoic acid synthesis gene ALDH1a2 is a candidate tumor suppressor in prostate cancer. Cancer Res. 2005;65:8118–8124. doi: 10.1158/0008-5472.CAN-04-4562. [DOI] [PubMed] [Google Scholar]
- 210.Trasino SE, Harrison EH, Wang TT. Androgen regulation of aldehyde dehydrogenase 1A3 (ALDH1A3) in the androgen-responsive human prostate cancer cell line LNCaP. Exp Biol Med (Maywood) 2007:232, 762–771. [PubMed] [Google Scholar]
- 211.Lin J, Haffner MC, Zhang Y, Lee BH, Brennen WN, Britton J, Kachhap SK, Shim JS, Liu JO, Nelson WG, Yegnasubra-manian S, Carducci MA. Disulfiram is a DNA demethylating agent and inhibits prostate cancer cell growth. Prostate. 2010 doi: 10.1002/pros.21247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Costa VL, Henrique R, Ribeiro FR, Carvalho JR, Oliveira J, Lobo F, Teixeira MR, Jeronimo C. Epigenetic regulation of Wnt signaling pathway in uro-logical cancer. Epigenetics. 2010;5:343–351. doi: 10.4161/epi.5.4.11749. [DOI] [PubMed] [Google Scholar]
- 213.Baylin SB, Ohm JE. Epigenetic gene silencing in cancer—a mechanism for early oncogenic pathway addiction? Nat Rev Cancer. 2006;6:107–116. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- 214.Lind GE, Thorstensen L, Lovig T, Meling GI, Hamelin R, Rognum TO, Esteller M, Lothe RA. A CpG island hypermethylation profile of primary colorectal carcinomas and colon cancer cell lines. Mol Cancer. 2004;3:28. doi: 10.1186/1476-4598-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bastian PJ, Palapattu GS, Yegnasubra-manian S, Lin X, Rogers CG, Mangold LA, Trock B, Eisenberger M, Partin AW, Nelson WG. Prognostic value of preoperative serum cell-free circulating DNA in men with prostate cancer undergoing radical prostatectomy. Clin Cancer Res. 2007;13:5361–5367. doi: 10.1158/1078-0432.CCR-06-2781. [DOI] [PubMed] [Google Scholar]
- 216.Bastian PJ, Yegnasubramanian S, Palapattu GS, Rogers CG, Lin X, De Marzo AM, Nelson WG. Molecular biomarker in prostate cancer: the role of CpG island hypermethylation. Eur Urol. 2004;46:698–708. doi: 10.1016/j.eururo.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 217.Richiardi L, Fiano V, Vizzini L, De Marco L, Delsedime L, Akre O, Tos AG, Merletti F. Promoter methylation in APC, RUNX3, and GSTP1 and mortality in prostate cancer patients. J Clin Oncol. 2009;27:3161–3168. doi: 10.1200/JCO.2008.18.2485. [DOI] [PubMed] [Google Scholar]
- 218.Gao X, Porter AT, Honn KV. Involvement of the multiple tumor suppressor genes and 12-lipoxygenase in human prostate cancer. Therapeutic implications. Adv Exp Med Biol. 1997;407:41–53. doi: 10.1007/978-1-4899-1813-0_7. [DOI] [PubMed] [Google Scholar]
- 219.Kito H, Suzuki H, Ichikawa T, Sekita N, Kamiya N, Akakura K, Igarashi T, Nakayama T, Watanabe M, Harigaya K, Ito H. Hypermethylation of the CD44 gene is associated with progression and metastasis of human prostate cancer. Prostate. 2001;49:110–115. doi: 10.1002/pros.1124. [DOI] [PubMed] [Google Scholar]
- 220.Lou W, Krill D, Dhir R, Becich MJ, Dong JT, Frierson HF, Jr, Isaacs WB, Isaacs JT, Gao AC. Methylation of the CD44 metastasis suppressor gene in human prostate cancer. Cancer Res. 1999;59:2329–2331. [PubMed] [Google Scholar]
- 221.Graziano F, Humar B, Guilford P. The role of the E-cadherin gene (CDH1) in diffuse gastric cancer susceptibility: from the laboratory to clinical practice. Ann Oncol. 2003;14:1705–1713. doi: 10.1093/annonc/mdg486. [DOI] [PubMed] [Google Scholar]
- 222.Li LC, Zhao H, Nakajima K, Oh BR, Ribeiro Filho LA, Carroll P, Dahiya R. Methylation of the E-cadherin gene promoter correlates with progression of prostate cancer. J Urol. 2001;166:705–709. [PubMed] [Google Scholar]
- 223.Saha B, Kaur P, Tsao-Wei D, Naritoku WY, Groshen S, Datar RH, Jones LW, Imam SA. Unmethylated E-cadherin gene expression is significantly associated with metastatic human prostate cancer cells in bone. Prostate. 2008;68:1681–1688. doi: 10.1002/pros.20836. [DOI] [PubMed] [Google Scholar]
- 224.Riou P, Saffroy R, Comoy J, Gross-Goupil M, Thiery JP, Emile JF, Azoulay D, Piatier-Tonneau D, Lemoine A, Debuire B. Investigation in liver tissues and cell lines of the transcription of 13 genes mapping to the 16q24 region that are frequently deleted in hepatocellular carcinoma. Clin Cancer Res. 2002;8:3178–3186. [PubMed] [Google Scholar]
- 225.Toyooka KO, Toyooka S, Virmani AK, Sathyanarayana UG, Euhus DM, Gilcrease M, Minna JD, Gazdar AF. Loss of expression and aberrant methylation of the CDH13(H-cadherin) gene in breast and lung carcinomas. Cancer Res. 2001;61:4556–4560. [PubMed] [Google Scholar]
- 226.Alumkal JJ, Zhang Z, Humphreys EB, Bennett C, Mangold LA, Carducci MA, Partin AW, Garrett-Mayer E, DeMarzo AM, Herman JG. Effect of DNA methylation on identification of aggressive prostate cancer. Urology. 2008;72:1234–1239. doi: 10.1016/j.urology.2007.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lee SW. H-cadherin, a novel cadherin with growth inhibitory functions and diminished expression in human breast cancer. Nat Med. 1996;2:776–782. doi: 10.1038/nm0796-776. [DOI] [PubMed] [Google Scholar]
- 228.Mashimo T, Watabe M, Cuthbert AP, Newbold RF, Rinker-Schaeffer CW, Helfer E, Watabe K. Human chromosome 16 suppresses metastasis but not tumorigenesis in rat prostatic tumor cells. Cancer Res. 1998;58:4572–4576. [PubMed] [Google Scholar]
- 229.Salama I, Malone PS, Mihaimeed F, Jones JL. A review of the S100 proteins in cancer. Eur J Surg Oncol. 2008;34:357–364. doi: 10.1016/j.ejso.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 230.Rehman I, Cross SS, Catto JW, Leiblich A, Mukherjee A, Azzouzi AR, Leung HY, Hamdy FC. Promoter hypermethylation of calcium binding proteins S100A6 and S100A2 in human prostate cancer. Prostate. 2005;65:322–330. doi: 10.1002/pros.20302. [DOI] [PubMed] [Google Scholar]
- 231.Gokaslan ZL, Chintala SK, York JE, Boyapati V, Jasti S, Sawaya R, Fuller G, Wildrick DM, Nicolson GL, Rao JS. Expression and role of matrix metalloproteinases MMP-2 and MMP-9 in human spinal column tumors. Clin Exp Metastasis. 1998;16:721–728. doi: 10.1023/a:1006580728338. [DOI] [PubMed] [Google Scholar]
- 232.Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol. 1997;74:111–122. [PubMed] [Google Scholar]
- 233.Imren S, Kohn DB, Shimada H, Blavier L, DeClerck YA. Overexpression of tissue inhibitor of metalloproteinases-2 retroviral-mediated gene transfer in vivo inhibits tumor growth and invasion. Cancer Res. 1996;56:2891–2895. [PubMed] [Google Scholar]
- 234.Mohanam S, Wang SW, Rayford A, Yamamoto M, Sawaya R, Nakajima M, Liotta LA, Nicolson GL, Stetler-Stevenson WG, Rao JS. Expression of tissue inhibitors of metalloproteinases: negative regulators of human glioblastoma invasion in vivo. Clin Exp Metastasis. 1995;13:57–62. doi: 10.1007/BF00144019. [DOI] [PubMed] [Google Scholar]
- 235.Pulukuri SM, Patibandla S, Patel J, Estes N, Rao JS. Epigenetic inactivation of the tissue inhibitor of metalloproteinase-2 (TIMP-2) gene in human prostate tumors. Oncogene. 2007;26:5229–5237. doi: 10.1038/sj.onc.1210329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ross JS, Kaur P, Sheehan CE, Fisher HA, Kaufman RA, Jr, Kallakury BV. Prognostic significance of matrix metalloproteinase 2 and tissue inhibitor of metalloproteinase 2 expression in prostate cancer. Mod Pathol. 2003;16:198–205. doi: 10.1097/01.MP.0000056984.62360.6C. [DOI] [PubMed] [Google Scholar]
- 237.Han X, Zhang H, Jia M, Han G, Jiang W. Expression of TIMP-3 gene by construction of a eukaryotic cell expression vector and its role in reduction of metastasis in a human breast cancer cell line. Cell Mol Immunol. 2004;1:308–310. [PubMed] [Google Scholar]
- 238.Deng X, Bhagat S, Dong Z, Mullins C, Chinni SR, Cher M. Tissue inhibitor of metalloproteinase-3 induces apoptosis in prostate cancer cells and confers increased sensitivity to paclitaxel. Eur J Cancer. 2006;42:3267–3273. doi: 10.1016/j.ejca.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 239.Finan KM, Hodge G, Reynolds AM, Hodge S, Holmes MD, Baker AH, Reynolds PN. In vitro susceptibility to the pro-apoptotic effects of TIMP-3 gene delivery translates to greater in vivo efficacy versus gene delivery for TIMPs-1 or -2. Lung Cancer. 2006;53:273–284. doi: 10.1016/j.lungcan.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 240.Smith E, De Young NJ, Tian ZQ, Caruso M, Ruszkiewicz AR, Liu JF, Jamieson GG, Drew PA. Methylation of TIMP3 in esophageal squamous cell carcinoma. World J Gastroenterol. 2008;14:203–210. doi: 10.3748/wjg.14.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Fizazi K. The role of Src in prostate cancer. Ann Oncol. 2007;18:1765–1773. doi: 10.1093/annonc/mdm086. [DOI] [PubMed] [Google Scholar]
- 242.Posadas EM, Al-Ahmadie H, Robinson VL, Jagadeeswaran R, Otto K, Kasza KE, Tretiakov M, Siddiqui J, Pienta KJ, Stadler WM, Rinker-Schaeffer C, Salgia R. FYN is overexpressed in human prostate cancer. BJU Int. 2009;103:171–177. doi: 10.1111/j.1464-410X.2008.08009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Sorensen KD, Borre M, Orntoft TF, Dyrskjot L, Torring N. Chromo-somal deletion, promoter hypermethylation and downregulation of FYN in prostate cancer. Int J Cancer. 2008;122:509–519. doi: 10.1002/ijc.23136. [DOI] [PubMed] [Google Scholar]
- 244.Usmani BA, Shen R, Janeczko M, Papandreou CN, Lee WH, Nelson WG, Nelson JB, Nanus DM. Methylation of the neutral endopeptidase gene promoter in human prostate cancers. Clin Cancer Res. 2000;6:1664–1670. [PubMed] [Google Scholar]
- 245.Osman I, Dai J, Mikhail M, Navarro D, Taneja SS, Lee P, Christos P, Shen R, Nanus DM. Loss of neutral endopeptidase and activation of protein kinase B (Akt) is associated with prostate cancer progression. Cancer. 2006;107:2628–2636. doi: 10.1002/cncr.22312. [DOI] [PubMed] [Google Scholar]
- 246.Osman I, Yee H, Taneja SS, Levinson B, Zeleniuch-Jacquotte A, Chang C, Nobert C, Nanus DM. Neutral endopeptidase protein expression and prognosis in localized prostate cancer. Clin Cancer Res. 2004;10:4096–4100. doi: 10.1158/1078-0432.CCR-04-0120. [DOI] [PubMed] [Google Scholar]
- 247.Friedberg EC. How nucleotide excision repair protects against cancer. Nat Rev Cancer. 2001;1:22–33. doi: 10.1038/35094000. [DOI] [PubMed] [Google Scholar]
- 248.Mullaart E, Lohman PH, Berends F, Vijg J. DNA damage metabolism and aging. Mutat Res. 1990;237:189–210. doi: 10.1016/0921-8734(90)90001-8. [DOI] [PubMed] [Google Scholar]
- 249.Wood RD, Mitchell M, Sgouros J, Lindahl T. Human DNA repair genes. Science. 2001;291:1284–1289. doi: 10.1126/science.1056154. [DOI] [PubMed] [Google Scholar]
- 250.Wood RD, Mitchell M, Lindahl T. Human DNA repair genes, 2005. Mutat Res. 2005;577:275–283. doi: 10.1016/j.mrfmmm.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 251.Park JY, Huang Y, Sellers TA. Single nucleotide polymorphisms in DNA repair genes and prostate cancer risk. Methods Mol Biol. 2009;471:361–385. doi: 10.1007/978-1-59745-416-2_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kim JI, Suh JT, Choi KU, Kang HJ, Shin DH, Lee IS, Moon TY, Kim WT. Inactivation of O6-methylguanine-DNA methyltransferase in soft tissue sarcomas: association with K-ras mutations. Hum Pathol. 2009;40:934–941. doi: 10.1016/j.humpath.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 253.Mack GS. Epigenetic cancer therapy makes headway. J Natl Cancer Inst. 2006;98:1443–1444. doi: 10.1093/jnci/djj447. [DOI] [PubMed] [Google Scholar]
- 254.Muller CI, Ruter B, Koeffler HP, Lubbert M. DNA hypermethylation of myeloid cells, a novel therapeutic target in MDS and AML. Curr Pharm Biotechnol. 2006;7:315–321. doi: 10.2174/138920106778521523. [DOI] [PubMed] [Google Scholar]
- 255.Oki Y, Aoki E, Issa JP. Decitabine-bedside to bench. Crit Rev Oncol Hematol. 2007;61:140–152. doi: 10.1016/j.critrevonc.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 256.Muller A, Florek M. 5-Azacytidine/Azacitidine. Recent Results Cancer Res. 2010;184:159–170. doi: 10.1007/978-3-642-01222-8_11. [DOI] [PubMed] [Google Scholar]
- 257.Woodson K, Gillespie J, Hanson J, Emmert-Buck M, Phillips JM, Linehan WM, Tangrea JA. Heterogeneous gene methylation patterns among pre-invasive and cancerous lesions of the prostate: a histopathologic study of whole mount prostate specimens. Prostate. 2004;60:25–31. doi: 10.1002/pros.20013. [DOI] [PubMed] [Google Scholar]


