Abstract
Type 2 diabetes mellitus (T2DM) is one of the leading causes of morbidity and mortality. While all ethnic groups are affected, the prevalence of T2DM in South Asians, both in their home countries and abroad, is extremely high and is continuing to rise rapidly. Innate biological susceptibilities coupled with rapid changes in physical activity, diet, and other lifestyle behaviors are contributing factors propelling the increased burden of disease in this population. The large scope of this problem calls for investigations into the cause of increased susceptibility and preventative efforts at both the individual and population level that are aggressive, culturally sensitive, and start early. In this review, we outline the biological and environmental factors that place South Asians at elevated risk for T2DM, compared with Caucasian and other ethnic groups.
Keywords: type 2 diabetes mellitus, South Asians, prevalence, ethnic comparison
Introduction
Type 2 diabetes mellitus (T2DM) currently affects approximately 366 million people worldwide.1 This includes individuals in developed countries, but also those living in urban and rural areas of developing countries.2–4 South Asians (those who live in or have their roots in India, Pakistan, Sri Lanka, Bangladesh, Nepal, Bhutan, or the Maldives)2 seem to be at especially high risk for developing T2DM. While the overall prevalence of T2DM in South Asia is high and increasing, there is considerable heterogeneity across South Asian countries (Table 1). Much of this heterogeneity can be attributed to differing states of socioeconomic development, variations in lifestyle factors, and differences in prevalence of undiagnosed versus diagnosed diabetes among countries. The majority of T2DM data from South Asia have come from India, the South Asian country with the largest diabetes burden, and where the prevalence has increased steadily over the past 40 years.2 The most recent national prevalence study collected data from three states and one union territory covering a population of over 200 million people. The overall weighted prevalence was 10.4% in Tamilnadu, 8.4% in Maharashtra, 5.3% in Jharkhand, and 13.6% in Chandigarh.5 If extrapolated nationwide, these estimates translate to 62.4 million individuals in India currently living with T2DM.5
Table 1.
South Asian diabetes estimates for 2011 by country
| Country | Diabetes cases | National prevalence of diabetes (%) |
|---|---|---|
| Bangladesh | 8,406,000 | 9.6 |
| Bhutan | 21,000 | 4.9 |
| India | 61,258,000 | 8.31 |
| Maldives | 15,000 | 7.6 |
| Nepal | 488,000 | 3.0 |
| Sri Lanka | 1,078,000 | 7.8 |
Source: International Diabetes Federation.3
By 2030 it is projected that there will be 120.9 million people with diabetes in South Asia (90–95% of these cases will be T2DM),3 more than double the number affected in North America or Europe (Fig. 1). The prevalence of T2DM is also high among South Asian migrant populations, with several studies noting a higher prevalence of T2DM in migrant South Asians than in other ethnic groups in the host countries.6–14 Nationally, representative studies in the United States have shown that regardless of BMI classification, South Asians have the highest BMI-specific prevalence of T2DM among all ethnic groups.7 A recent randomized population based study of South Asians in the United States reported an overall T2DM prevalence of 17.4% in South Asian adults. These prevalence estimates greatly exceed those in non-Hispanic whites (7.8%), non-Hispanic blacks (13%), and Hispanic Latinos (10.2%).8 Similar patterns have also been observed in other diaspora countries, including the United Kingdom, Fiji, South Africa, Norway, and Singapore (Fig. 2).9–14 Furthermore, although the data are limited, it appears that T2DM incidence is much higher in South Asians compared with groups in Europe and the United States (20.2 per 1,000 person-years in a study in India15 compared with 6.9 per 1,000 person-years in the United States,16 and 7.6 and 10.8 cases per 1,000 person-years for studies in Italy17 and Spain,18 respectively).
Figure 1.
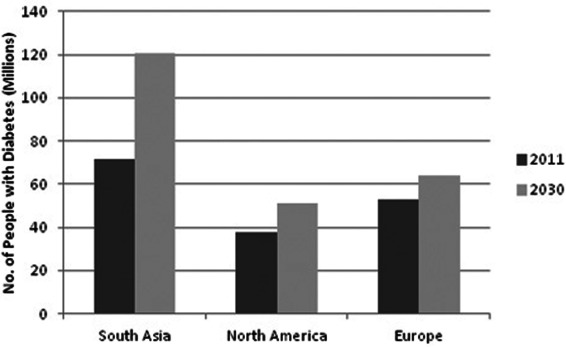
Projected number of people with diabetes (millions) by year—2011 and 2013.
Figure 2.
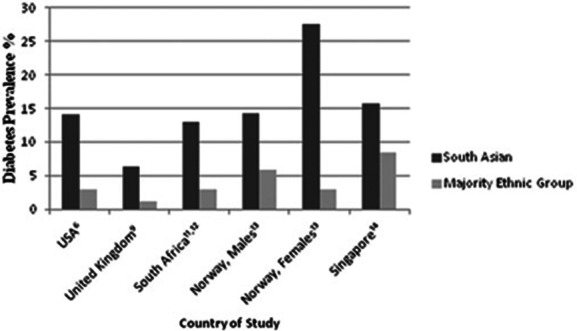
Differences in prevalence among South Asians and other ethnic groups.
The higher prevalence and incidence of T2DM in South Asians populations worldwide is likely indicative of underlying biological factors in South Asians, coupled with recent rapid changes in dietary, activity, and other lifestyle behaviors. Herein, we discuss the current literature comparing South Asians with other populations with regard to biological (e.g., pathophysiology and genetics) and environmental factors (e.g., lifestyle) associated with T2DM. A synopsis of the paper is presented in Table 2.
Table 2.
Comparison of diabetes risk factors among South Asians, Caucasians, and other ethnic groups
| Pathophysiology | |
| • Insulin resistance | • Insulin resistance is a pathophysiological factor and precursor for diabetes in all ethnic populations. However, South Asians are shown to be shown to be more insulin resistant than Caucasian populations even at younger ages and lower levels of BMI. Some of this increased propensity for insulin resistance in South Asians might be attributed to greater deposition of visceral fat in South Asians as compared to Caucasians. |
| • Pancreatic β-cell function | • While declines in pancreatic β-cell function are involved in diabetes pathogenesis for all ethnic groups, there may be ethnic differences in the timing and relative contribution of β-cell decline. Preliminary data from South Africa indicates an early impairment of β-cell function as an underlying pathophysiological abnormality in South Asians. However, the complete mechanistic pathway determining T2DM in South Asians as compared to other ethnic groups remains unclear, and early declines in β-cell function are an important pathophysiological factor to investigate. |
| Risk Factors | |
| • Genetics | • Approximately 60 genes have been implicated in T2DM development;43,44 however, most studies were conducted in European populations. While the actual genes established may not be different, some differences exist in allele frequencies and polymorphisms. |
| • Age | • Increasing age elevates T2DM risk in all populations; however, South Asians develop the disease at younger ages. Mean age at diagnosis was lowest in South Asians (49 years), followed by Chinese (55 years), Blacks (57 years), and Whites (58 years) living in Canada.57 To date there have been no studies directly comparing the age-specific prevalence and incidence of diabetes among South Asians living in South Asia and members of other ethnic groups living in the United States and Europe. |
| • Anthropometry | • Higher BMI, overweight, and obesity are associated with increased risk for diabetes in all ethnic groups. However, despite similar levels of BMI, South Asians have more total abdominal and visceral fat compared to Caucasians.33,58 These differences are seen even in childhood and early adolescence.61–63 |
| • Prospective studies from South Asia indicate that participants who were underweight as children have a higher prevalence of overweight and obesity as adults.67 Furthermore, in South Asians with hyperglycemia in adulthood, a relationship was seen with low BMI before age 2, followed by accelerated increases in BMI throughout adulthood.68 Being that 40% of the world's low-birth-weight babies are born in India,65 this is an important area for future research. | |
| • Biomarkers | • ROS, leptin, and C-reactive protein (CRP) have all been associated with an increased risk for diabetes, while adiponectin has been associated with a decreased risk.51,74–77 Compared to Caucasians, South Asians have exhibited elevated levels of leptin and CRP and decreased levels of adiponectin.75–78 However, to our knowledge, there have been no studies assessing levels of oxidative stress in South Asians. |
| • Physical inactivity | • Reductions in physical activity increase the risk of diabetes in all ethnic groups; however, South Asians appear to be even less physically active than their Caucasian counterparts.85,86 South Asians have demonstrated physical activity levels that were 50–75% lower than those of Europeans.87 |
| • Diet | • Higher intakes of refined carbohydrates, saturated fats, and trans fats have been shown to increase diabetes risk in all populations, while low glycemic index foods and foods high in dietary fiber have been shown to decrease the risk.89,90 In general, a typical South Asian meal has a higher caloric intake and a higher percentage of carbohydrate than does a European meal.92 In South Asians who have migrated to Western countries, acculturation has led to an increased consumption of foods, such as potato chips and colas, and a decrease in consumption of fiber.95,96 |
| • Tobacco use | • Tobacco use increases diabetes risk in all populations.100–102 While there is a higher prevalence of tobacco use in South Asian than in the United States and Europe,104–107 there are no studies directly comparing the relationship between diabetes risk and smoking in South Asians as compared to Caucasians. |
| • Sleep | • Studies have shown that sleep duration above or below average or disruptions in sleep can increase the risk for diabetes.108–110 However, to date, no comparative studies have been conducted on duration of sleep and or/sleep disruptions in South Asians as compared to other ethnic groups. |
| • Persistent organic pollutants | • Persistent organic pollutants are of recent concern regarding their association with diabetes risk.114 While studies in the United States have noted an association between exposure to POPs and elevated risk for diabetes,55 no such studies have been conducted in South Asia. However, there is some evidence of high levels of POP contaminants in South Asian waters.116 |
Biological risk factors for T2DM
Pathophysiology
While some studies note that the pathogenesis of T2DM begins with adiposity-induced insulin resistance followed by a subsequent decline in pancreatic β-cell function,19–21 other data point to the role of earlier declines in β-cell function, suggesting that this may be the primary defect predisposing individuals and populations to T2DM.22–26 It is possible that variances in both the propensity to develop insulin resistance as well as the compensatory ability of β cells may explain some ethnic differences in T2DM susceptibility.27–30 There is strong evidence to suggest that South Asians are more insulin resistant than Caucasians, even at younger ages and comparative levels of BMI. A study by Mohan et al. demonstrated increased insulin levels in South Asians as compared to Europeans, both with and without diabetes.31 A later study from Europe found South Asians with T2DM to be significantly more insulin resistant then their European counterparts, despite their younger age,32 while studies from the United States have noted a significantly lower insulin sensitivity in South Asians as compared to Caucasians, regardless of the level of total body fat.33
It appears that some of the increased propensity for South Asians to develop insulin resistance could be attributable to greater accumulation of visceral fat.34,35 Data from a subset of the Chennai Urban Rural Epidemiology Study (CURES) indicated that a progressive increase in visceral fat was associated with increasing glucose intolerance in South Asians.35 Furthermore, when compared to Caucasians, South Asians demonstrate significantly lower glucose disposal while also exhibiting significantly greater total abdominal and visceral fat.33 Recent studies have shown that even in nondiabetic South Asians, greater visceral fat is associated with increased insulin resistance.36 However, It is possible that gender differences may exist regarding body fat distribution and insulin resistance. A recent study comparing young South Asian and Caucasian women matched for BMI noted no differences in body fat distribution and insulin sensitivity between groups.37 While this study introduced novel findings, the sample size was fairly small, and all participants were healthy, young (mean age 24), and premenopausal. Therefore, generalizations cannot be made about women of all ages and BMI categories, and further studies exclusive to South Asian women are warranted.
In addition to an increased propensity for insulin resistance, South Asians may also experience early declines in β-cell function, compared with other ethnic groups. A study examining both insulin resistance and β-cell function in a group of East Asians, South Asians, Blacks, and Caucasians found that despite being matched on lifestyle factors and BMI the prevalence of insulin resistance in South Asian men was threefold to fourfold greater than lean men of other ethnic groups.38 Upon assessment of β-cell function in a subgroup of South Asian and Caucasian men it was observed that South Asians had a 30% increase in basal β-cell responsiveness; however, this increase in β-cell function was not sufficient to compensate for the degree of insulin resistance, as shown by a 60% reduction in disposition index (a measure of β-cell response to insulin resistance), in South Asian men.38 Additionally, a prospective study of Asian Indians in South Africa with impaired glucose tolerance (IGT) reported that, compared with controls, participants with IGT exhibited delayed insulin responses despite similar plasma glucose levels,24 indicating that early β-cell dysfunction is an underlying pathophysiological abnormality of impaired glucose tolerance in this population. Therefore, while it is apparent that South Asians have a higher degree of insulin resistance than do members of other ethnic groups, an early impairment in β-cell function could also be a key pathophysiological mechanism in T2DM development in South Asians.
It has been proposed that early impairment in β-cell function could be the result of intrauterine under nutrition that leads to abnormal pancreatic development.39 However, data supporting this hypothesis are inconclusive. A study comparing insulin secretion in 12 insulin-resistant adults who were born with intrauterine growth restriction (IUGR) to 13 controls adults failed to observe any evidence suggesting a defect in insulin secretion in young adults born with IUGR.40 Similarly, a study of 8-year-old Indian children noted that lower birth weight was associated with increased insulin resistance but it was not related to reduced β-cell function.41 However, a study of middle aged Danes found that participants with low birth weight had elevated concentrations of post-load serum insulin, and after adjustment for insulin sensitivity, also exhibited a reduction in β-cell function.42 Therefore, it is possible that intrauterine undernutrition may indeed lead to impairments in pancreatic development, and subsequent β-cell dysfunction, which could be exacerbated by insulin resistance. This could have severe implications for T2DM development in South Asians due to the high prevalence of low birth weight babies born in the region. It is also possible that impaired β-cell function is the result of genetics, epigenetics, or endocrine disruptors, such as pollution. However, the complete mechanistic pathway determining T2DM in this population remains to be elucidated and further studies are warranted.
Genetics
Genome-wide association (GWA) studies have identified approximately 60 genes associated with T2DM risk.43,44 However, most of these studies have been conducted in European populations,43,44 and few studies have attempted to replicate the findings of GWA studies in South Asians.37 Two recent replication studies in South Asians determined that common type 2 diabetes variants previously found in European populations, such as PPARG, TCF7L2, FTO, and CDKN2A, are also associated with T2DM in South Asians.45,46 However, it appears that factors that mediate genetic effects, allele frequencies, or varying polymorphisms may differ between groups for at least some of these genes. The FTO gene, for example, is associated with T2DM in both Europeans and South Asians.47–50 In Europeans the association is entirely mediated by BMI.20 However, in South Asians, the associations among T2DM, FTO, and obesity are inconsistent. While some studies have noted associations between T2DM and FTO that were mediated by obesity,49 others have reported associations with FTO variants that were not entirely mediated by BMI, central adiposity, or obesity.47,48 Furthermore, a recent study from North India reported FTO associations with obesity in South Asians, yet none of the variants tested in this population were associated with T2DM.51 The differences in associations among FTO, T2DM, and obesity between South Asians and Europeans suggest ethnic differences in the mechanisms of association between FTO genes and T2DM development. However, there is also considerable heterogeneity as measured by associations among different South Asian populations, which calls for further large scale studies on South Asians of varying cultural, geographic, and economic backgrounds.
Another possibility is that varying genetic polymorphisms could contribute to differences in T2DM risk. Adiponectin is a protein associated with glucose modulation,52 and studies have noted lower levels of circulating plasma adiponectin in South Asians, compared with Caucasians.52 This difference could be, in part, attributed to polymorphisms in the adiponectin gene, as a study comparing 2,000 normal glucose tolerant patients to 2,000 patients with T2DM reported that a polymorphism of the adiponectin gene is associated with T2DM and obesity in South Asians.53 However, this association has yet to be tested in other populations, and thus the functional significance remains unknown.
GWA studies targeting South Asian populations may provide valuable information about novel genetic associations underlying T2DM risk. Recently, a GWA study of T2DM carried out in South Asians found 20 independent single nucleotide polymorphisms associated with T2DM, and common genetic variants were identified at six new loci associated with T2DM in South Asians.43 The results of this study indicate that additional genetic associations with T2DM can be made by studying populations of non-European ancestry.
Age
It is a widely held belief that T2DM onset occurs at a much earlier age in South Asians than in other ethnic populations.54 A national survey conducted in India noted that the onset of diabetes occurred before age 50 in 54.1% of cases.55 In contrast, in the United States, only 37.6% of cases occurred before age 50.56 A longitudinal study of diabetes incidence in Ontario, Canada reported that the median age at diagnosis was lowest among South Asians (49 years), followed by Chinese (55 years), Blacks (57 years), and Whites (58 years).57 However, to date there have been no studies directly comparing the age-specific prevalence of T2DM in South Asians living in their home countries to members of other ethnic groups living in the United States or in Europe. There is also a lack of information regarding differences in age-specific prevalence between South Asians living in South Asia and those who have migrated to other countries. Such studies may be able to elucidate whether South Asians are at increased innate susceptibility or whether environmental factors related to migration and nutrition transitions are more at play.
Anthropometry
While South Asians have lower rates of obesity compared with other ethnic groups, as defined by BMI, they tend to have larger waist measurements and waist to hip ratios, and therefore a greater degree of central obesity. This is seen at any given level of BMI and has been associated with higher plasma insulin levels, increased insulin resistance, and a higher prevalence of T2DM.58 A study comparing 12 South Asians and 12 Caucasians matched for age and BMI noted that, compared with Caucasians, South Asians had significantly greater total abdominal and visceral fat.33 These results are similar to a previous study comparing South Asian and Caucasian men. Again, despite similar total body fat content, South Asians had greater abdominal adiposity than did Caucasians.59 South Asians also exhibit greater abdominal fat as compared to other high-risk ethnic groups. A cross-sectional study examining body size, body composition, and fat distribution among European, Maori, Pacific Island, and South Asian adults reported that South Asians had more total fat, both overall and in the abdominal region, compared with Europeans and Pacific Islanders.60
Such differences in body fat composition are seen even in childhood and adolescence. Cross-sectional studies have shown that South Asian children have significantly more body fat than European children, with a greater degree of central fat.61 Furthermore, despite having lower birth weight, South Asian babies have more central adiposity than their Caucasian counterparts.62,63 This predisposition may, in part, be attributed to epigenetic changes in gene expression, resulting from undernutrition related stressors during intrauterine development.64 On a global scale, the prevalence of low birth weight is highest in the South Asian region, with India accounting for nearly 40% of global low-birth-weight infants.65 Maternal undernutrition during pregnancy is a known cause of low birth weight,66 and it is possible that such fetal undernutrition may increase the risk of T2DM development later in life. Prospective studies from India have shown that participants who were underweight as children had a high prevalence of overweight, obesity, and central obesity as young adults.67 Additional studies from India have demonstrated that participants with impaired glucose tolerance or T2DM in adulthood had low body mass indices before the age of two, followed by a subsequent early adiposity rebound and then an accelerated increase in BMI through adulthood.68 Such studies lend support to the notion that fetal programming in utero can promote fat preservation. Furthermore, exposure to excess caloric energy later in life can lead to metabolic imbalances, thus increasing the risk for T2DM in adulthood, as several studies have noted that participants who were small at birth and became obese in childhood have and increased risk of developing insulin resistance.69 It is possible that small size at birth can also be predictive of future diabetes development in parents of low-birth-weight babies, as a recent study from Mysore, India noted a linear inverse association between birth weight with both maternal and paternal diabetes development at nine years of follow-up,70 thereby indicating possible genetic or epigenetic associations between parental diabetes risk and reduced fetal growth in their offspring.
Biomarkers
Several biomarkers, such as leptin, adiponectin, C-reactive protein (CRP), and markers of oxidative stress may indicate processes that may influence the increased prevalence of T2DM in South Asians compared to other ethnic groups. Oxidative stress resulting from an increased content of reactive oxygen species (ROS) has been implicated in the parthenogenesis of T2DM development.71,72 While no studies have been conducted to assess levels of oxidative stress biomarkers in South Asians, it has been shown that oxidative stress has been linked to higher levels of visceral fat, which could place South Asians at an increased risk compared to other ethnic groups.73
Adiponectin and leptin are proteins linked with T2DM development. Circulating adiponectin concentrations are decreased in those with T2DM, while leptin levels increase with increasing BMI.51,74 Associations among adiponectin, leptin and T2DM have been found in South Asians,51 and recent studies have demonstrated decreased levels of adiponectin and increased levels of leptin in South Asians compared to Caucasians, despite there being no differences in BMI, waist circumference, or hip circumference.75–77 Such results highlight the possibility of defects in adipose tissue metabolism in South Asians that extend beyond elevated total abdominal fat.
CRP is a marker of inflammation that is associated with T2DM development in both Western and South Asian populations.78,79 A study comparing healthy South Asian and European men and women in West London, United Kingdom reported that CRP levels in South Asian women were nearly double that of European women.78 A similar study found CRP levels to be 17% higher in healthy South Asian men than in European Caucasians and were associated with greater levels of insulin resistance and central adiposity.79 A study from the United States compared CRP levels in 82 South Asian and 55 Caucasian males and reported that on average, Asian Indians had significantly higher plasma CRP levels than did Caucasians.80 This difference became even more significant after adjustment for total body fat and waist circumference and suggests an underlying proinflamatory state in South Asians, which could be another important contributing factor toward increased T2DM risk.
Environmental risk factors for T2DM
Lifestyle behaviors
In recent decades important demographic and environmental shifts have occurred in South Asia. Life expectancy is increasing while birth rates are declining, resulting in a significant proportion of older individuals.81 Additionally, the individual income and per capita expenditure have risen, as has migration from villages to cities.81 All of these factors have contributed toward lifestyle changes that now include physical inactivity, a shift away from traditional dietary habits, higher rates of tobacco use, and less sleep.
Physical inactivity
It is well established that reductions in physical activity increase the risk of T2DM in all ethnic groups.82–84 While physical activity levels do not meet recommended guidelines in either Caucasians or South Asians, South Asians appear to be even less physically active than their Caucasian counterparts.85,86 A systematic review of studies describing levels of physical activity and fitness in UK South Asians identified 12 studies examining physical activity in adults. The differences in physical activity levels in South Asians, compared with general the population, were substantial; South Asian groups reported physical activity levels that were 50–75% lower than those of Europeans.87 These results are similar to those from a study analyzing data from the Newcastle Heart Project, indicating that 52% of European men did not meet current guidelines for physical activity, compared with 71% of Asian Indians, 88% of Pakistanis, and 87% of Bangladeshis.88
Dietary changes
In addition to lower physical activity levels, higher intakes of refined carbohydrates and saturated and trans fats have been shown to increase T2DM risk by adversely affecting glucose metabolism and insulin resistance. In contrast, low glycemic index foods and foods high in dietary fiber have been shown to reduce glycemic and insulinemic responses, thereby reducing the risk of T2DM.89,90 In South India, intake of polished white rice, with a high glycemic index, have been linked to T2DM prevalence after adjusting for potential confounders.90 The typical South Asian diet is high in carbohydrates, trans fats, and saturated fat.91 A comparative study reported higher overall caloric intake, as well as a greater percentage of carbohydrate content in a typical South Asian meal, compared with standard European meals.92 Over the past several decade there have been increases in consumption of animal products, sugars, and fats in South Asian diets.93 Furthermore, among those who have migrated to Western countries, acculturation has led to a more frequent selection of nontraditional foods.94 Specifically, consumption of margarine, juice, chips, colas, alcohol, and fast food has been shown to increase upon migration to Western countries, while consumption of fruits, vegetables, and fiber has been shown to decrease.95,96 A recent study from Norway reported that after migration, Pakistanis and Sri Lankans increased their consumption of oil, meat, and dairy products, while decreasing their consumption of beans and lentils.95 It is also possible that differences in micronutrient intakes could contribute to varying risk in T2DM development. Several micronutrients such as magnesium, calcium, vitamin C, and folate have been thought to play a beneficial role in glucose homeostasis.97,98 A study from the UK comparing the dietary intakes South Asian, black African-Caribbean, and white European children reported higher total energy intake among South Asian children compared with white Europeans, but lower intake of vitamin C, D, calcium, and iron.98 A study assessing dietary intake of Asian Indian adults living in the United States reported insufficient intake of magnesium in males and insufficient intake of calcium in females.99 The long-term implications of micronutrient deficiencies in relationship to T2DM in South Asians, compared with other ethnic groups, remains to be elucidated and further studies are required.
Tobacco use
Several studies have established the association between cigarette smoking and increased risk for T2DM.100–102 The association between smokeless tobacco and diabetes risk is less well established.103 While tobacco use is a global epidemic, developing countries account for a disproportionate amount of worldwide tobacco consumption.104 Recent estimates report that 47% of men over the age of 15 and 14% of women in India are tobacco users,105 with a large percentage of users consuming smokeless tobacco products.106 For men, the prevalence of tobacco use in India is nearly double that in the United States.107 Therefore, comparative studies assessing smoking prevalence and associated T2DM risk across ethnic populations could help determine if excess T2DM risk in South Asians is in part due to greater exposure to tobacco, or if other factors weigh more heavily.
Sleep duration
Duration of sleep, both above and below average, has been shown to play a role in T2DM risk. Studies have noted that sleep restriction to only four hours caused decreases in the appetite suppressing hormone leptin and increases in the appetite inducing hormone ghrelin.108 In a study of 900 individuals without diabetes followed for up to five years, 146 participants developed incident T2DM. Sleep duration of less than seven hours was a significant predictor of T2DM in Caucasians and Hispanics, even after adjusting for insulin sensitivity and insulin resistance.109 In addition to duration, disturbances in sleep have also been shown to be associated with diabetes incidence. A prospective, population based study from Sweden followed nondiabetic participants for approximately 15 years. Study results indicate that difficulties falling asleep or regular use of hypnotics were associated with an increased risk of developing diabetes.110 While there have been no studies directly comparing sleep duration or disturbances and diabetes incidence in South Asians and their Western counterparts, a cross-sectional study from India noted a high prevalence of snoring (40%) and daytime sleepiness (59%) in a normal weight urban South Indian population,111 both of which showed a significant positive relationship with impaired glucose metabolism. Furthermore, a study examining the associations between sleep apnea and risk factors for metabolic syndrome in North India suggested that obstructive sleep apnea was independently positively associated with fasting insulin levels.112 Such associations could be due to neuro–endocrine–metabolic associations related to sleep apnea that also might contribute to the development of T2DM.113 Therefore, while it is possible that decreased or increased sleep duration as well as sleep disturbances increase T2DM risk in all ethnic populations, comparative studies are needed in order to determine if sleep duration or disturbance contributes to excess risk for T2DM in South Asians as compared to other ethnic groups.
Environmental pollutants
Persistent organic pollutants (POPs) encompass a variety of man-made chemicals and are of recent concern with regard to T2DM risk. Several studies in the United States and Europe have noted associations between POP exposure and type 2 diabetes.114 A cross-sectional study using U.S. National Health and Examination Survey data reported strong associations between insulin resistance and serum concentrations of POPs.115 The findings of this study noted that the association between obesity and diabetes was diminished in people with low POP concentrations. However, the association between obesity and diabetes was strengthened as POP levels in the blood increased. To our knowledge, there have been no studies examining the relationship between POP exposure and diabetes risk in South Asian populations. However, there have been studies noting detectable levels of POP in mussels collected from the coastal waters of India.116 High levels of POPs have also been observed in Indian municipal dumping sites.117 Given the already increased risk for T2DM in South Asians, it may be useful to examine the association between POP exposure and insulin resistance in cross-sectional and prospective studies conducted on South Asian populations.
Conclusion
Current evidence suggests that the prevalence of T2DM in South Asians is high and rising both in South Asian countries, as well as in the diaspora. These increases are due, in part, to higher T2DM incidence rates in South Asians compared with Caucasians, which suggests an increased propensity for South Asians to develop the disease. This notion is highlighted by evidence indicating that South Asians (1) are more insulin resistant than Caucasians even at similar levels of BMI and total body fat percent, (2) demonstrate early impairments in β-cell function, (3) exhibit greater tendencies toward visceral fat deposition, even as neonates, and (4) have lower levels of circulating plasma adiponectin and higher levels of plasma leptin.
In addition to possible innate predisposition, South Asians are currently experiencing changes in lifestyle behaviors due to migration or nutritional transitions, resulting in physical inactivity and a shift away from traditional dietary habits to those that include greater overall carbohydrates, saturated, and trans fats and lower amounts of dietary fiber. Coupled with an increased propensity for T2DM, the recent shifts in lifestyle behaviors only serve to exacerbate the risk for disease. Future research is needed regarding the etiology and pathophysiology of disease in South Asians, compared with other ethnic groups. Attention should be paid to the complete mechanistic pathway and the relative contributions of both insulin resistance and β-cell function, mechanisms related to early T2DM onset, and variations in genetic polymorphisms and epigenetic processes. Studies focusing on the contributions of tobacco use, sleep duration, and environmental pollutants are also warranted. Furthermore, there is a great need for primary prevention. This need will persist, especially as South Asians continue to become more affluent, have greater access to high-fat foods, adopt more sedentary lifestyles, experience a growing population of aging individuals, and migrate to diaspora countries. Evidence indicates that lifestyle interventions, including increases in physical activity and improvements in dietary quality, are effective at preventing or delaying the development of T2DM in high-risk groups.118 While those with polycystic ovarian syndrome, gestational diabetes mellitus, and stress-induced hyperglycemia are all considered at high risk for developing T2DM, evidence suggests that the most cost-effective method for T2DM prevention is to target individuals with prediabetes (fasting glucose between 100 and 125 mg/dL or 2-h post-challenge glucose between 140 and 199 mg/dL).119 Results from a population based cohort study indicated that while individuals with prediabetes accounted for 16% of the population, they contribute to over 60% of incident T2DM cases, thereby accounting for a significant proportion of those at high risk.120 Two randomized trials in persons with impaired glucose tolerance, The Finish Diabetes Prevention Study and the US Diabetes Prevention Program (DPP), demonstrated that the three year risk of developing T2DM was reduced by 58% in those receiving intensive lifestyle interventions.121,122 Components in such interventions included lessons on behavior change, physical activity requirement of at least 150 minutes per week, well-balanced diets rich in whole grains, fruit and vegetables, with <30% total fat and no more than 10% saturated fat, and weight loss of at least 5–7%. Randomized controlled trials are now taking place to assess the effectiveness, cost-effectiveness, generalizability, and sustainability of such interventions in South Asians.123 If shown to be effective, such preventative efforts need to be directed both at individual and population levels, should be culturally sensitive and aggressive, and should start early in order to reduce the risk of T2DM in this highly susceptible population.
Acknowledgments
We acknowledge NIH Grant 5T32DK007298-33 and NHLBI Grant HHSN2682009900026C for support. We thank Jessica Marcinkevage for her thoughtful review of the manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Whiting DR, et al. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes. Res. Clin. Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Mohan V. Why are Indians more prone to diabetes. J. Assoc. Physicians India. 2004;52:468–474. [PubMed] [Google Scholar]
- 3.International Diabetes Federation. IDF Diabetes Atlas. 5th ed. Brussels, Belgium: International Diabetes Federation; 2011. 2011. [PubMed] [Google Scholar]
- 4.Hwang CK, et al. Rural diabetes prevalence quintuples over twenty-five years in low-and middle-income countries: a systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2012;96:271–285. doi: 10.1016/j.diabres.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Anjana RM, et al. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: phase I results of the Indian Council of Medical Research-INdiaDIABetes (ICMR-INDIAB) study. Diabetologia. 2011;54:3022–3027. doi: 10.1007/s00125-011-2291-5. [DOI] [PubMed] [Google Scholar]
- 6.Gupta L, et al. Prevalence of Diabetes in New York City, 2002–2008: comparing foreign-born South Asians and other Asians with U.S.-born whites, blacks, and Hispanics. Diab. Care. 2011;34:1791–1793. doi: 10.2337/dc11-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oza-Frank R, et al. Asian Americans: diabetes prevalence across U.S. and World Health Organization weight classifications. Diab. Care. 2009:1644–1646. doi: 10.2337/dc09-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Misra R, et al. Prevalence of diabetes, metabolic syndrome, and cardiovascular risk factors in US Asian Indians: results from a national study. J. Diab. Complicat. 2010;24:145–153. doi: 10.1016/j.jdiacomp.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Mather HM, Keen H. The Southall Diabetes Survey: prevalence of known diabetes in Asians and Europeans. Br. Med. J. (Clin. Res. Ed.) 1985;291:1081–1084. doi: 10.1136/bmj.291.6502.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zimmet P, et al. Prevalence of diabetes and impaired glucose tolerance in the biracial (Melanesian and Indian) population of Fiji: a rural-urban comparison. Am. J. Epidemiol. 1983;118:673–688. doi: 10.1093/oxfordjournals.aje.a113678. [DOI] [PubMed] [Google Scholar]
- 11.Omar MA, et al. South African Indians show a high prevalence of NIDDM and bimodality in plasma glucose distribution patterns. Diab. Care. 1994;17:70–73. doi: 10.2337/diacare.17.1.70. [DOI] [PubMed] [Google Scholar]
- 12.Seedat YK, et al. Risk factors for coronary heart disease in the white community of Durban. South Afr. Med. J. 1994;84:257–262. [PubMed] [Google Scholar]
- 13.Jenum AK, et al. Ethnicity and sex are strong determinants of diabetes in an urban Western society: implications for prevention. Diabetologia. 2005;48:435–439. doi: 10.1007/s00125-005-1668-8. [DOI] [PubMed] [Google Scholar]
- 14.Lee WR. The changing demography of diabetes mellitus in Singapore. Diab. Res. Clin. Pract. 2000;50(Suppl 2):S35–S39. doi: 10.1016/s0168-8227(00)00184-4. [DOI] [PubMed] [Google Scholar]
- 15.Mohan V, et al. Incidence of diabetes and pre-diabetes in a selected urban south Indian population (CUPS-19) J. Assoc. Physicians India. 2008;56:152–157. [PubMed] [Google Scholar]
- 16.Geiss L, et al. Changes in incidence of diabetes in U.S. adults, 1997–2003. Am. J. Prev. Med. 2006;30:371–377. doi: 10.1016/j.amepre.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Bonora E, et al. Population-based incidence rates and risk factors for type 2 diabetes in white individuals: the Bruneck study. Diabetes. 2004;53:1782–1789. doi: 10.2337/diabetes.53.7.1782. [DOI] [PubMed] [Google Scholar]
- 18.Valdes S, et al. Population-based incidence of type 2 diabetes in northern Spain: the Asturias Study. Diab. Care. 2007;30:2258–2263. doi: 10.2337/dc06-2461. [DOI] [PubMed] [Google Scholar]
- 19.Kasuga M. Insulin resistance and pancreatic beta cell failure. J. Clin. Invest. 2006;116:1756–1760. doi: 10.1172/JCI29189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahn SE, et al. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia. 2003;46:3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 21.Saad MF, et al. A two-step model for development of non-insulin-dependent diabetes. Am. J. Med. 1991;90:229–235. [PubMed] [Google Scholar]
- 22.Cnop M, et al. Progressive loss of beta-cell function leads to worsening glucose tolerance in first degree relatives of subjects with type 2 diabetes. Diabet. Care. 2007;30:677–682. doi: 10.2337/dc06-1834. [DOI] [PubMed] [Google Scholar]
- 23.Cali AM, et al. Primary defects in beta-cell function further exacerbated by worsening of insulin resistance mark the development of impaired glucose tolerance in obese adolescents. Diabet. Care. 2009;32:456–461. doi: 10.2337/dc08-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Motala AA, et al. Evidence for impaired pancreatic beta cell function in South African Indians with impaired glucose tolerance. Diab. Med. 1994;11:437–444. doi: 10.1111/j.1464-5491.1994.tb00303.x. [DOI] [PubMed] [Google Scholar]
- 25.Kahn SE. Clinical review 135: the importance of β-cell failure in the development and progression of type 2 diabetes. J. Clin. Endocrinol. Metabol. 2001;86:4047–4058. doi: 10.1210/jcem.86.9.7713. [DOI] [PubMed] [Google Scholar]
- 26.Polonsky K, et al. Seminars in Medicine of the Beth Israel Hospital, Boston: non-Insulin-Dependent Diabetes Mellitus—A Genetically Programmed Failure of the Beta Cell to Compensate for Insulin Resistance. N. Engl. J. Med. 1996;334:777–783. doi: 10.1056/NEJM199603213341207. [DOI] [PubMed] [Google Scholar]
- 27.Torrens JI, et al. Ethnic differences in insulin sensitivity and beta-cell function in premenopausal or early perimenopausal women without diabetes: the Study of Women's Health Across the Nation (SWAN) Diab. Care. 2004;27:354–361. doi: 10.2337/diacare.27.2.354. [DOI] [PubMed] [Google Scholar]
- 28.Bi Y, et al. Association of β-cell function and insulin sensitivity with fasting and 2-h plasma glucose in a large Chinese population. Diab. Obesity Metabol. 2012;14:174–180. doi: 10.1111/j.1463-1326.2011.01504.x. [DOI] [PubMed] [Google Scholar]
- 29.Abdul-Ghani MA, et al. The relative contributions of insulin resistance and beta cell failure to the transition from normal to impaired glucose tolerance varies in different ethnic groups. Diab. Met. Syn. Res. Rev. 2007;1:105–112. [Google Scholar]
- 30.Chiu KC, et al. Insulin sensitivity differs among ethnic groups with a compensatory response in beta-cell function. Diab. Care. 2000;23:1353–1358. doi: 10.2337/diacare.23.9.1353. [DOI] [PubMed] [Google Scholar]
- 31.Mohan V, et al. Serum immunoreactive insulin responses to a glucose load in Asian Indian and European Type 2 (non-insulin dependent) diabetic patients and control subjects. Diabetologia. 1986;29:235–237. doi: 10.1007/BF00454882. [DOI] [PubMed] [Google Scholar]
- 32.Sharp PS, et al. Insulin resistance in patients of Asian Indian and European origin with non-insulin dependent diabetes. Hormone Metabol. Res. 1987;19:84–85. doi: 10.1055/s-2007-1011745. [DOI] [PubMed] [Google Scholar]
- 33.Raji A, et al. Body fat distribution and insulin resistance in healthy Asian Indians and Caucasians. J. Clin. Endocrinol. Metabol. 2001;86:5366–5371. doi: 10.1210/jcem.86.11.7992. [DOI] [PubMed] [Google Scholar]
- 34.Banerji MA, et al. Body composition, visceral fat, leptin, and insulin resistance in Asian Indian men. J. Clin. Endocrinol. Metabol. 1999;84:137–144. doi: 10.1210/jcem.84.1.5371. [DOI] [PubMed] [Google Scholar]
- 35.Indulekha K, et al. Association of visceral and subcutaneous fat with glucose intolerance, insulin resistance, adipocytokines and inflammatory markers in Asian Indians (CURES-113) Clin. Biochem. 2011;44:281–287. doi: 10.1016/j.clinbiochem.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 36.Sandeep S, et al. Visceral & subcutaneous abdominal fat in relation to insulin resistance & metabolic syndrome in non-diabetic south Indians. Indian J. Med. Res. 2010;131:629–635. [PubMed] [Google Scholar]
- 37.Szuszkiewicz-Garcia M, et al. Fat distribution and insulin resistance in young adult nonobese Asian Indian women. Metabol. Syndrome Related Disorders. 2012;10:326–330. doi: 10.1089/met.2012.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petersen KF, et al. Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. Proc. Nat. Acad. Sci. USA. 2006;103:18273–18277. doi: 10.1073/pnas.0608537103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hales CN, et al. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303:1019–1022. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaquet D, et al. No evidence for a major beta-cell dysfunction in young adults born with intra-uterine growth retardation. Pediatric Diab. 2000;1:181–185. doi: 10.1046/j.1399543x.2000.010402.x. [DOI] [PubMed] [Google Scholar]
- 41.Bavdekar A, et al. Insulin resistance syndrome in 8-year-old Indian children: small at birth, big at 8 years, or both. Diabetes. 1999;48:2422–2429. doi: 10.2337/diabetes.48.12.2422. [DOI] [PubMed] [Google Scholar]
- 42.Pilgaard K, et al. Low birthweight and premature birth are both associated with type 2 diabetes in a random sample of middle-aged Danes. Diabetologia. 2010;53:2526–2530. doi: 10.1007/s00125-010-1917-3. [DOI] [PubMed] [Google Scholar]
- 43.Kooner JS, et al. Genome-wide association study in individuals of South Asian ancestry identifies six new type 2 diabetes susceptibility loci. Nature Genet. 2011;43:984–989. doi: 10.1038/ng.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cho YS, et al. Meta-analysis of genome-wide association studies identifies eight new loci for type 2 diabetes in east Asians. Nat. Genet. 2012;44:67–72. doi: 10.1038/ng.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rees SD, et al. Replication of 13 genome-wide association (GWA)-validated risk variants for type 2 diabetes in Pakistani populations. Diabetologia. 2011;54:1368–1374. doi: 10.1007/s00125-011-2063-2. [DOI] [PubMed] [Google Scholar]
- 46.Chauhan G, et al. Impact of common variants of PPARG, KCNJ11, TCF7L2, SLC30A8, HHEX, CDKN2A, IGF2BP2, and CDKAL1 on the risk of type 2 diabetes in 5,164 Indians. Diabetes. 2010;59:2068–2074. doi: 10.2337/db09-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yajnik CS, et al. FTO gene variants are strongly associated with type 2 diabetes in South Asian Indians. Diabetologia. 2009;52:247–252. doi: 10.1007/s00125-008-1186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanghera DK, et al. Impact of nine common type 2 diabetes risk polymorphisms in Asian Indian Sikhs: PPARG2 (Pro12Ala), IGF2BP2, TCF7L2 and FTO variants confer a significant risk. BMC Med. Genet. 2008;9:59. doi: 10.1186/1471-2350-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramya K, et al. Genetic variations in the FTO gene are associated with type 2 diabetes and obesity in south Indians (CURES-79) Diab. Technol. Therap. 2011;13:33–42. doi: 10.1089/dia.2010.0071. [DOI] [PubMed] [Google Scholar]
- 50.Frayling TM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chauhan G, et al. Common variants of FTO and the risk of obesity and type 2 diabetes in Indians. J. Hum. Genet. 2011;56:720–726. doi: 10.1038/jhg.2011.87. [DOI] [PubMed] [Google Scholar]
- 52.Wasim H, et al. Relationship of serum adiponectin and resistin to glucose intolerance and fat topography in South-Asians. Cardiovasc. Diabetol. 2006;5:10. doi: 10.1186/1475-2840-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vimaleswaran KS, et al. Peroxisome proliferators-activated receptor-co-activator-1 (PGC-1) gene polymorphisms and their relationship to Type 2 diabetes in Asian Indians. (CURES-14) Diab. Med. 2005;22:1516–1521. doi: 10.1111/j.1464-5491.2005.01709.x. [DOI] [PubMed] [Google Scholar]
- 54.Qiao Q, et al. Age- and sex-specific prevalence of diabetes and impaired glucose regulation in 11 Asian cohorts. Diab. Care. 2003;26:1770–1780. doi: 10.2337/diacare.26.6.1770. [DOI] [PubMed] [Google Scholar]
- 55.Ramachandran A, et al. High prevalence of diabetes and impaired glucose tolerance in India: National Urban Diabetes Survey. Diabetologia. 2001;44:1094–1101. doi: 10.1007/s001250100627. [DOI] [PubMed] [Google Scholar]
- 56.Center for Disease Control and Prevention. Diabetes Data & Trends. Available at http://www.cdc.gov/diabetes/statistics/incidence_national.htm. Accessed on June 1, 2012.
- 57.Chiu M, et al. Deriving ethnic-specific BMI cutoff points for assessing diabetes risk. Diab. Care. 2011;34:1741–1748. doi: 10.2337/dc10-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mohan V, et al. Epidemiology of type 2 diabetes: Indian scenario. Indian J. Med. Res. 2007;125:217–230. [PubMed] [Google Scholar]
- 59.Chandalia M, et al. Relationship between generalized and upper body obesity to insulin resistance in Asian Indian men. J. Clin. Endocrinol. Metabol. 1999;84:2329–2335. doi: 10.1210/jcem.84.7.5817. [DOI] [PubMed] [Google Scholar]
- 60.Rush EC, et al. Body size, body composition and fat distribution: comparative analysis of European, Maori, Pacific Island and Asian Indian adults. Br. J. Nutr. 2009;102:632–641. doi: 10.1017/S0007114508207221. [DOI] [PubMed] [Google Scholar]
- 61.Shaw NJ, et al. Ethnic and gender differences in body fat in British school children as measured by DXA. Arch. Dis. Child. 2007;92:872–875. doi: 10.1136/adc.2007.117911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krishnaveni GV, et al. Truncal adiposity is present at birth and in early childhood in South Indian children. Indian Pediatr. 2005;42:527–538. [PubMed] [Google Scholar]
- 63.Yajnik CS, et al. Neonatal anthropometry: the thin-fat Indian baby. The Pune Maternal Nutrition Study. Int. J. Obes. Relat. Metabol. Disord. 2003;27:173–180. doi: 10.1038/sj.ijo.802219. [DOI] [PubMed] [Google Scholar]
- 64.Chan J, et al. Diabetes in Asia: epidemiology, Risk Factors, and Pathophysiology. JAMA. 2009;301:2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 65.Ramachandran P. Nutrition and child survival in India. Indian. J. Pediatr. 2010;77:301–305. doi: 10.1007/s12098-010-0038-9. [DOI] [PubMed] [Google Scholar]
- 66.Ramakrishnan U. Nutrition and low birth weight: from research to practice. Am. J. Clin. Nutr. 2004;79:17–21. doi: 10.1093/ajcn/79.1.17. [DOI] [PubMed] [Google Scholar]
- 67.Sachdev HS, et al. Anthropometric indicators of body composition in young adults: relation to size at birth and serial measurements of body mass index in childhood in the New Delhi birth cohort. Am. J. Clin. Nutr. 2005;82:456–466. doi: 10.1093/ajcn.82.2.456. [DOI] [PubMed] [Google Scholar]
- 68.Bhargava SK, et al. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. New England J. Med. 2004;350:865–875. doi: 10.1056/NEJMoa035698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Levy-Marchal C, Jaquet D. Long-term metabolic consequences of being born small for gestational age. Pediatric Diab. 2004;5:147–153. doi: 10.1111/j.1399-543X.2004.00057.x. [DOI] [PubMed] [Google Scholar]
- 70.Veena SR, et al. Newborn size and body composition as predictors of insulin resistance and diabetes in the parents: Parthenon Birth Cohort Study, Mysore, India. Diab. Care. 2012;35:1884–1890. doi: 10.2337/dc12-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maritim AC, et al. Diabetes, oxidative stress, and antioxidants: a review. J. Biochem. Mol. Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 72.Evans JL, et al. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction. Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- 73.Pou KM, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 74.Indulekha K, et al. Association of visceral and subcutaneous fat with glucose intolerance, insulin resistance, adipocytokines and inflammatory markers in Asian Indians (CURES-113) Clin. Biochem. 2011;44:281–287. doi: 10.1016/j.clinbiochem.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 75.Mohan V, et al. Association of C-reactive protein with body fat, diabetes and coronary artery disease in Asian Indians: the Chennai Urban Rural Epidemiology Study (CURES-6) Diab. Med. 2005;22:863–870. doi: 10.1111/j.1464-5491.2005.01541.x. [DOI] [PubMed] [Google Scholar]
- 76.Pradhan AD, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 77.Abate N, et al. Adipose tissue metabolites and insulin resistance in nondiabetic Asian Indian men. J. Clin. Endocrinol. Metabol. 2004;89:2750–2755. doi: 10.1210/jc.2003-031843. [DOI] [PubMed] [Google Scholar]
- 78.Forouhi NG, et al. Relation of C-reactive protein to body fat distribution and features of the metabolic syndrome in Europeans and South Asians. Int. J. Obes. Relat. Metabol. Disord. 2001;25:1327–1331. doi: 10.1038/sj.ijo.0801723. [DOI] [PubMed] [Google Scholar]
- 79.Chambers JC, et al. C-reactive protein, insulin resistance, central obesity, and coronary heart disease risk in Indian Asians from the United Kingdom compared with European whites. Circulation. 2001;104:145–150. doi: 10.1161/01.cir.104.2.145. [DOI] [PubMed] [Google Scholar]
- 80.Chandalia M, et al. Elevated plasma high-sensitivity C-reactive protein concentrations in Asian Indians living in the United States. J. Clin. Endocrinol. Metabol. 2003;88:3773–3776. doi: 10.1210/jc.2003-030301. [DOI] [PubMed] [Google Scholar]
- 81.Shetty PS. Nutrition transition in India. Public Health Nutr. 2002;5:175–182. doi: 10.1079/PHN2001291. [DOI] [PubMed] [Google Scholar]
- 82.Helmrich SP, et al. Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. New England J. Med. 1991;325:147–152. doi: 10.1056/NEJM199107183250302. [DOI] [PubMed] [Google Scholar]
- 83.Laaksonen DE, et al. Physical activity in the prevention of type 2 diabetes: the Finnish diabetes prevention study. Diabetes. 2005;54:158–165. doi: 10.2337/diabetes.54.1.158. [DOI] [PubMed] [Google Scholar]
- 84.Mohan V, et al. Association of physical inactivity with components of metabolic syndrome and coronary artery disease—the Chennai Urban Population Study (CUPS no. 15) Diabet. Med. 2005;22:1206–1211. doi: 10.1111/j.1464-5491.2005.01616.x. [DOI] [PubMed] [Google Scholar]
- 85.Williams ED, et al. Assessment of physical activity levels in South Asians in the UK: findings from the Health Survey for England. J. Epidemiol. Commun. Health. 2011;65:517–521. doi: 10.1136/jech.2009.102509. [DOI] [PubMed] [Google Scholar]
- 86.Lip GY, et al. Ethnic differences in public health awareness, health perceptions and physical exercise: implications for heart disease prevention. Ethn. Health. 1996;1:47–53. doi: 10.1080/13557858.1996.9961769. [DOI] [PubMed] [Google Scholar]
- 87.Fischbacher CM, et al. How physically active are South Asians in the United Kingdom? A literature review. J. Public Health (Oxf.) 2004;26:250–258. doi: 10.1093/pubmed/fdh158. [DOI] [PubMed] [Google Scholar]
- 88.Hayes L, et al. Patterns of physical activity and relationship with risk markers for cardiovascular disease and diabetes in Indian, Pakistani, Bangladeshi and European adults in a UK population. J. Public Health Med. 2002;24:170–178. doi: 10.1093/pubmed/24.3.170. [DOI] [PubMed] [Google Scholar]
- 89.Hu FB, et al. Diet and risk of Type II diabetes: the role of types of fat and carbohydrate. Diabetologia. 2001;44:805–817. doi: 10.1007/s001250100547. [DOI] [PubMed] [Google Scholar]
- 90.Mohan V, et al. Dietary carbohydrates, glycemic load, food groups and newly detected type 2 diabetes among urban Asian Indian population in Chennai, India (Chennai Urban Rural Epidemiology Study 59) Br. J. Nutr. 2009;102:1498–1506. doi: 10.1017/S0007114509990468. [DOI] [PubMed] [Google Scholar]
- 91.Misra A, et al. South Asian diets and insulin resistance. Br. J. Nutr. 2009;101:465–473. doi: 10.1017/S0007114508073649. [DOI] [PubMed] [Google Scholar]
- 92.Burden ML, et al. A comparison of the glycemic and insulinaemic effects of an Asian and a European meal. Pract. Diab. Int. 1994;11:208–211. [Google Scholar]
- 93.Raj S, et al. Dietary habits of Asian Indians in relation to length of residence in the United States. J. Am. Diet. Assoc. 1999;99:1106–1108. doi: 10.1016/S0002-8223(99)00266-7. [DOI] [PubMed] [Google Scholar]
- 94.Kulkarni K. Food, culture, and diabetes in the United States. Clin. Diab. 2004;22:190–192. [Google Scholar]
- 95.Wandel M, et al. Changes in food habits after migration among South Asians settled in Oslo: the effect of demographic, socio-economic and integration factors. Appetite. 2008;50:376–385. doi: 10.1016/j.appet.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 96.Garduno-Diaz SD, Khokhar S. Prevalence, risk factors and complications associated with type 2 diabetes in migrant South Asians. Diab. Metabol. Res. Rev. 2012;28:6–24. doi: 10.1002/dmrr.1219. [DOI] [PubMed] [Google Scholar]
- 97.Isharwal S, et al. Diet & insulin resistance: a review & Asian Indian perspective. Indian J. Med. Res. 2009;129:485–499. [PubMed] [Google Scholar]
- 98.Donin AS, et al. Nutritional composition of the diets of South Asian, black African-Caribbean and white European children in the United Kingdom: the Child Heart and Health Study in England (CHASE) Br. J. Nutr. 2010;104:276–285. doi: 10.1017/S000711451000070X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shah T, et al. Prevalence of metabolic syndrome risk factors among young adult Asian Indians. Jimmigr. Health. 2005;7:117–126. doi: 10.1007/s10903-005-2645-5. [DOI] [PubMed] [Google Scholar]
- 100.Rimm EB, et al. Prospective study of cigarette smoking, alcohol use, and the risk of diabetes in men. BMJ. 1995;310:555–559. doi: 10.1136/bmj.310.6979.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rimm EB, et al. Cigarette smoking and the risk of diabetes in women. Am. J. Public Health. 1993;83:211–214. doi: 10.2105/ajph.83.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Frati AC, et al. Acute effect of cigarette smoking on glucose tolerance and other cardiovascular risk factors. Diab. Care. 1996;19:112–118. doi: 10.2337/diacare.19.2.112. [DOI] [PubMed] [Google Scholar]
- 103.Persson PG, et al. Cigarette smoking, oral moist snuff use and glucose intolerance. J. Intern. Med. 2000;248:103–110. doi: 10.1046/j.1365-2796.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- 104.Baris E, et al. Research priorities for tobacco control in developing countries: a regional approach to a global consultative process. Tob. Control. 2000;9:217–223. doi: 10.1136/tc.9.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rani M, et al. Tobacco use in India: prevalence and predictors of smoking and chewing in a national cross sectional household survey. Tob. Control. 2003;12:e4. doi: 10.1136/tc.12.4.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gupta PC, Ray CS. Smokeless tobacco and health in India and South Asia. Respirology. 2003;8:419–431. doi: 10.1046/j.1440-1843.2003.00507.x. [DOI] [PubMed] [Google Scholar]
- 107.Govino G. Epidemiology of tobacco use in the United States. Oncogene. 2002;21:7326–7340. doi: 10.1038/sj.onc.1205808. [DOI] [PubMed] [Google Scholar]
- 108.Spiegel K, et al. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Int. Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 109.Beihl DA, et al. Sleep duration as a risk factor for incident type 2 diabetes in a multiethnic cohort. Ann. Epidemiol. 2009;19:351–357. doi: 10.1016/j.annepidem.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 110.Nilsson PM, et al. Incidence of diabetes in middle-aged men is related to sleep disturbances. Diab. Care. 2004;27:2464–2469. doi: 10.2337/diacare.27.10.2464. [DOI] [PubMed] [Google Scholar]
- 111.Roopa M, et al. Prevalence of sleep abnormalities and their association with metabolic syndrome among Asian Indians: Chennai Urban Rural Epidemiology Study (CURES-67) J. Diab. Sci. Technol. 2010;4:1524–1531. doi: 10.1177/193229681000400630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bhushan B, et al. Obstructive sleep apnea is independently associated with the metabolic syndrome in obese Asian Indians in northern India. Metabol. Syndrome Related Disord. 2010;8:431–435. doi: 10.1089/met.2009.0125. [DOI] [PubMed] [Google Scholar]
- 113.Barone MT, Menna-Barreto L. Diabetes and sleep: a complex cause-and-effect relationship. Diab. Res. Clin. Pract. 2011;91:129–137. doi: 10.1016/j.diabres.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 114.Carpenter DO. Environmental contaminants as risk factors for developing diabetes. Rev. Environ. Health. 2008;23:59–74. doi: 10.1515/REVEH.2008.23.1.59. [DOI] [PubMed] [Google Scholar]
- 115.Lee DH, et al. A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: results from the National Health and Examination Survey 1999–2002. Diab. Care. 2006;29:1638–1644. doi: 10.2337/dc06-0543. [DOI] [PubMed] [Google Scholar]
- 116.Monirith I, et al. Asia-Pacific mussel watch: monitoring contamination of persistent organochlorine compounds in coastal waters of Asian countries. Mar. Pollut. Bull. 2003;46:281–300. doi: 10.1016/S0025-326X(02)00400-9. [DOI] [PubMed] [Google Scholar]
- 117.Minh NH, et al. Contamination by persistent organic pollutants in dumping sites of Asian developing countries: implication of emerging pollution sources. Arch. Environ. Contaminat. Toxicol. 2006;50:474–481. doi: 10.1007/s00244-005-1087-3. [DOI] [PubMed] [Google Scholar]
- 118.Weber MB, et al. Lifestyle interventions and the prevention and treatment of Type 2 diabetes. Am. J. Lifestyle Med. 2010;4:468–480. [Google Scholar]
- 119.Narayan KM, Williamson DF. Prevention of type 2 diabetes: risk status, clinic, and community. J. Gen. Internal Med. 2010;25:154–157. doi: 10.1007/s11606-009-1148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.deVegt F, et al. Relation of impaired fasting and postload glucose with incident type 2diabetes in a Dutch population: the Hoorn study. JAMA. 2001;285:2109–2113. doi: 10.1001/jama.285.16.2109. [DOI] [PubMed] [Google Scholar]
- 121.Knowler WC, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lindstrom J, et al. The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diab. Care. 2003;20:537–544. doi: 10.2337/diacare.26.12.3230. [DOI] [PubMed] [Google Scholar]
- 123.Weber MB, et al. A model of translational research for diabetes prevention in low and middle-income countries: The Diabetes Community Lifestyle Improvement Program (D-CLIP) trial. Primary Care Diab. 2012;6:3–9. doi: 10.1016/j.pcd.2011.04.005. [DOI] [PubMed] [Google Scholar]


