Summary
There is mounting evidence that serous tubal intraepithelial carcinoma (STIC) may be the immediate precursor of ovarian high-grade serous carcinoma (HGSC) but the criteria for its diagnosis are not well established as highlighted in a recent study showing that interobserver reproducibility, even among expert gynecologic pathologists, was moderate at best. Given the clinical significance of a diagnosis of STIC in a patient who has no other evidence of ovarian carcinoma, this is a serious issue that we felt needed to be addressed. Although it is not clear, at this time, whether such a patient should or should not be treated, the importance of an accurate and reproducible diagnosis of precursors of ovarian carcinoma cannot be underestimated. We hypothesized that an elevated Ki-67 labeling index may aid the diagnosis of STIC. Accordingly, we compared the Ki-67 index of STIC and HGSC to normal fallopian tube epithelium (FTE) in the same patients and to a control group of patients without carcinoma, matched for age. A total of 41 STICs were analyzed, of which 35 were associated with a concurrent HGSC. In FTE, immunoreactivity for Ki-67 was restricted to a few scattered cells (mean 2.0%). No statistically significant difference was found between patients with and without HGSC (P>0.05). However, both STICs and HGSC had significantly higher Ki-67 indices than normal FTE (P<0.0001). STICs uniformly had an elevated Ki-67 labeling index that ranged from 11.7% to 71.1% (average 35.6%). There was no correlation of the Ki-67 labeling index in the STICs and the associated HGSC, as the labeling index was lower in STIC in 18/35 (51.4%) whereas it was higher in 17/35 (48.6%) (P=0.86). In conclusion, the findings in this study indicate that compared with FTE, STICs have a significantly higher Ki-67 index similar to HGSC. Accordingly, the Ki-67 index can aid the diagnosis of intraepithelial tubal proliferations suspicious for STIC. Therefore, we propose that a Ki-67 index of 10% is a useful diagnostic tool to distinguish STICs from normal FTE.
Keywords: Ovarian cancer, Serous tubal intraepithelial carcinoma, Ki-67, Serous, STIC
There is mounting evidence that serous tubal intraepithelial carcinoma (STIC) is the immediate precursor of ovarian high-grade serous carcinoma (HGSC) (1–4) but the criteria for its diagnosis are not well established. A recent study, in fact, showed that interobserver reproducibility, even among expert gynecologic pathologists, was moderate at best (5). As more women with a family history of ovarian carcinoma undergo risk-reducing salpingo oophorectomy and as the SEE-FIM protocol, which allows more thorough evaluation of the fallopian tubes (6), becomes more widely used, this problem will become increasingly important. Pathologists will encounter not only STICs but other tubal abnormalities that may either be precursors of STICs or benign mimickers. In view of the difficulties in establishing a diagnosis on the basis of morphology alone, we have undertaken a number of studies to determine the most sensitive and specific methods of diagnosing STICs. We first investigated alterations in p53, because somatic mutation of TP53 is the most common molecular genetic change of HGSC. TP53 mutational status was correlated with p53 immunoreactivity and clinicopathologic characteristics (7). We found that STICs harbor TP53 mutations in the majority of the cases (91.3%) and these were the identical mutations found in the associated HGSC. Positive immunostaining for p53, however, depended on whether the mutations were missense mutations that resulted in strong staining or nonsense mutations that resulted in a truncated protein not recognized by the p53 antibody and therefore there was complete absence of staining. For these latter cases, in particular, we reasoned that the Ki-67 labeling index might be a useful adjunct to morphology and p53 immunostaining, because Mib1 staining would provide a sensitive and potentially quantifiable method of assessing proliferation in these lesions. Some previous studies have reported an increase in proliferation in STIC (range from 10% to 95%) compared with epithelium of the normal fallopian tube, but the range of the index in normal fallopian tube epithelium (FTE) has not been systematically evaluated so far (8,9). We therefore carried out the present study to determine the baseline Ki-67 proliferation index using Ki-67 staining in normal FTE compared with STICs.
Materials and Methods
Cases Selection
A total of 41 STICs and 35 associated concurrent HGSCs were analyzed for their proliferative activity on the basis of immunohistochemistry using the Mib-1 antibody to detect Ki-67 antigen. All STICs were discrete from HGSC. In addition, FTE from 42 patients who underwent total hysterectomy and salpingo-oophorectomy for benign diseases were included as controls. The age of the patients with STIC ranged from 42 to 88 yr, with a mean of 59 and a median of 57 yr. The age of the patients with normal tubes ranged from 24 to 92 yr, with a mean of 53 and a median of 56 yr. All the cases were collected from the Johns Hopkins Hospital (Baltimore, MD), Legacy Health System (Portland, OR), and Memorial Sloan Kettering Cancer Center (New York, NY). Histologic diagnosis of STICs was made according to previously reported morphologic criteria and joint review by all 4 authors (10,11). In summary, the diagnosis of STIC made on the basis of cytologic features (nuclear enlargement, nuclear hyperchromasia, irregularly distributed chromatin, prominent nucleoli, and presence of mitoses or apoptotic bodies), architectural features (loss of polarity, epithelial tufted, and pluristratification); moreover, all STICs were adjoining to normal-appearing tubal epithelium and not continuous to HGSC in any slide analyzed. Tissue collection conformed to the guidelines of the Institutional Research Board of the Johns Hopkins Hospital, Legacy Health System, and the Memorial Sloan Kettering Cancer Center.
Immunohistochemistry
Sections were deparaffinized in xylene and rehydrated in graded alcohols. Antigen retrieval was performed by steaming the sections in citrate buffer (pH 6.0) for 20 min and then immersing in 3% hydrogen peroxide in distilled water for 20 min, followed by incubation in 10% fetal bovine serum diluted in distilled water at room temperature. A DAKO autostainer was used for immunostaining with the Mib-1 antibody (Ventana) (directly from the kit without dilution) at room temperature for 2 hr, followed by the addition of 3,3′-diaminobenzidine. A positive reaction was detected by the EnVision + System (DAKO, Carpinteria, CA).
Quantitative Analysis
The Olympus DP20 digital system was used to take 312 photomicrographs at 200 × magnification. One pathologist (E.K.) recorded the cells showing an unequivocal nuclear positivity in relation to the total number of epithelial cells present in the captured image using Image J. A minimum of 250 cells was counted within FTE, STIC, or the ovarian carcinoma in each patient and the results were reported as a percentage index (Ki-67 index) on the basis of the average values from 3 randomly selected fields.
Statistical Analysis
A two-tail unpaired t test was used to determine the statistical significance of the Ki-67 labeling index between groups. Receiver-operator characteristic curves were applied to assess the performance (sensitivity and specificity) of the Ki-67 labeling index in distinguishing normal FTE from STIC. P<0.05 was defined as statistically significant. To define the Ki-67 index at the high end of normal FTE, we used the mean Ki-67 index + 3 SD that yielded a confidence level of 99.7%.
Results
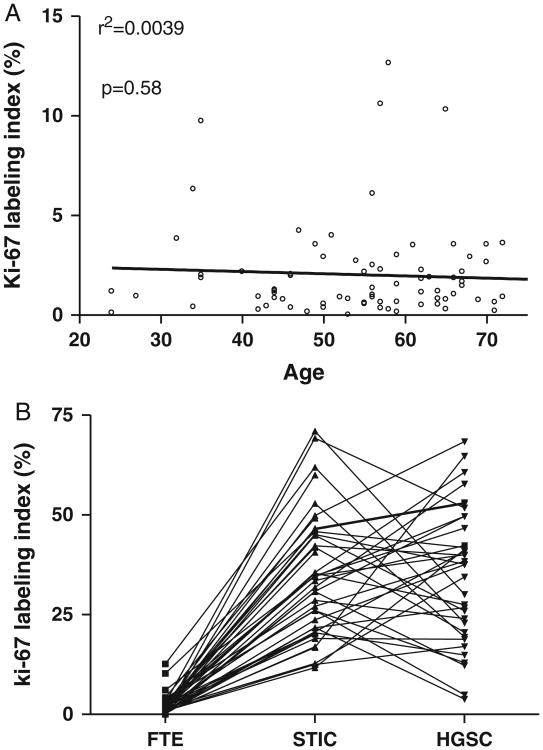
Immunoreactivity for Ki-67 in normal-appearing FTE in specimens containing a STIC was restricted to a few scattered nuclei with a median index of 1.6% and a mean ± 1 SD of 2.2% ± 2.8%. Representative cases with various Ki-67 staining patterns are shown in Figure 1. All the Ki-67-positive nuclei were detected in nonciliated cells and scattered lymphocytes in the epithelium just above the basement membrane. Occasionally, the Ki-67-positive nuclei were located immediately next to each other, suggesting that their progenitor had just undergone cell division (Fig. 1). In normal FTE, the distribution and number of Ki-67-positive nuclei was the same throughout all portions of the tube. To determine whether the Ki-67 labeling index in the FTE from patients with STIC differed from FTE in patients without STIC, we compared the Ki-67 labeling index in normal FTE from 19 premenopausal and 23 postmenopausal women who underwent salpingo-oophorectomy or hysterectomy and bilateral salpingo-oophorectomy for benign disease and whose tubes were completely examined (using the SEE-FIM protocol) and who did not have STICs. We failed to demonstrate any significant difference between those 2 groups (P = 0.802). We also observed no difference in the labeling index in FTE between premenopausal and postmenopausal women with STIC and of women without STIC (P = 0.454) (Table 1). There was also no significant difference in the Ki-67 labeling index in normal FTE by patient age (Fig. 2A).
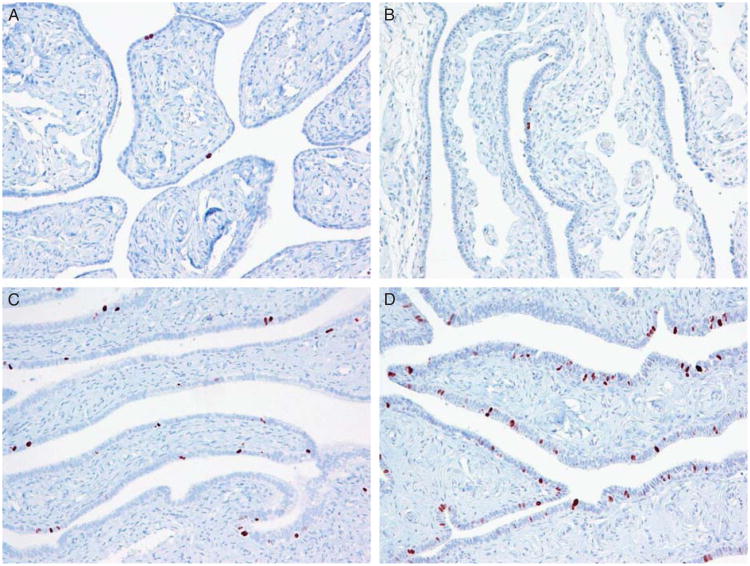
Fig. 1.
Ki-67 immunoreactivity in normal-appearing fallopian tube epithelium. The Ki-67-positive cells scatter in the tubal epithelium with a labeling index ranging widely from <1% in (A) and (B), whereas the index increases in (C) (approximately 6%) and (D) (approximately 10%).
Table 1. Ki-67 index in fallopian tube epithelium, serous tubal intraepithelial carcinoma, and high-grade serous carcinoma and patient age.
| Case number | Ki-67 index | Age | |||
|---|---|---|---|---|---|
|
|
|
||||
| Average (%) | SD (%) | Average | SD | ||
| FTE | 83 | 2.02 | 2.39 | 56 | 13.7 |
| All without STIC | 42 | 1.83 | 2.27 | 53 | 16.6 |
| Premenopausal | 19 | 1.92 | 2.40 | 38 | 7.6 |
| Postmenopausal | 23 | 1.74 | 2.20 | 68 | 9.7 |
| All with STIC | 41 | 2.22 | 2.75 | 59 | 9.2 |
| STIC | 41 | 34.55 | 15.30 | 59 | 9.2 |
| HGSC | 35 | 35.20 | 16.93 | 59 | 9.3 |
FTE indicates normal-appearing fallopian tubal epithelium; HGSC, high-grade serous carcinoma; STIC, serous tubal intra-epithelium carcinoma.
Fig. 2.
Ki-67 labeling index for all cases examined in this study. (A) In normal tubal epithelium, no correlation is found between the Ki-67 labeling index and patients' age (P = 0.66 and r2 = 0.0042). (B) Comparison of the Ki-67 index among normal fallopian tube epithelium (FTE), concurrent serous tubal intraepithelial carcinoma (STIC), and high-grade serous carcinoma (HGSC). Connecting lines indicate the specimens from the same patients. The Ki-67 labeling index is significantly higher in STIC than that in normal fallopian tube epithelium (P<0.0001). However, there is no statistically significant difference in the Ki-67 labeling index between STIC and HGSC.
STICs and HGSC had a significantly higher Ki-67 index than normal FTE (P<0.0001) (Fig. 2B). The Ki-67 index was not significantly different between STICs with or without an associated HGSC (P>0.05). STICs had a Ki-67 index ranging from 11.7% to 71.1%, with a median index of 33.5% and a mean ± 1 SD of 34.6% ± 15.3%. Among 41 STICs, 11 (26.8%) were flat and consisted of a single layer of highly atypical epithelium, whereas 10 (24.4%) were composed of stratified epithelium and 20 (48.8%) were tufted. The Ki-67-positive cells were uniformly distributed in STICs composed of a single layer of epithelium and were predominantly located in the basal-parabasal layer in STICs that were stratified and tufted. The morphologic and Ki-67 immunostaining features in a representative STIC are shown in Figure 3. Tufted STICs tended to have a lower Ki-67 index compared with STICs composed of a single layer but the difference was not statistically significant (P = 0.09). The Ki-67 index in HGSCs ranged from 3.9% to 68.4% (median was 37.6%) and the mean ± 1 SD was 35.2% ± 16.9%. There was no significant correlation of the Ki-67 labeling index in STICs compared with HGSC (P = 0.86). Specifically, 18 (51.4%) of 35 cases had a lower Ki-67 index in STIC than in HGSC, and 17 (48.6%) of 35 cases had a higher index in STIC than in HGSC. Interestingly, the Ki-67 index in STICs showing a single-layer architecture was significantly higher than in the concurrent HGSC, whereas the index in STICs that were stratified or tufted was lower than the HGSC (P = 0.0031). In many cases, the Ki-67 labeling index varied widely in different areas within HGSCs, reflecting the existence of tumor subclones with different proliferative activity.
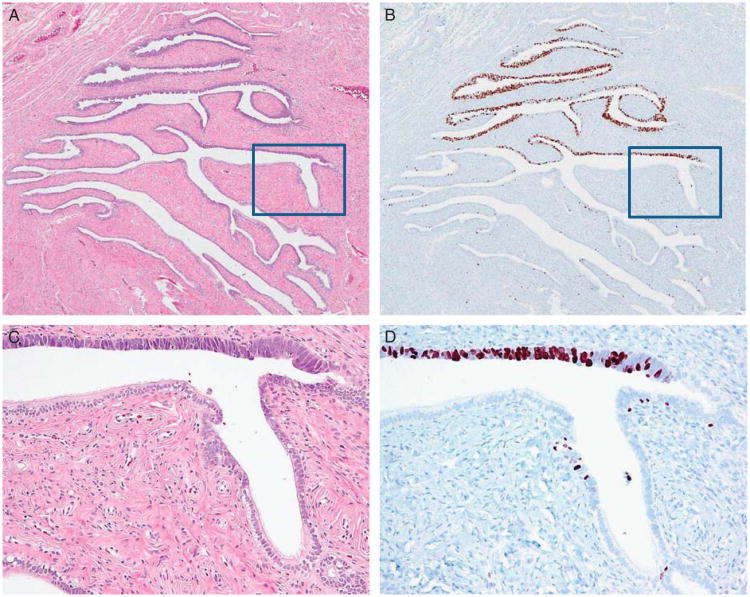
Fig. 3.
Ki-67 immunoreactivity in a serous tubal intraepithelial carcinoma (STIC). (A) Hematoxylin and eosin-stained section shows a discrete STIC occupying approximately 40% of epithelium in the cross-section of a tube. (B) The Ki-67 immunoreactivity is confined largely to the STIC. (C and D) A higher magnification view demonstrating morphologic features of the STIC that contains highly atypical epithelial cells and has a very high Ki-67 labeling index, whereas the adjacent normal-appearing tubal epithelium has a low index. The squares in (A) and (B) show the magnified views in (C) and (D).
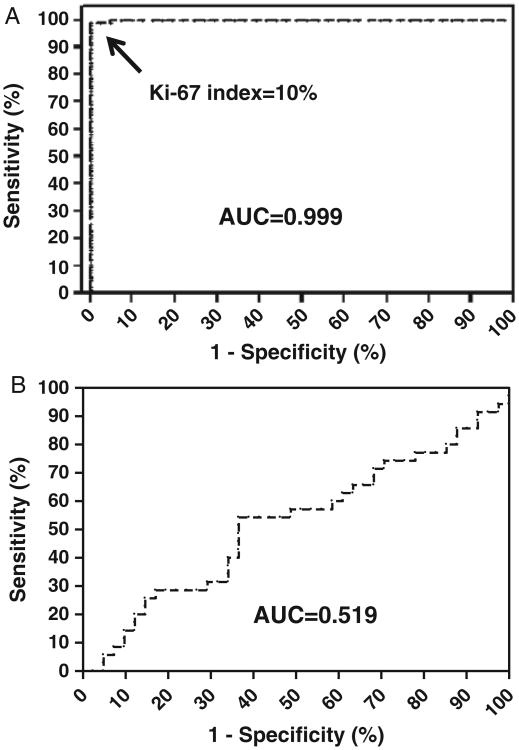
A receiver-operator characteristic curve analysis was performed to determine the cut point between a STIC and normal FTE. A Ki-67 labeling index of 10% (approximately the mean+3 SD) yielded a sensitivity of 100% and a specificity of 96.4% (area under the curve = 0.999) (Fig. 4A). Only 4 FTEs showed Ki-67 labeling index ≥10%: 2 FTE from women with STICs and 2 controls (1 premenopausal and 1 postmenopausal). Interestingly, the clinical information available for the last 2 patients showed that the postmenopausal patient was known to harbor BRCA2 germline mutations and the premenopausal woman was 35 yr old and with prior breast carcinoma. Finally, the Ki-67 labeling index was not statistically different between STIC and HGSC as the area under the curve was only 0.519 (Fig. 4B).
Fig. 4.
Receiver operation curve analysis of the Ki-67 labeling index. (A) Ki-67 labeling index can distinguish a serous tubal intraepithelial carcinoma from a normal fallopian tube with high sensitivity and specificity and the area under the curve (AUC) is 0.999. (B) Ki-67 labeling index is not useful in distinguishing a serous tubal intraepithelial carcinoma from a high-grade serous carcinoma with an AUC of only 0.519.
Discussion
The morphologic differentiation of STIC from normal FTE is not difficult; however, as fallopian tubes are being more carefully scrutinized, a variety of morphologic alterations, including focal areas of stratification, mainly due to tangentially cut epithelium, hyperplasia, and a variety of “atypical” proliferations that are not clearly normal but fall short of a definitive diagnosis of STIC are now being recognized. These morphologic alterations are subtle and of unknown clinical significance. However, bona fide diagnosis of STIC, the putative precursor of ovarian HGSC, is important. Establishment of reproducible criteria for the diagnosis of STIC is essential.
We reasoned that the Ki-67 labeling index might be a useful adjunct to morphology and p53 immunostaining in making the diagnosis of STIC and therefore carried out a quantitative analysis of the Ki-67 index in STICs and normal FTE to determine a cut point that could be used in assisting in the diagnosis of a STIC. In the present study, the receiver-operator characteristic curve showed that high sensitivity and specificity could be achieved in distinguishing a STIC from normal FTE using the Ki-67 labeling index. STICs had a significantly higher Ki-67 labeling index than normal FTE. We identified a cutoff index of 10% as the upper threshold of normal on the basis of the mean labeling index of normal FTE plus 3SD. Our rationale for using 3SD was to have a high confidence interval (99.7%), which would result in a false-positive rate of <0.3%, that is, a normal fallopian tube with Ki-67 index ≥10%. Using this cut point, the sensitivity and specificity in diagnosing STIC as distinct from normal FTE was 100% and 96.6%, respectively. Because intraepithelial lesions are usually small, assessment of the Ki-67 labeling index in a suspicious lesion should be based on counting all the epithelial cells within the lesion.
The morphologic appearance of normal FTE varies considerably in different portions of the tube and with age and hormonal status. For example, with increasing age, the plicae generally become blunter and the architecture becomes less complex. In addition, the height of the epithelium diminishes. Ciliated cells are more frequently observed in the fimbria and may be fewer in number in hypoestrogenic states. Despite these differences, we found that the Ki-67 labeling index did not differ significantly in different portions of the fallopian tube, in premenopausal versus menopausal women, or whether a STIC was present in the tube. In premenopausal women, information on the time in the menstrual cycle when the fallopian tubes were removed was not available and therefore we were unable to accurately assess whether there was variation in the proliferation index on the basis of hormonal status. Nonetheless, because the proliferation index in normal tubes was essentially the same in all premenopausal patients, it is unlikely that there is a significant difference in the proliferative and secretory phases.
Our findings indicate that a Ki-67 labeling index cut point of 10% can aid in the diagnosis of a STIC, irrespective of the patients' age. Indeed, in our study, out of 83 FTEs analyzed, only 4 FTEs exceeded the Ki-67 labeling index of 10%. Two of these patents did not have a STIC elsewhere, one had a BRCA2 germ line mutation, and the other had breast cancer at a relatively young age. These observations suggest that a higher Ki-67 index may be related to BRCA germ line mutations, as reported in previous studies (12,13). It would be important for future studies to further investigate whether the normal-appearing FTE has a higher Ki-67 labeling index in tubes from a high-risk group including those with and without BRCA germ line mutations.
An interesting finding in this study was the detection of Ki-67-positive nuclei in both normal FTE and STICs with tufted patterns that tended to localize in the basal-parabasal portion of the tubal mucosa (Fig. 5), raising the possibility that these cells may be derived from somatic stem cells that are able to renew themselves and generate new tubal epithelial cells. Future studies are required to determine whether this basally located tubal epithelium represents the somatic stem cells. Another interesting observation was that there was no progressive increase in the Ki-67 index in a STIC and the associated HGSC, suggesting that increasing proliferation is not necessarily a feature of tumor progression in HGSC.
Fig. 5.
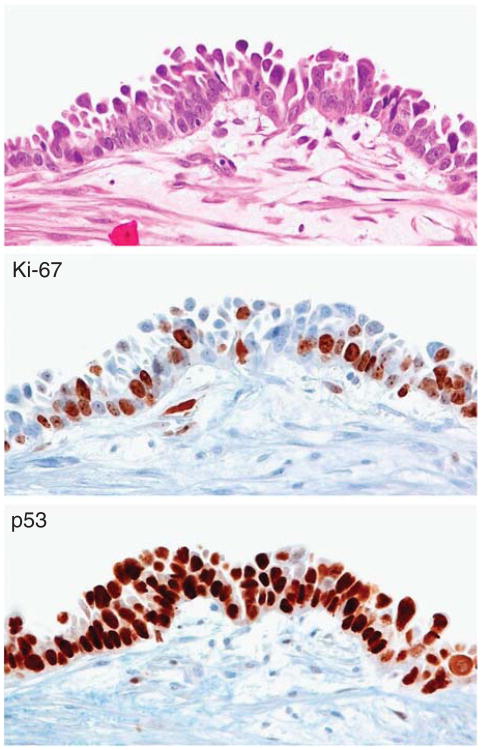
Ki-67 immunoreactivity in a serous tubal intraepithelial carcinoma. A tufted and multilayer serous tubal intraepithelial carcinoma shows that many Ki-67-positive cells are located in the basal-parabasal compartment. The lesion is also diffusely positive for p53.
The Ki-67 labeling index clearly distinguishes normal FTE from a STIC but the more difficult problem is the distinction of a STIC from tubal lesions with cytologic atypia that is greater than normal FTE but less than that of a STIC that either display strong, diffuse p53 immunostaining and have a Ki-67 labeling index <10% or wild-type p53 immunostaining with a Ki-67 labeling index ≥10%. Similar atypical “intermediate” lesions have been designated “tubal intraepithelial lesions in transition (TILT)” (14) by others but we prefer the term “serous tubal intraepithelial lesions (STILs)” because the nature of these lesions, their relationship with STIC, and therefore their clinical significance, if any, are not known. They may be precursors of STICs or they may be reactive lesions that are unrelated to STICs. Alternatively, they may comprise a heterogeneous group of lesions, some of which are precursor lesions and others that are benign reactive changes. We have recently developed an algorithm that uses morphology and both p53 and Ki-67 immunostaining to aid in the differential diagnosis of these lesions (11).
In conclusion, we determined that a Ki-67 labeling index of 10% is the optimal cut point to define an atypical tubal lesion as a STIC. It is important to note, however, that this feature alone is insufficient to make a diagnosis of a STIC. Indeed, our preliminary findings suggest that a Ki-67 labeling index ≥10% in conjunction with morphologic criteria of malignancy and expression of p53 is useful in classifying a lesion as a STIC. These findings serve as initial steps in the application of immunohistochemistry toward defining STIC for both diagnostic and research purposes.
Acknowledgments
The authors thank Dr R. Soslow in providing cases.
This study is supported by a CDMRP Ovarian Cancer Consortium grant (OC100517) from the US Department of Defense.
Footnotes
The authors declare no conflict of interest.
References
- 1.Kuhn E, Kurman RJ, Shih IM. Ovarian cancer is an imported disease- fact or fiction? Current Ob Gyn Report. doi: 10.1007/s13669-011-0004-1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gross AL, Kurman RJ, Vang R, et al. Precursor lesions of high-grade serous ovarian carcinoma: morphological and molecular characteristics. J Oncol. doi: 10.1155/2010/126295. Epub April 27, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kindelberger DW, Lee Y, Miron A, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–9. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 4.Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–43. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlson JW, Jarboe EA, Kindelberger D, et al. Serous tubal intraepithelial carcinoma: diagnostic reproducibility and its implications. Int J Gynecol Pathol. 2010;29:310–4. doi: 10.1097/PGP.0b013e3181c713a8. [DOI] [PubMed] [Google Scholar]
- 6.Medeiros F, Muto MG, Lee Y, et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol. 2006;30:230–6. doi: 10.1097/01.pas.0000180854.28831.77. [DOI] [PubMed] [Google Scholar]
- 7.Kuhn E, Kurman RJ, Vang R, et al. TP53 mutations in serous tubal intraepithelial carcinoma and concurrent pelvic high-grade serous carcinoma-evidence supporting the clonal relationship of the two lesions. J Pathol. 2012;226:421–6. doi: 10.1002/path.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jarboe E, Folkins A, Nucci MR, et al. Serous carcinogenesis in the fallopian tube: a descriptive classification. Int J Gynecol Pathol. 2008;27:1–9. doi: 10.1097/pgp.0b013e31814b191f. [DOI] [PubMed] [Google Scholar]
- 9.Sehdev AS, Kurman RJ, Kuhn E, et al. Serous tubal intraepithelial carcinoma upregulates markers associated with high-grade serous carcinomas including Rsf-1 (HBXAP), cyclin E and fatty acid synthase. Mod Pathol. 2010;23:844–55. doi: 10.1038/modpathol.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhn E, Meeker A, Wang TL, et al. Shortened telomeres in serous tubal intraepithelial carcinoma: an early event in ovarian high-grade serous carcinogenesis. Am J Surg Pathol. 2010;34:829–36. doi: 10.1097/PAS.0b013e3181dcede7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Visvanathan K, Vang R, Shaw P, et al. Diagnosis of serous tubal intraepithelial carcinoma (STIC) based on morphologic and immunohistochemical features- a reproducibility study. Am J Surg Pathol. 2011;35:1766–75. doi: 10.1097/PAS.0b013e31822f58bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norquist BM, Garcia RL, Allison KH, et al. The molecular pathogenesis of hereditary ovarian carcinoma: alterations in the tubal epithelium of women with BRCA1 and BRCA2 mutations. Cancer. 2010;116:5261–71. doi: 10.1002/cncr.25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piek JM, van Diest PJ, Zweemer RP, et al. Dysplastic changes in prophylactically removed fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195:451–6. doi: 10.1002/path.1000. [DOI] [PubMed] [Google Scholar]
- 14.Folkins AK, Jarboe EA, Roh MH, et al. Precursors to pelvic serous carcinoma and their clinical implications. Gynecol Oncol. 2009;113:391–6. doi: 10.1016/j.ygyno.2009.01.013. [DOI] [PubMed] [Google Scholar]