Abstract
An MR-electrophysiology (EP) catheter is presented that provides full diagnostic EP functionality and a high level of radiofrequency safety achieved by custom-designed transmission lines. Highly resistive wires transmit intracardiac electrograms and currents for intracardiac pacing. A transformer cable transmits the localization signal of a tip coil. Specific absorption rate simulations and temperature measurements at 1.5 T demonstrate that a wire resistance > 3 kΩ/m limits dielectric heating to a physiologically irrelevant level. Additional wires do not increase tip specific absorption rate significantly, which is important because some clinical catheters require up to 20 electrodes. It is further demonstrated that radiofrequency-induced and pacing-induced resistive heating of the wires is negligible under clinical conditions. The MR-EP catheters provided uncompromised recording of electrograms and cardiac pacing in combination with a standard EP recorder in MR-guided in vivo EP studies, and the tip coil enabled fast and robust catheter localization. In vivo temperature measurements during such a study did not detect any device-related heating, which confirms the high level of safety of the catheter, whereas unacceptable heating was found with a standard EP catheter. The presented concept for the first time enables catheters with full diagnostic EP functionality and active tracking and at the same time a sufficient level of radiofrequency safety for MRI without specific absorption rate-related limitations.
Keywords: catheter, electrophysiology, MR safety, interventional devices
Interventional cardiac electrophysiology (EP) has grown rapidly in recent years as a means of studying and treating cardiac arrhythmias, which are afflicting millions of people world-wide (1). The catheter ablation procedure developed in this discipline requires the introduction of catheter devices that can measure intracardiac electrograms (IEGMs), perform cardiac pacing and tissue ablation (2). While many EP procedures can be performed efficiently under X-ray fluoroscopy guidance, complex procedures as for atrial fibrillation and ventricular tachycardia still suffer from poor outcome (3). It is discussed that these procedures may bear a large potential of improvement when performed under MR guidance for several reasons. Active MR catheter tracking and visualization in combination with cardiac MR imaging provides 3D navigation of the catheter relative to myocardial tissue (4), whereas conventional X-ray-guided procedures mainly visualize catheters and provide only minimal tissue information. An important example is ventricular tachycardia, where MR-delayed enhancement imaging can visualize scar tissue, which is often the substrate for ventricular tachycardia (5,6), providing additional information to the electroanatomic data, which may be used to guide the ablation. Even more important is the potential of MRI to visualize ablated structures (7–11), which may eventually provide means to titrate the ablation and to guide repeat procedures. Additionally, complex EP procedures with long duration and respective radiation dose will benefit from the avoidance of any ionizing radiation for patients and physicians.
EP procedures require highly specialized catheters with a range of functions to perform electroanatomic mapping and ablation procedures. For this, the devices must support the reception and transmission of electrical signals while measuring the position of the catheter tip. While the principal feasibility of several types of MR-guided EP procedures has been successfully demonstrated previously (4,7,12,13), the problem of radiofrequency (RF) safety of MR-EP catheters has not yet been addressed in depth before.
It is the objective of this article to present an MR-catheter prototype that provides diagnostic EP functionality and is equipped with active tip tracking for fast and precise positioning (14). It is a further objective to demonstrate that such an MR-EP catheter can be constructed to maintain RF safety even for imaging sequences with high specific absorption rate (SAR) as desirable for real-time image guidance and lesion imaging.
Materials and Methods
Simulations and Phantom Experiments for Design of RF-Safe MR-EP Catheters
EP catheters require electrodes for recording of the IEGM (amplitude ≤10 mV, frequency ≤1 kHz). Additionally, intracardiac pacing is performed with these electrodes (currents ≤10 mA, frequency ≤100 kHz). In conventional EP catheters, these signals are transmitted by copper cables, which are impermissible in MR due to potential RF heating (15–18). Highly resistive (HR) wires with a resistance in the order of kΩ have been shown to dampen such resonances effectively (19). A resistance in the order of 100 Ω was reported to result in reduced but clinically still unacceptable RF heating for a global SAR of 2.1 W/kg (13). On the other hand, transmission of IEGM should not be compromised by a too high resistance. To determine an appropriate resistance and further design parameters of the MR-EP catheter, local SAR simulations at the EP electrodes as well as respective RF-heating experiments with initial catheter prototypes were performed. Additionally, resistive heating along the wires due to induced RF currents and pacing currents was investigated. Finally, the effect of increased resistance on the quality of IEGM transmission was investigated.
The SAR simulations were performed with the method of moments tool FEKO (EM Software & Systems, Stellenbosch, South Africa). A model of a 1-m long 6F catheter with one tip and up to 12 ring electrodes all connected with internal wires was used. The simulations were performed with the device in contact with phantom tissue (σ = 0.5 S/m, ε = 81) inside a quadrature body coil model (band pass, 600 mm diameter, 16 rods of 400 mm length). The mean SAR within a 1 mm3 volume at each electrode was calculated for various wire resistances, wire lengths, number of wires, and as a function of the lateral distance of wires inside the catheter tube.
For validation of the simulations, RF-heating experiments were performed with catheter prototypes located in a basin filled with saline (size 2.0 × 0.12 × 0.08 m3, σ = 0.5 S/m, ε = 81), which was positioned parallel to the amplitude of the static (polarizing) field inside the MR system (Philips Achieva I/T 1.5T, Philips Healthcare, The Netherlands). A lateral position as close as possible to the body coil (approx. 10 cm) was chosen to maximize the electric field interaction. Measurements were carried out for single electrode catheters containing wires of various lengths made of copper (0.55 Ω/m resistance), various HR alloys as well as custom-manufactured gold-plated nylon threads with a resistance of up to 20 kΩ/m. Scanning was performed with a high SAR sequence (spoiled gradient echo, console-reported whole body SAR = 3.9 W/kg). The temperature was monitored using a fiber-optic thermometer (Luxtron 790, Santa Clara, CA) whose sensors made contact with the electrodes and were surrounded by a gel block to avoid convective heat transfer.
Resistive heating due to induced RF currents was studied by FEKO simulations of the dissipated power as a function of wire resistance and by temperature measurements in a setup as above but with temperature probes attached to the outside of the catheter shaft. Resistive heating due to pacing currents was studied for a single wire with a resistance of 8.5 kΩ/m suspended in air and imaged by a thermocamera (VarioTherm, Infratec GmbH, Dresden).
For initial evaluation of the quality of the IEGM transmission by HR wires, bipolar electrocardiogram (ECG) signals of an ECG simulator were recorded after transmission via pairs of 1-m long samples of all wires mentioned above.
To minimize RF heating associated with the active tracking components of the MR-EP catheter, the tip tracking coil of the catheter was connected to a transformer-based cable. Such cables have been shown to suppress common mode RF currents associated with RF heating by a low capacity between the primary and secondary loop of the transformers (20). For the MR-EP catheter, the design of the transformer-based cable was improved by studying the optimal distribution of the transformers, which were originally positioned equidistantly. For this purpose, the SAR near the tracking coil was simulated as a function of the positions of the four transformers distributed in the cable.
Construction of MR-EP Catheters
All MR-EP catheters for the preclinical experiments were built using the results of the above design study. The catheters were constructed from nonbraided 7F nylon tubing providing a usable length of 100 cm. To limit the effort of catheter construction, the catheters were equipped with a single pair of Pt/Ir ring electrodes with a spacing of 4 mm with the distal ring placed 1 mm from the catheter tip. Each electrode was connected to a HR wire with a resistance of 7.4 kΩ. This value including some safety margin was motivated by the results of the above EM simulations and phantom measurements. The HR wires were contacted to standard EP leads in the custom-made hub of the catheter and terminated with standard EP plugs.
The catheters were equipped with a solenoid tip tracking coil (10 turns of 150 mm copper wire) located 3 mm from the proximal ring electrode. The coil was tuned and matched with miniature capacitors (size 0.3 × 0.3 × 0.6 mm3, Murata, Japan). This receive circuit was connected to a transformer-based cable consisting of a coaxial cable (50 Ω, 0.53 mm diameter, MMK 5001, Elspec, Germany) and four custom-made miniature transformers (maximal cross-section 0.6 × 0.5 mm2). The positions of the transformers were chosen according to the results of the respective simulations. The transformer-based cable was connected to a standard RF cable in the hub of the catheter, routing the signal to one of the MR receivers. The external cable sections were equipped with resonant RF chokes.
In Vivo Proof of RF Safety of MR-EP Catheter and Comparison to Standard EP Catheter
In addition to the phantom experiments, in vivo temperature measurements in the MR system were performed with the MR-EP catheter and with a standard diagnostic EP catheter (SPIKE LC S, bipolar, 6F, Fiab Medical Devices, Italy). These and the experiments on the evaluation of the EP functionality of the MR-EP catheter were performed with a setup similar to the one described in Ref. 4 with the difference that all components were located inside the MR scan room. For the in vivo experiments in this article, eight domestic pigs weighing 60 kg were used. The animals were presedated with an intramuscular injection of 1 mL/kg azaperone and 1 mL/kg midazolam. After induction of anesthesia with intravenous propofol (5 mg/kg) and endotracheal intubation, anesthesia was maintained with intravenous propofol (12 mg/ [kg h]) and fentanyl (2 mg/[kg h]) and animals were mechanically ventilated. The study was approved by the local ethics committee for animal care and use.
For the in vivo temperature measurements performed in one animal, the MR-EP catheter was equipped with fiber-optic temperature probes (Luxtron 790) at both ring electrodes, at the active tracking coil, and at the distal end of the distal transformer. The fibers of the probes were fixed to the catheter with ultrathin shrink tube (6.4 mm wall thickness), and the shrink tube material was removed locally to expose the probes to blood. The MR-EP catheter was inserted via a 9F sheath, and temperature increase was measured for an inserted length of 28 and 58 cm. At each position, the baseline temperature was recorded without RF transmission, and then the temperature increase was measured during the application of a high-SAR balanced gradient echo sequence (pulse repetition time 2.4 msec, α = 65°, SAR =4 W/kg). Finally, the catheter was pulled back at 0.5 cm/sec during continued RF transmission to detect potential RF resonances for different inserted lengths. After the experiment, it was confirmed that none of the temperature probes had been dislocated.
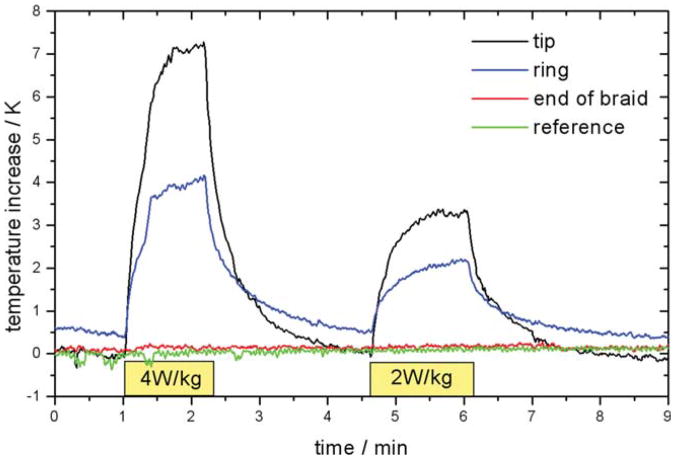
Additional temperature measurements were performed with the standard EP catheter equipped with probes at the tip and the ring electrode at the end of the braided section (8 cm proximal from the tip). A fourth probe was inserted separately through the insertion sheath and used as a reference. After recording of the baseline temperature with the catheter just inserted into the sheath, the catheter was advanced during RF transmission at 4 W/kg. As soon as a temperature increase of more than 5 K was observed, RF transmission was stopped to allow cool down, and then restarted to allow for a local SAR evaluation. After repeated cool down, RF transmission with 2 W/kg was performed.
Evaluation of EP Functionality of MR-EP Catheter
The EP recording and pacing functions of the MR-EP catheter were evaluated in two steps. First, the function of the MR-EP catheter was compared with a conventional quadripolar diagnostic EP catheter (Supreme Quad, JSN, 5F; St Jude Medical, St.Paul, MN) in two animals in a standard EP lab equipped with an X-ray unit. Second, the MR-EP catheters were used for MR-guided mapping and pacing studies in the MR scanner in these two and six additional animals. In all experiments, a conventional EP recorder (EP Tracer, CardioTek, Netherlands) was used for recording and pacing. Real-time imaging with a clinical five-element cardiac synergy coil (balanced gradient echo, pulse repetition time = 2.5 msec, α = 45°, 2.4 × 2.4 mm2 resolution, field of view = 310 mm) as well as active tip tracking were used for MR-guided catheterization of the right atrium (RA) and right ventricle (RV). Bipolar IEGMs were acquired at corresponding locations (including RA lateral wall, RV apex, tricuspid valve ring, and bundle of His).
For evaluation of the pacing function, two MR-EP catheters were guided into the RA and into the RV apex. Atrial as well as ventricular pacing at a rate of 400 msec−1 was performed while recording with the other catheter, respectively.
Results
RF-Safe Design of the MR-EP Catheter
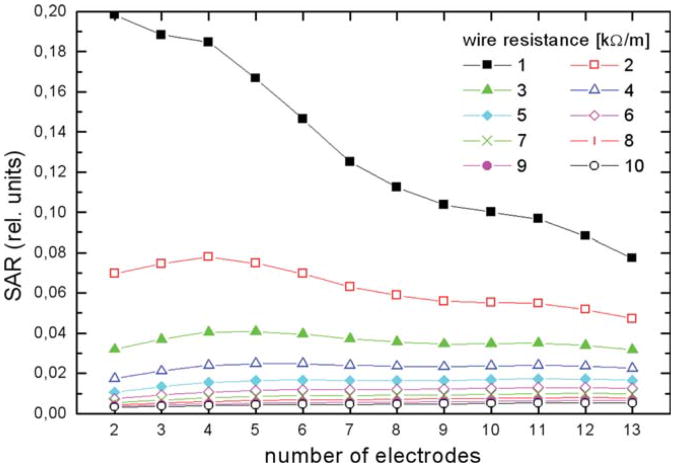
The simulation of tip SAR for catheters with one to 13 wired electrodes showed that tip SAR is reduced very effectively for a wire resistance of 3 kΩ/m and higher (Fig. 1). In this regime the tip SAR does not vary noticeably with the number of wires. Relative SAR is given because absolute values depend sensitively on the device position in the body coil, its design, exact location and shape of probed volume and other parameters, while the main purpose here is to derive parameters for the design of a MR-EP catheter. Tip SAR of a four-electrode catheter model did not change by more than 20% with the variation of the lateral distance of the wires arranged on a square pattern centered in the tube. The diagonal size of the square was varied between 10% and 90% of the tube diameter. Tip SAR decreased with increasing wire distance.
Fig. 1.
Simulation of SAR at tip electrode as a function of the number of wired electrodes and the wire resistance. For a dual electrode catheter equipped with wires of at least 3 kΩ/m, the tip SAR is reduced to less than 3% in comparison with copper leads (SAR normalized to one). Above this resistance, the tip SAR does not depend noticeably on the number of electrodes, which indicates that the HR wire concept is also effective for multielectrode catheters. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
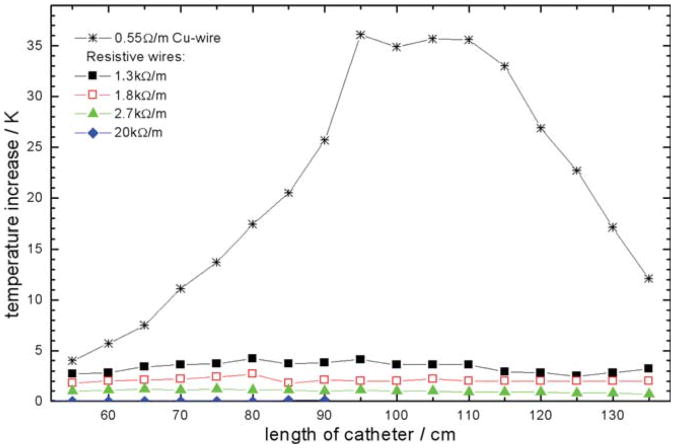
The RF-heating experiments with single electrode catheters equipped with various wires confirmed that HR wiring reduces the temperature increase at the tip electrode effectively. The temperature increase at the tip electrode after reaching thermal steady state versus catheter length is shown in Fig. 2. The catheter with copper wiring showed a pronounced temperature increase for a length of 110 cm due to resonance so that scanning was stopped already after 1.7 sec before reaching thermal steady state for all measurements with that wiring. HR wires with 2.7 kW/m or higher did not result in tip heating of more than 1.5 K in thermal steady state.
Fig. 2.
Temperature increase measured at the tip of a single wire EP catheter as a function of catheter length and wire resistance. For the Cu wire, RF heating of more than 35 K was observed already after 1.7 sec of RF transmission, so that transmission was stopped. For all HR wires, tip heating was evaluated after 46 sec in thermal steady state. HR wires with 2.7 kΩ/m or higher did not exhibit significant tip heating.
The measurements to assess resistive heating due to RF-induced currents performed with a catheter containing 1.8 kW/m wires of resonant length (defined by maximum tip heating) resulted in a maximum temperature increase of 1.7 K. Simulations of resistive heating power per unit length showed that in the resistance range required to limit RF tip heating, the resistive thermal power decreases about linearly with increasing resistance of the HR wires. The measurements to assess resistive heating by pacing currents performed with a single wire carrying a constant 10 mA current showed a maximum temperature increase of 1.8 K.
The initial evaluation of ECG transmission via HR wires with resistance up to 20 kΩ did not result in any noticeable decrease of signal quality.
The simulations on the optimal locations of the transformers within the transformer-based cable showed that minimum SAR near the tracking coil is achieved when two transformers are placed as close to the tracking coil as possible and the third and fourth one are arranged with a spacing of 8 and 12 cm, respectively. This distribution resulted in a 9.4% reduction of SAR at the tip coil in comparison with an equidistant arrangement.
In Vivo Proof of RF Safety of MR-EP Catheter and Comparison to Standard EP Catheter
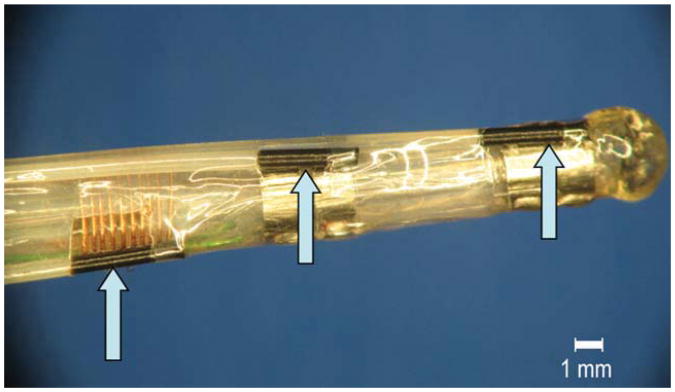
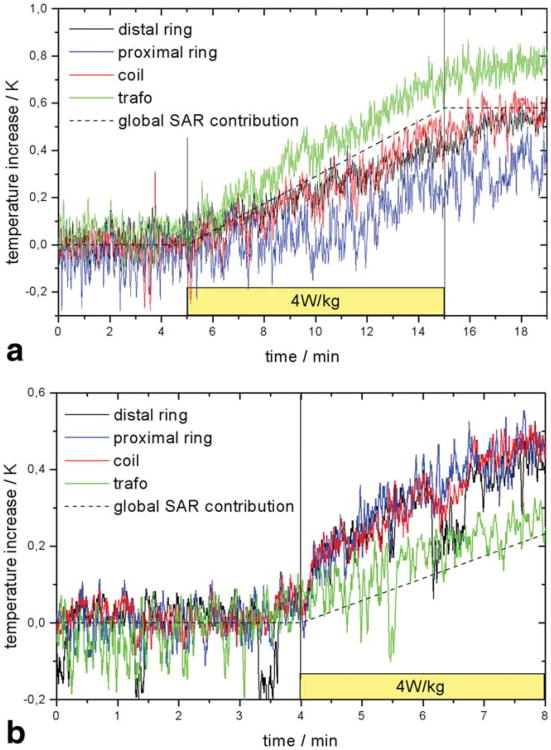
For the MR-EP catheter (Fig. 3) inserted by 28 cm, the temperature increased by 0.7 K at the distal transformer and less at the EP electrodes and the tracking coil during 10 min of RF transmission at 4 W/kg. The temperatures at each location remained at their elevated levels after switching off RF transmission for several minutes (Fig. 4a). For an inserted length of 58.5 cm, the maximum temperature increase of 0.35 K in 4 min occurred equally at coil and electrodes (Fig. 4b). No temperature increase was detected during complete and very slow pull back of the catheter.
Fig. 3.
MR-EP catheter equipped with fiber-optic sensors for in vivo temperature measurement fixed to the tip coil and proximal and distal ring electrodes (arrows). A fourth sensor was fixed to the distal transformer (not shown).
Fig. 4.
In vivo temperature increase for MR-EP catheter inserted by 28 cm (a) and 58 cm (b). The global temperature increase expected for a sequence with a SAR of 4 W/kg is plotted for comparison.
For the standard EP catheter, an increase of 7.5 K at the tip and 3.5 K at the ring were observed in 80 sec of RF transmission with 4 W/kg (Fig. 5). Transmission at 2 W/kg resulted in a reduction of the temperature increases by about 50%. Negligible heating occurred at the end of the braid section and at a reference probe floating freely in the vena cava.
Fig. 5.
In vivo temperature increase measured with standard EP catheter inserted by 20 cm and reference temperature probe inserted separately.
EP Functionality of MR-EP Catheter
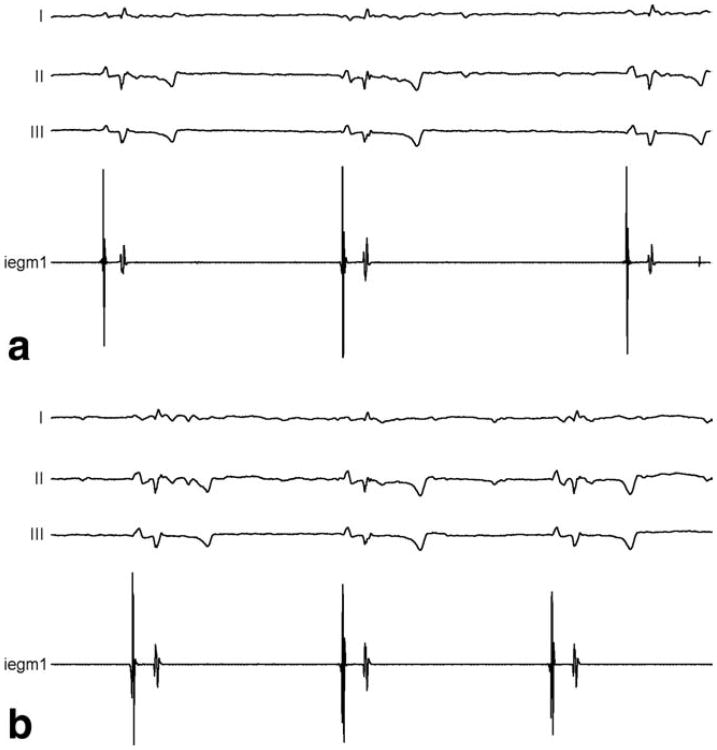
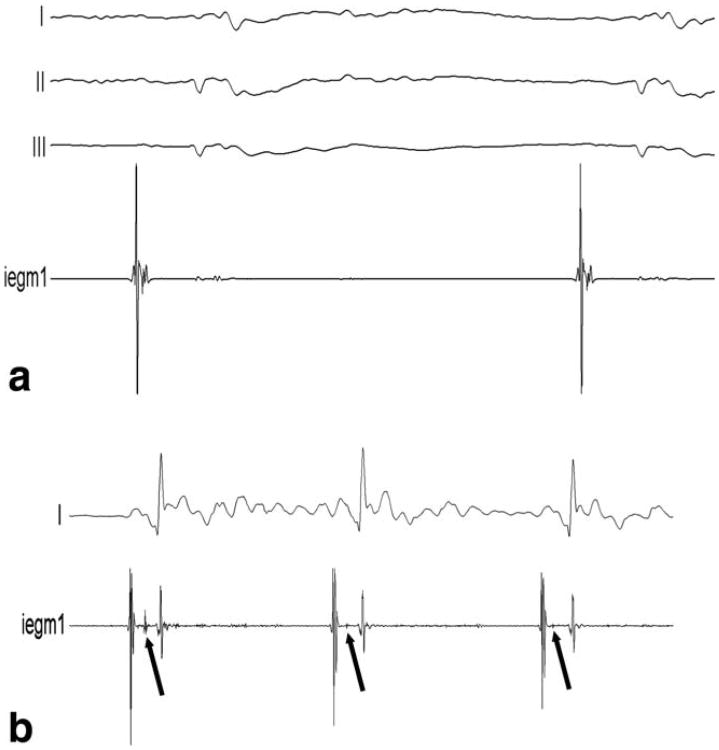
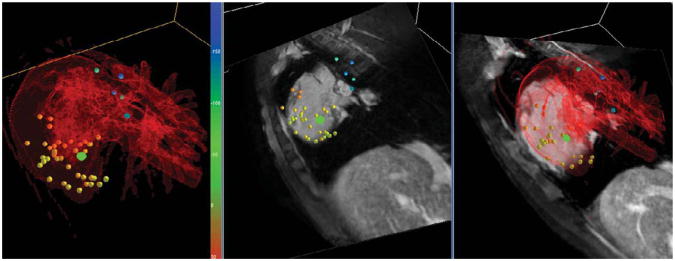
IEGMs recorded in two animals with both the standard EP catheter and the MR-EP catheter equipped with a pair of HR wires of 7.4 kΩ were of high and comparable quality. Two exemplary atrial signals acquired in the same animal are displayed in Fig. 6. IEGMs recorded in several mapping studies of the right heart performed in the MR bore did not show any difference in quality. Respective IEGMs from the RA and from the His region are displayed in Fig. 7. Active tip tracking and real-time imaging with active catheter visualization were used alternatively for catheter guidance, and the rendering of catheter positions in 3D relative to preacquired volume datasets provided by a MR-EP workstation improved catheter navigation considerably in comparison with early experiments based on the tip coil position indicated on a 2D slice. Figure 8 provides a screenshot from a mapping session.
Fig. 6.
Comparison of right atrial IEGMs acquired in the standard EP suite with the conventional catheter (a) and the MR-EP catheter equipped with HR wires (b), both in the same animal.
Fig. 7.
a: Right atrial IEGM acquired with the MR-EP catheter in the MR. b: High-fidelity IEGM acquired after guiding the MR-EP catheter to the His position. The lower trace shows typical groups of atrial peaks followed by His deflections (arrows) and ventricular peaks.
Fig. 8.
Display of real-time catheter tip position (green dot) relative to previously mapped points (yellow to blue) and cardiac anatomy in a mapping study. The surface model (red) has been created from a Gd-enhanced MRA dataset, and the imaging slices have been reformatted from a balanced gradient echo dataset, both acquired at the beginning of the mapping study.
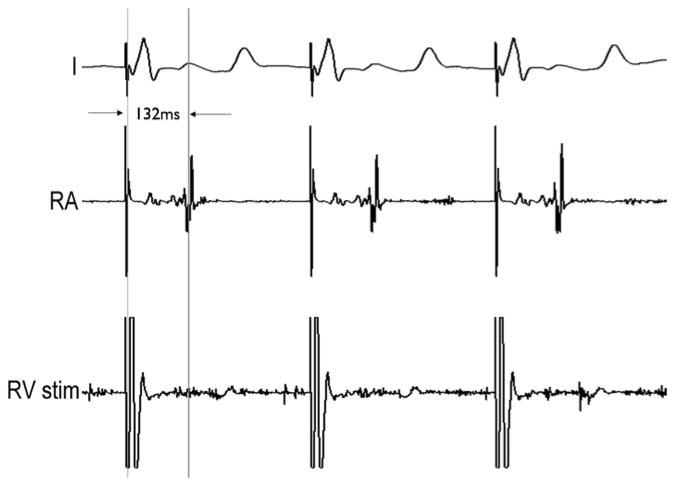
For the pacing study, both, the RA and the RV were catheterized with two actively tracked MR-EP catheters. Capture of the heart could be achieved during both atrial as well as ventricular apical pacing with currents ranging from 1.3 to 1.9 mA. Capture during atrial pacing was confirmed with the ventricular catheter and vice versa (Fig. 9).
Fig. 9.
Pacing study performed with two MR-EP catheters. A first catheter stimulates the RV (lower trace) triggering an R peak in the external ECG (upper trace). The second catheter in the RA records the pacing pulse and the delayed atrial contraction (center trace).
Discussion
RF-Safe Design of the EP Components of the MR-EP Catheter
The phantom temperature measurements with the single electrode catheter indicated that the HR wire concept limits dielectric tip heating to a physiologically irrelevant level even under the tested worst case conditions, if the wire has a resistance of at least 2.7 kΩ/m. The simulations indicate that for such a resistance and higher the addition of further wires and electrodes does not increase tip SAR significantly, which is clinically important because many EP catheters are equipped with four to 20 electrodes. Simulated tip SAR decreases with the number of wires and electrodes for the case of 1 kΩ/m wires, which may be due to a distribution of SAR to the ring electrodes. According to further simulations, the exact arrangement of four wires of more than 5 kΩ/m in the catheter is uncritical, but the safety margin can be increased by a high spacing between the wires. The above phantom temperature measurements were done under worst case conditions (10 cm from bore wall with a length that results in a resonance in case of a copper wire and at maximum clinically allowable global SAR). Therefore, dielectric heating with catheters equipped with wires of sufficient resistance is expected to be physiologically irrelevant under clinical use conditions. This indication was confirmed by the in vivo temperature measurements for the dual electrode catheter equipped with two 7.4 kΩ wires.
Resistive heating of the wires themselves due to induced RF currents was limited to below 2 K in the worst case setup for wires of 1.8 kΩ/m. Note, that these measurements include the effect of dielectric heating of water, because the temperature probe was placed in a gel block at different positions along the outside of the catheter rather than directly at the wire inside the catheter for experimental simplicity and clinical relevance. Due to the almost linear dependence of resistive heating on the wire resistance as shown in simulation, resistive heating is expected to be much lower in catheters with wires of more than 3 kΩ/m used under clinical conditions.
Resistive heating due to pacing currents was evaluated for a 10 mA constant current. The respective ohmic power in the wire exceeds clinically used average pacing powers by about three orders of magnitude because clinical pacing protocols typically apply pulses of 3 mA in a duty cycle of 1:10. Consequently, resistive heating will be negligible in a clinical setting.
In Vivo RF Safety of MR-EP Catheter Prototype and Standard EP Catheter
While simulations and phantom experiments had already indicated the effects of the HR wires and the transformer-based cable on RF safety separately, the temperature measurements with the MR-EP catheter confirm these results for the combination of HR wires and transformer-based cable in one device under in vivo conditions.
The application of a sequence with a whole body SAR of 4 W/kg for 10 min is expected to cause a global increase of body temperature by 0.58 K if cooling is neglected. The temperature increase observed for the MR-EP catheter at both catheter positions almost corresponds to this expected global increase. The fact that the temperatures at all four probed locations remain at their individually elevated levels after termination of RF transmission supports the conclusion, that a global increase of the body temperature is the dominant effect. Residual local device-related heating can be estimated to be less than 0.2 K from the data. The absence of any temperature increase during slow pull back of the catheter indicates that RF resonances did not occur for any inserted length. This confirms findings from the simulations and phantom experiments as in Fig. 2, in which the HR wires dampen out any resonance. With respect to the tracking components, it has been shown earlier that the transformer-based cable shifts the lowest resonance frequency to far above the Larmor frequency even for a fully immersed catheter (21), which is confirmed here by the absence of temperature increase at the tracking coil during slow pull back.
In contrast to the MR-EP catheter, considerable RF heating was observed for the standard EP catheter. The high temperature increase and the exponential behavior with time constants in the order of less than 1 min clearly indicate a local and device-related effect. RF heating at the tip electrode is probably underestimated because the temperature probe could only be attached laterally to and not in front of the tip electrode in the in vivo situation. This limitation does not apply for the ring electrode of the catheter and for the ring electrodes of the MR-EP catheter. In a previous study on RF heating with a standard EP catheter, a temperature increase of more than 40 K at the tip for a SAR of 2.1 W/kg in a physiological configuration was reported (13). These higher temperatures were measured in a gel phantom without convection and blood flow and they allowed optimal placement of the probe in front of the tip.
A major part of this study was dedicated to simulations and phantom experiments on the design and realization of actively tracked MR-EP catheters that provide a high level of RF safety using a combination of the HR wire concept and the transformer-based cable. Passively visualized MR-EP catheters wired with carbon fibers, which have an elevated resistance, have been realized previously in a comprehensive study on passive image guidance of MR-EP procedures, but in this study much lower resistances were realized (13). As a consequence, residual RF heating for these MR-EP catheters of up to 13 K for a global SAR of 2.1 W/kg occurred in phantom, so that a scan SAR reduction was required for the in vivo study, resulting in compromised image quality. Similarly, in a diagnostic study with a passively visualized MR-EP catheter using copper leads for EP signal transmission, RF heating had to be limited by decreasing the SAR of the MRI sequences used for image guidance (12). The results presented here indicate that the construction of an actively tracked diagnostic MR-EP catheter with sufficient RF safety is possible when using sufficiently high resistances, so that optimal image quality uncompromised by SAR limitations can be achieved. Interestingly, we did not observe a relevant influence of the configuration of connection cables on tip heating during phantom and in vivo experiments. This can be explained by the fact, that all wires located in the transmission zone of the body coil, i.e., the wires inside the catheter, represent a high common mode impedance.
EP Functionality
The uncompromised quality of the IEGMs recorded via HR wires can be explained by the fact that the resistance of the wires is still much lower than the input impedance of the EP recorder. Also, the thermal noise of a 20 kΩ resistance at 1 kHz bandwidth is in the order μV, which is much smaller than the amplitude of IEGM signals. No MRI was performed during recording of IEGMs in this study. However, MR-induced artifacts on IEGMs can be suppressed efficiently by filtering (4,12). The pacing study was successfully performed via HR wires with a standard clinical recorder/stimulator. Because the maximum allowable pacing voltage of such stimulators is limited, pacing currents as required in patients may only be realized with a respective modification, which should not represent a technical problem.
Active Tracking Functionality
Active tip tracking enabled precise and fast EP mapping, i.e., steering of the catheter to the target locations and combined recording of IEGM and respective 3D position. Fifty mapping points were recorded in 20 min. In comparison, catheter manipulation based on real-time imaging of a passively visualized device was reported to require targeting times in the order of 2–5 min per mapping, point (13). During the series of animal experiments, passive tracking was also tested repeatedly. Based on this experience, active tracking is considered to be an indispensable prerequisite to create time and voltage maps as state-of-the-art for conventional clinical EP procedures. Equally important is the functionality to provide an intuitive overview of already mapped locations in relation to catheter and anatomy as electrophysiologists are used to work with (Fig. 8). Such an overview supports the identification of the next point to be targeted. The value of the 3D overview for guidance became particularly apparent when in one mapping session it was immediately recognizable that the catheter had entered the LA despite venous access. This was confirmed by IEGMs and caused by an unintentional crossover into the LA via a patent foramen ovale.
Catheter Mechanics and Maneuverability
Both the HR wires as well as the transformer-based cable allow easy integration into catheters for several reasons. First, these components are highly flexible so that they do not add noticeably to bending stiffness of EP catheters. Second, they have a low profile and in our case occupied only 14% of the cross-sectional area of the catheter lumen (diameter 1.73 mm), which provides space for the integration of more than two HR wires for multielectrode versions. Finally, the components are durable, which was demonstrated by reliable tracking and sensing functionality including repeated use of catheters in several animals.
The presented MR-EP catheter prototype is based on relatively stiff 7F nylon tubing because nonconductive braiding was not available. The nylon tubing provided enough kink resistance and torque transfer to approach several different regions in the RA and the RV, but the absence of a braid, a flexible distal section, and active tip deflection active tip deflection limited maneuverability, limited maneuverability, making it difficult to approach certain regions during the mapping sessions. However, braids and active tip deflection mechanics made from nonconductive components have been demonstrated previously (12,22).
Clinical Relevance, Study Limitations, and Future Work
Clinical MR-guided vascular interventions in general and EP interventions in particular have been hampered by the nonavailability of devices of sufficient safety. To our knowledge, the presented concept for the first time enables catheters that comprise full diagnostic EP functionality and active tracking as required for precise MR guidance and at the same time a sufficient level of RF safety for scanning without SAR-related limitations. Considering the demands for imaging sequences during MR-guided interventions, namely real-time imaging with high contrast and spatiotemporal resolution, any constraint on imaging parameters is disadvantageous. This work concentrated on realization of diagnostic EP and tracking functions in view of RF safety. The clinically evenly important mechanical performance is clearly the most important limitation in our prototype. In addition, none of the presented concepts allows construction of an ablation device with sufficient RF safety, which will be subject of future work.
Conclusions
Diagnostic MR-EP catheters with full EP functionality and active tip tracking can be constructed that achieve a high level of safety with respect to RF heating. The use of HR wires of adequate resistance allows for uncompromised IEGM recording and cardiac pacing. The use of a transformer-based cable connected to a tip coil enables fast and precise position detection. Both components are highly flexible and perform reliably. The integration of such safety measures into one device permits the use of high SAR imaging sequences, which allows for high quality real-time image guidance and lesion imaging.
Acknowledgments
The authors would like to thank Dr. H. Halperin from Johns Hopkins University for helpful discussions.
Grant sponsor: British Technology Strategy Board's Collaborative Research and Development program; Grant number: TP/3/IMG/6/I/17352.
Footnotes
Parts of this work have been presented on the ISMRM 2007 in Berlin and the interventional MRI Symposium 2008 in Baltimore.
References
- 1.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 2.Scheinman MM, Morady F, Hess DS, Gonzalez R. Catheter-induced ablation of the atrioventricular junction to control refractory supraventricular arrhythmias. JAMA. 1982;248:851–855. [PubMed] [Google Scholar]
- 3.Marine JE. Catheter ablation therapy for supraventricular arrhythmias. JAMA. 2007;298:2768–2778. doi: 10.1001/jama.298.23.2768. [DOI] [PubMed] [Google Scholar]
- 4.Dukkipati SR, Mallozzi R, Schmidt EJ, Holmvang G, d'Avila A, Guhde R, Darrow RD, Slavin G, Fung M, Malchano Z, Kampa G, Dando JD, McPherson C, Foo TK, Ruskin JN, Dumoulin CL, Reddy VY. Electroanatomic mapping of the left ventricle in a porcine model of chronic myocardial infarction with magnetic resonance-based catheter tracking. Circulation. 2008;118:853–862. doi: 10.1161/CIRCULATIONAHA.107.738229. [DOI] [PubMed] [Google Scholar]
- 5.Nazarian S, Bluemke DA, Lardo AC, Zviman MM, Watkins SP, Dickfeld TL, Meininger GR, Roguin A, Calkins H, Tomaselli GF, Weiss RG, Berger RD, Lima JA, Halperin HR. Magnetic resonance assessment of the substrate for inducible ventricular tachycardia in non-ischemic cardiomyopathy. Circulation. 2005;112:2821–2825. doi: 10.1161/CIRCULATIONAHA.105.549659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bello D, Fieno DS, Kim RJ, Pereles FS, Passman R, Song G, Kadish AH, Goldberger JJ. Infarct morphology identifies patients with substrate for sustained ventricular tachycardia. J Am Coll Cardiol. 2005;45:1104–1108. doi: 10.1016/j.jacc.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 7.Lardo AC, McVeigh ER, Jumrussirikul P, Berger RD, Calkins H, Lima J, Halperin HR. Visualization and temporal/spatial characterization of cardiac radiofrequency ablation lesions using magnetic resonance imaging. Circulation. 2000;102:698–705. doi: 10.1161/01.cir.102.6.698. [DOI] [PubMed] [Google Scholar]
- 8.Dickfeld T, Kato R, Zviman M, Lai S, Meininger G, Lardo AC, Roguin A, Blumke D, Berger R, Calkins H, Halperin H. Characterization of radiofrequency ablation lesions with gadolinium-enhanced cardiovascular magnetic resonance imaging. J Am Coll Cardiol. 2006;47:370–378. doi: 10.1016/j.jacc.2005.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters DC, Wylie JV, Hauser TH, Kissinger KV, Botnar RM, Essebag V, Josephson ME, Manning WJ. Detection of pulmonary vein and left atrial scar after catheter ablation with three-dimensional navigatorgated delayed enhancement MR imaging: initial experience. Radiology. 2007;243:690–695. doi: 10.1148/radiol.2433060417. [DOI] [PubMed] [Google Scholar]
- 10.McGann CJ, Kholmovski EG, Oakes RS, Blauer JJ, Daccarett M, Segerson N, Airey KJ, Akoum N, Fish E, Badger TJ, DiBella EV, Parker D, MacLeod RS, Marrouche NF. New magnetic resonance imaging-based method for defining the extent of left atrial wall injury after the ablation of atrial fibrillation. J Am Coll Cardiol. 2008;52:1263–1271. doi: 10.1016/j.jacc.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 11.Reddy V, Schmidt EJ, Holmvang G, Fung M. Arrhythmia recurrence after atrial fibrillation ablation: can magnetic resonance imaging identify gaps in atrial ablation lines? J Cardiovasc Electrophysiol. 2008;19:434–437. doi: 10.1111/j.1540-8167.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- 12.Nazarian S, Kolandaivelu A, Zviman MM, Meininger GR, Kato R, Susil RC, Roguin A, Dickfeld TL, Ashikaga H, Calkins H, Berger RD, Bluemke DA, Lardo AC, Halperin HR. Feasibility of real-time magnetic resonance imaging for catheter guidance in electrophysiology studies. Circulation. 2008;118:223–229. doi: 10.1161/CIRCULATIONAHA.107.742452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nordbeck P, Bauer WR, Fidler F, Warmuth M, Hiller KH, Nahrendorf M, Maxfield M, Wurtz S, Geistert W, Broscheit J, Jakob PM, Ritter O. Feasibility of real-time MRI with a novel carbon catheter for interventional electrophysiology. Circ Arrhythm Electrophysiol. 2009;2:258–267. doi: 10.1161/CIRCEP.108.778357. [DOI] [PubMed] [Google Scholar]
- 14.Dumoulin CL, Souza SP, Darrow RD. Real-time position monitoring of invasive devices using magnetic resonance. Magn Reson Med. 1993;29:411–415. doi: 10.1002/mrm.1910290322. [DOI] [PubMed] [Google Scholar]
- 15.Maier SE, Wildermuth S, Darrow RD, Watkins RD, Debatin JF, Dumoulin CL. Safety of MR tracking catheters. Proceedings of the 12th Annual Joint Meeting of SMR/ESMRMB; Nice, France. 1995; p. 497. [Google Scholar]
- 16.Ladd ME, Quick HH, Debatin JF, von Schulthess GK, McKinnon GC. Resonant heating of intravascular RF coils. Proceedings of the 6th Annual Meeting of ISMRM; Sydney, Australia. 1998; p. 473. [Google Scholar]
- 17.Konings MK, Bartels LW, Smits HFM, Bakker CJG. Heating around intravascular guidewires by resonating RF waves. J Magn Reson Imaging. 2000;12:79–85. doi: 10.1002/1522-2586(200007)12:1<79::aid-jmri9>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 18.Nitz WR, Oppelt A, Renz W, Manke C, Lenhart M, Link J. On the heating of linear conductive structures as guide wires and catheters in interventional MRI. J Magn Reson Imaging. 2001;13:105–114. doi: 10.1002/1522-2586(200101)13:1<105::aid-jmri1016>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 19.Wirtz D, Lips O, David B, Krueger S, Weiss S. Diagnostic MR-electro-physiology catheter with highly resistive wires for reduction of RF-heating. Proceedings of the 15th Scientific Meeting of the ISMRM; Berlin, Germany. 2007; p. 738. [Google Scholar]
- 20.Weiss S, Vernickel P, Schaeffter T, Schulz V, Gleich B. Transmission line for improved RF safety of interventional devices. Magn Reson Med. 2005;54:182–189. doi: 10.1002/mrm.20543. [DOI] [PubMed] [Google Scholar]
- 21.Vernickel P, Schulz V, Weiss S, Gleich B. A safe transmission line for MRI. IEEE Trans BME. 2005;52:1094–1102. doi: 10.1109/TBME.2005.846713. [DOI] [PubMed] [Google Scholar]
- 22.Saikus CE, Bell JA, Ratnayaka K, Wu V, Sonmez M, Lederman RJ, Kocaturk O. Deflectable catheter for interventional cardiovascular MRI. Proceedings of the 18th Scientific Meeting of the ISMRM; Stockholm, Sweden. 2010; p. 4155. [Google Scholar]