Abstract
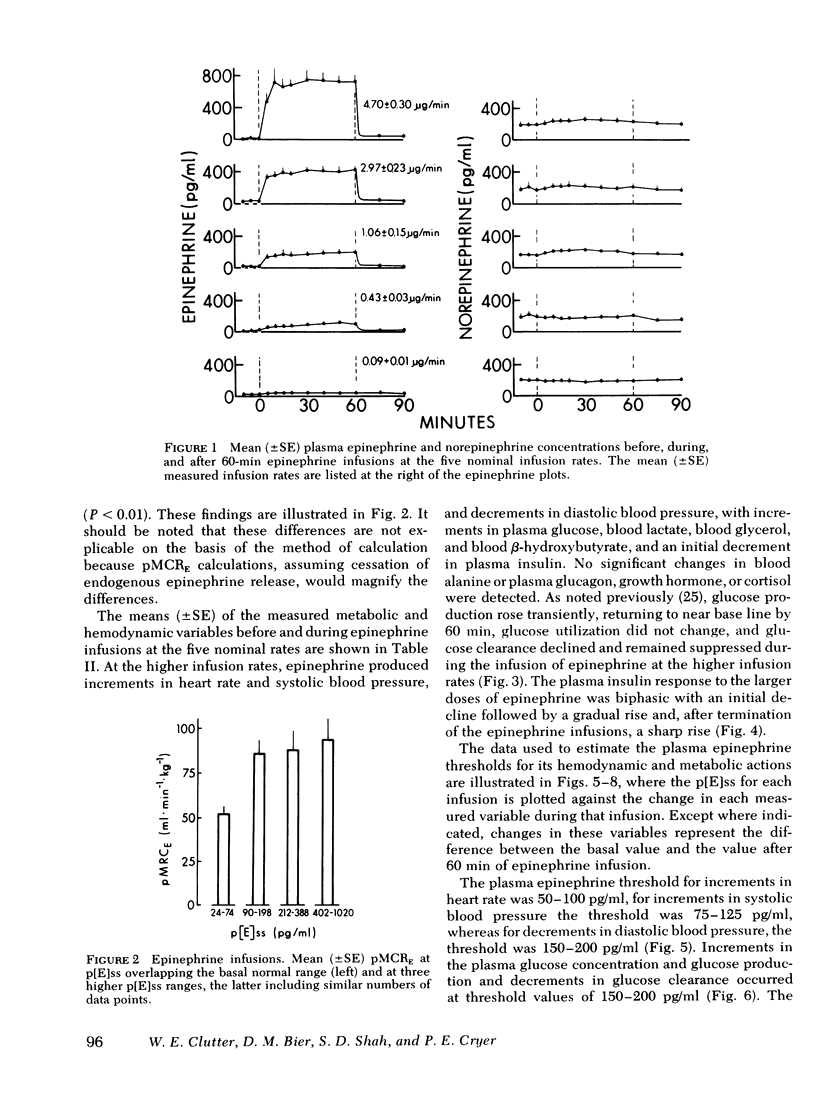
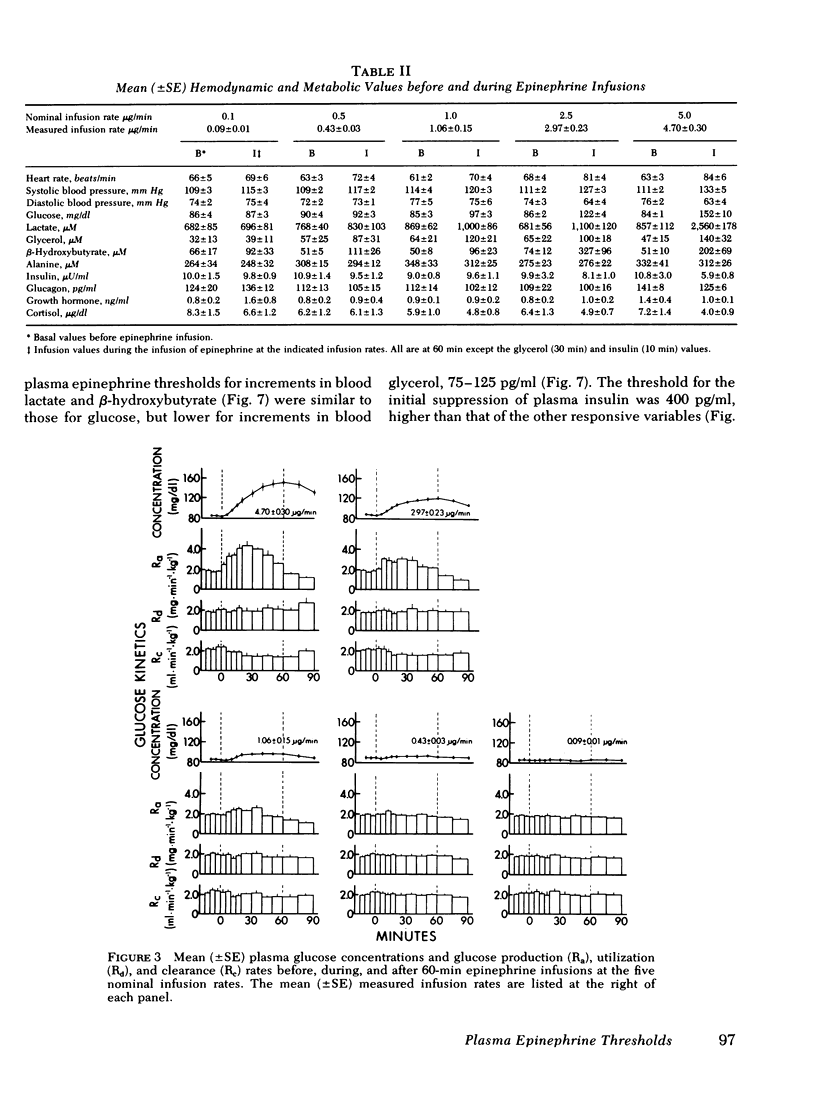
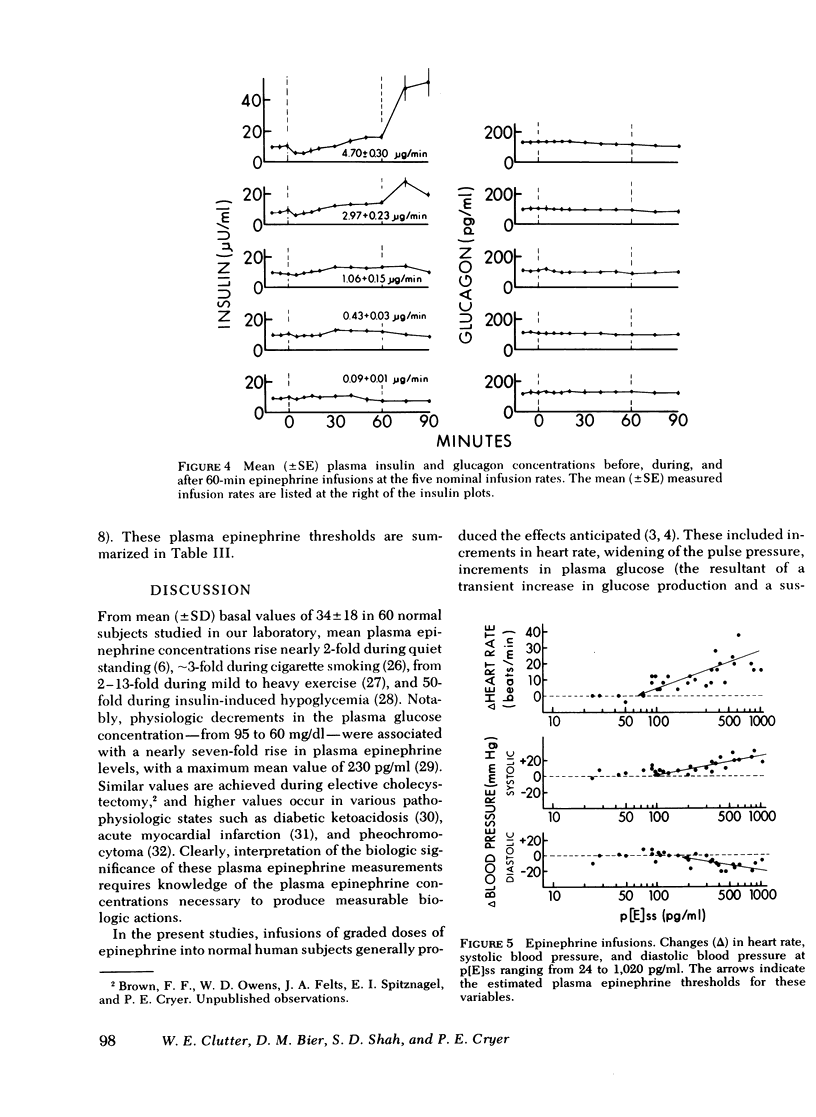
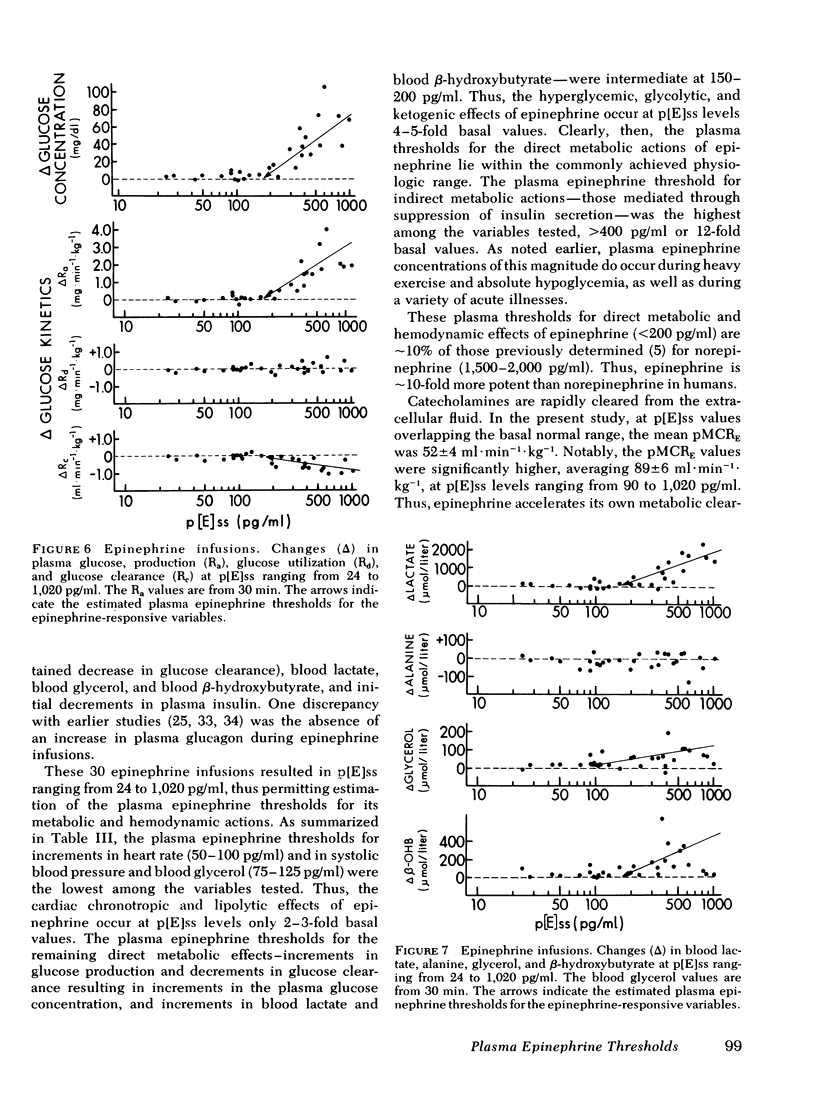
To determine the plasma epinephrine thresholds for its metabolic and hemodynamic actions and plasma epinephrine metabolic clearance rates, 60-min intravenous epinephrine infusions at nominal rates of 0.1, 0.5, 1.0, 2.5, and 5.0 microgram/min were performed in each of six normal human subjects. These 30 infusions resulted in steady-state plasma epinephrine concentrations ranging from 24 to 1,020 pg/ml. Plasma epinephrine thresholds were 50-100 pg/ml for increments in heart rate, 75-125 pg/ml for increments in blood glycerol and systolic blood pressure, 150-200 pg/ml for increments in plasma glucose (the resultant of increments in glucose production and decrements in glucose clearance), blood lactate, blood beta-hydroxybutyrate, and diastolic blood pressure, and greater than 400 pg/ml for early decrements in plasma insulin. Changes in blood alanine, plasma glucagon, plasma growth hormone, and plasma cortisol were not detected. At steady-state plasma epinephrine concentrations of 24-74 pg/ml, values overlapping the basal normal range, the mean (+/-SE) plasma metabolic clearance rate of epinephrine was 52 +/- 4 ml x min-1 x kg-1; this value rose to 89 +/- 6 ml x min-1 x kg-1 (P less than 0.01) at steady-state epinephrine concentrations of 90-1,020 pg/ml. We conclude that in human subjects: (a) the plasma epinephrine thresholds for its hemodynamic and metabolic actions lie within the physiologic range, (b) epinephrine and norepinephrine accelerate their own metabolic clearance, and (c) epinephrine is 10 times more potent than norepinephrine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beitins I. Z., Shaw M. H., Kowarski A., Migeon C. J. Comparison of competitive protein binding radioassay of cortisol to double isotope dilution and Porter Silber methods. Steroids. 1970 Jun;15(6):765–776. doi: 10.1016/s0039-128x(70)80045-9. [DOI] [PubMed] [Google Scholar]
- Bier D. M., Arnold K. J., Sherman W. R., Holland W. H., Holmes W. F., Kipnis D. M. In-vivo measurement of glucose and alanine metabolism with stable isotopic tracers. Diabetes. 1977 Nov;26(11):1005–1015. doi: 10.2337/diab.26.11.1005. [DOI] [PubMed] [Google Scholar]
- Bier D. M., Leake R. D., Haymond M. W., Arnold K. J., Gruenke L. D., Sperling M. A., Kipnis D. M. Measurement of "true" glucose production rates in infancy and childhood with 6,6-dideuteroglucose. Diabetes. 1977 Nov;26(11):1016–1023. doi: 10.2337/diab.26.11.1016. [DOI] [PubMed] [Google Scholar]
- Cahill G. F., Jr, Herrera M. G., Morgan A. P., Soeldner J. S., Steinke J., Levy P. L., Reichard G. A., Jr, Kipnis D. M. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966 Nov;45(11):1751–1769. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrington A. D., Williams P. E., Harris M. S. Relationship between the plasma glucose level and glucose uptake in the conscious dog. Metabolism. 1978 Jul;27(7):787–791. doi: 10.1016/0026-0495(78)90213-5. [DOI] [PubMed] [Google Scholar]
- Christensen N. J. Plasma norepinephrine and epinephrine in untreated diabetics, during fasting and after insulin administration. Diabetes. 1974 Jan;23(1):1–8. doi: 10.2337/diab.23.1.1. [DOI] [PubMed] [Google Scholar]
- Cowan J. S., Hetenyi G., Jr Glucoregulatory responses in normal and diabetic dogs recorded by a new tracer method. Metabolism. 1971 Apr;20(4):360–372. doi: 10.1016/0026-0495(71)90098-9. [DOI] [PubMed] [Google Scholar]
- Cryer P. E., Haymond M. W., Santiago J. V., Shah S. D. Norepinephrine and epinephrine release and adrenergic mediation of smoking-associated hemodynamic and metabolic events. N Engl J Med. 1976 Sep 9;295(11):573–577. doi: 10.1056/NEJM197609092951101. [DOI] [PubMed] [Google Scholar]
- Cryer P. E. Isotope-derivative measurements of plasma norepinephrine and epinephrine in man. Diabetes. 1976 Nov;25(11):1071–1082. doi: 10.2337/diab.25.11.1071. [DOI] [PubMed] [Google Scholar]
- Cryer P. E., Santiago J. V., Shah S. Measurement of norepinephrine and epinephrine in small volumes of human plasma by a single isotope derivative method: response to the upright posture. J Clin Endocrinol Metab. 1974 Dec;39(6):1025–1029. doi: 10.1210/jcem-39-6-1025. [DOI] [PubMed] [Google Scholar]
- Cryer P. E., Silverberg A. B., Santiago J. V., Shah S. D. Plasma catecholamines in diabetes. The syndromes of hypoadrenergic and hyperadrenergic postural hypotension. Am J Med. 1978 Mar;64(3):407–416. doi: 10.1016/0002-9343(78)90220-6. [DOI] [PubMed] [Google Scholar]
- Esler M., Jackman G., Bobik A., Kelleher D., Jennings G., Leonard P., Skews H., Korner P. Determination of norepinephrine apparent release rate and clearance in humans. Life Sci. 1979 Oct 22;25(17):1461–1470. doi: 10.1016/0024-3205(79)90371-0. [DOI] [PubMed] [Google Scholar]
- Galbo H., Holst J. J., Christensen N. J. Glucagon and plasma catecholamine responses to graded and prolonged exercise in man. J Appl Physiol. 1975 Jan;38(1):70–76. doi: 10.1152/jappl.1975.38.1.70. [DOI] [PubMed] [Google Scholar]
- Garber A. J., Cryer P. E., Santiago J. V., Haymond M. W., Pagliara A. S., Kipnis D. M. The role of adrenergic mechanisms in the substrate and hormonal response to insulin-induced hypoglycemia in man. J Clin Invest. 1976 Jul;58(1):7–15. doi: 10.1172/JCI108460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerich J. E., Karam J. H., Forsham P. H. Stimulation of glucagon secretion by epinephrine in man. J Clin Endocrinol Metab. 1973 Sep;37(3):479–481. doi: 10.1210/jcem-37-3-479. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Lorenzi M., Tsalikian E., Karam J. H. Studies on the mechanism of epinephrine-induced hyperglycemia in man. Evidence for participation of pancreatic glucagon secretion. Diabetes. 1976 Jan;25(1):65–71. doi: 10.2337/diab.25.1.65. [DOI] [PubMed] [Google Scholar]
- Karl I. E., Pagliara A. S., Kipnis D. M. A microfluorometric enzymatic assay for the determination of alanine and pyruvate in plasma and tissues. J Lab Clin Med. 1972 Sep;80(3):434–441. [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- Leichter S. B., Pagliara A. S., Grieder M. H., Pohl S., Rosai J., Kipnis D. M. Uncontrolled diabetes mellitus and hyperglucagonemia associated with an islet cell carcinoma. Am J Med. 1975 Feb;58(2):285–293. doi: 10.1016/0002-9343(75)90579-3. [DOI] [PubMed] [Google Scholar]
- Miura Y., Haneda T., Sato T., Miyazawa K., Sakuma H., Kobayashi K., Minai K., Shirato K., Honna T., Takishima T. Plasma catecholamine levels in the coronary sinus, aorta and femoral vein of subjects undergoing cardiac catheterization at rest and during exercise. Jpn Circ J. 1976 Aug;40(8):929–934. doi: 10.1253/jcj.40.929. [DOI] [PubMed] [Google Scholar]
- Passon P. G., Peuler J. D. A simplified radiometric assay for plasma norepinephrine and epinephrine. Anal Biochem. 1973 Feb;51(2):618–631. doi: 10.1016/0003-2697(73)90517-4. [DOI] [PubMed] [Google Scholar]
- Pinter J. K., Hayashi J. A., Watson J. A. Enzymic assay of glycerol, dihydroxyacetone, and glyceraldehyde. Arch Biochem Biophys. 1967 Aug;121(2):404–414. doi: 10.1016/0003-9861(67)90094-x. [DOI] [PubMed] [Google Scholar]
- Radziuk J., Norwich K. H., Vranic M. Experimental validation of measurements of glucose turnover in nonsteady state. Am J Physiol. 1978 Jan;234(1):E84–E93. doi: 10.1152/ajpendo.1978.234.1.E84. [DOI] [PubMed] [Google Scholar]
- Rizza R., Haymond M., Cryer P., Gerich J. Differential effects of epinephrine on glucose production and disposal in man. Am J Physiol. 1979 Oct;237(4):E356–E362. doi: 10.1152/ajpendo.1979.237.4.E356. [DOI] [PubMed] [Google Scholar]
- SCHALCH D. S., PARKER M. L. A SENSITIVE DOUBLE ANTIBODY IMMUNOASSAY FOR HUMAN GROWTH HORMONE IN PLASMA. Nature. 1964 Sep 12;203:1141–1142. doi: 10.1038/2031141a0. [DOI] [PubMed] [Google Scholar]
- Silverberg A. B., Shah S. D., Haymond M. W., Cryer P. E. Norepinephrine: hormone and neurotransmitter in man. Am J Physiol. 1978 Mar;234(3):E252–E256. doi: 10.1152/ajpendo.1978.234.3.E252. [DOI] [PubMed] [Google Scholar]
- Steele R., Rostami H., Altszuler N. A two-compartment calculator for the dog glucose pool in the nonsteady state. Fed Proc. 1974 Jul;33(7):1869–1876. [PubMed] [Google Scholar]
- Young J. B., Landsberg L. Catecholamines and intermediary metabolism. Clin Endocrinol Metab. 1977 Nov;6(3):599–631. doi: 10.1016/s0300-595x(77)80073-x. [DOI] [PubMed] [Google Scholar]
- Young J. B., Landsberg L. Catecholamines and the regulation of hormone secretion. Clin Endocrinol Metab. 1977 Nov;6(3):657–695. doi: 10.1016/s0300-595x(77)80075-3. [DOI] [PubMed] [Google Scholar]


