Abstract
Background
Previous studies have not confirmed associations between some current performance measures for inpatient heart failure processes of care and postdischarge outcomes. It is unknown if alternative measures are associated with outcomes.
Methods
Using data for 20,441 Medicare beneficiaries in the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) from March 2003 through December 2004, which we linked to Medicare claims data, we examined associations between hospital-level processes of care and patient outcomes. Performance measures included any beta-blocker for patients with left ventricular systolic dysfunction (LVSD); evidence-based beta-blocker for patients with LVSD; warfarin for patients with atrial fibrillation; aldosterone antagonist for patients with LVSD; implantable cardioverter-defibrillator for patients with ejection fraction ≤ 35%; and referral to disease management. Outcome measures were unadjusted and adjusted associations of each process measure with 60-day and 1-year mortality and cardiovascular readmission at the hospital-level.
Results
Adjusted hazard ratios for 1-year mortality with a 10% increase in hospital-level adherence were 0.94 for any beta-blocker (95% confidence interval [CI], 0.90–0.98; P = .004), 0.95 for evidence-based beta-blocker (95% CI, 0.92–0.98; P = .004); 0.97 for warfarin (95% CI, 0.92–1.03; P = .33); 0.94 for aldosterone antagonists (95% CI, 0.91–0.98; P = .006); 0.92 for implantable cardioverter-defibrillator (95% CI, 0.87–0.98; P = .007); and 1.01 for referral to disease management (95% CI, 0.99–1.03; P = .21).
Conclusions
Several evidence-based processes of care are associated with improved outcomes, can discriminate hospital-level quality of care, and could be considered as clinical performance measures.
Introduction
Heart failure typifies the challenges that chronic diseases pose for the US health care system. As the most common diagnosis for hospitalizations among Medicare beneficiaries, more dollars are spent for the management of heart failure than for any other diagnosis, and heart failure is the leading reason for hospital readmission.1 Postdischarge mortality among patients with heart failure is 11% at 30 days and 37% at 1 year,2 and these rates have not improved in the past 15 years. Quality of care varies across hospitals, and there is a substantial lag between the incorporation of evidence into professional guidelines and the delivery of evidence-based care.3
Government agencies, health care payers, and accreditation organizations have instituted programs to facilitate improvements in clinical care for patients hospitalized with heart failure. For example, public reporting programs aim to improve hospital accountability by reporting core performance measures and clinical outcomes.4 Pay-for-performance programs aim to improve clinical care and outcomes through financial incentives. A major component of these efforts is the development and implementation of standard, guideline-based performance measures.
Selection of appropriate performance measures for use in public profiling or financial incentives is critically important because of the potential implications for the health system, patient outcomes, and administrative burden. Public reporting of the quality of heart failure care at the hospital level affects payments to medical centers and physicians,5 so it is essential that the selected performance measures be associated with patient outcomes. Previous studies have found little or no association between current performance measures for inpatient heart failure care and outcomes, suggesting the need to identify other process measures that are linked to outcomes.6–8 We combined data from a large clinical registry with Medicare claims data to examine relationships between adherence to several emerging process measures and clinical outcomes.9
Methods
Data Sources
The study population was from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF) registry, which contains data on processes of care for patients hospitalized with heart failure.10 Participating US hospitals (n = 259) enrolled 48,612 patients from March 1, 2003, through December 31, 2004, using a case-ascertainment approach similar to that used by the Joint Commission.11 Eligible patients were those who presented with heart failure symptoms during a hospitalization for which heart failure was the primary discharge diagnosis and those for whom the primary reason for the admission was an episode of worsening heart failure and the primary discharge diagnosis was heart failure.
The OPTIMIZE-HF registry includes patients from all regions of the United States and from institutions of a wide variety of types and sizes. The institutional review board of each hospital, or a central institutional review board, approved the protocol. Staff at participating centers used a Web-based case report form to record patients’ demographic characteristics, comorbid conditions, vital signs, and drug therapies. This system used automatic electronic data checks to prevent out-of-range and duplicate entries, and an audit of the database used prespecified criteria to verify the data against source documents for a 5% random sample of the first 10,000 patients.
In this study, we linked data from the OPTIMIZE-HF registry to research-identifiable Medicare Part A inpatient claims data from the Centers for Medicare & Medicaid Services (CMS). We matched the data by date of birth, sex, hospital, and admission and discharge dates.12 Among 36,265 hospitalizations of patients aged 65 years or older, we matched 29,301 hospitalizations (80.8%) representing 25,901 distinct patients to the Medicare claims data. We excluded 1218 patients who died before discharge, 1143 patients who were ineligible for any of the process measures of interest (described below), and 790 patients in 88 hospitals that had fewer than 25 eligible patients—an approach taken in previous studies to improve the stability of the process measure estimates.6 The final data set contained data for 20,441 patients from 141 hospitals.
Performance Measures and Outcomes
For this analysis, we selected 6 performance measures based on evidence-based therapies or structural aspects of clinical care that received class I recommendations in the 2005 American College of Cardiology/American Heart Association (ACC/AHA) guidelines13 but are not among the heart failure performance measures currently used by CMS: (a) any beta-blocker at discharge for patients with left ventricular systolic dysfunction (LVSD); (b) evidence-based beta-blocker (ie, carvedilol, metoprolol succinate, or bisoprolol) at discharge for patients with LVSD; (c) warfarin at discharge for patients with atrial fibrillation; (d) aldosterone antagonist for patients with LVSD and low serum creatinine level (ie, serum creatinine ≤ 2.0 mg/dL for men and ≤ 2.5 mg/dL for women); (e) implantable cardioverter-defibrillator (ICD) for patients with left ventricular ejection fraction ≤ 35%; and (f) referral to a heart failure disease management program, defined as any multidisciplinary program for the chronic management of heart failure after hospitalization, including clinic-based, home-based, and telemanagement programs.
We measured adherence to each process of care among eligible patients who had no documented contraindications, intolerance, or other physician documentation of a reason not to use the process of care. Patients who died, were transferred to another acute care hospital, were discharged to hospice or a federal hospital, or left the hospital against medical advice were not considered eligible to receive any of the processes of care. These eligibility criteria are the same as those used by the Joint Commission.11 For each measure, we first identified which eligible patients were documented to have received the related process of care, hereafter termed patient-level performance measure. We then quantified each performance measure at the hospital level by dividing the number of patients for whom the process of care was documented by the number of patients who were eligible, hereafter termed hospital-level performance measure.
The main outcome measure was mortality at 1 year after discharge. Other outcome measures included 1-year cardiovascular readmission rate and 60-day mortality and cardiovascular readmission rates. Dates of death through December 31, 2006, were available in the CMS data. Cardiovascular readmission referred to the first subsequent hospital admission for a cardiovascular reason as identified in Medicare Part A claims and defined by diagnosis related group (DRG) codes 104–112, 115–118, 121–125, 127–145, 476, 514–518, 525–527, 535–536, and 547–558, excluding transfers or admissions for rehabilitation.
Covariates
The analysis included several baseline patient demographic and clinical characteristics from the OPTIMIZE-HF registry, including age, race, history of acute myocardial infarction, diabetes mellitus, prior cerebrovascular disease, peripheral vascular disease, depression, hyperlipidemia, chronic obstructive pulmonary disease, atrial arrhythmia, mean serum creatinine and hemoglobin levels, systolic and diastolic blood pressure, and weight. Values for serum creatinine, hemoglobin, systolic and diastolic blood pressure, and weight were missing for 1% to 6% of the study population. We imputed the mean values of the overall cohort for these missing values. For each hospital, we used CMS data to calculate the total number of heart failure hospitalizations and the percentage of total hospital discharges that were heart failure hospitalizations.
Statistical Analysis
Descriptive statistics included frequencies and means for baseline demographic characteristics, comorbid conditions, and clinical characteristics for the entire study population. We also report hospital-level performance measures for the 141 hospitals using medians and interquartile ranges.
The primary analysis examined associations between hospital-level performance measures and patient-level outcomes. We used Cox proportional hazards models to estimate unadjusted and adjusted relationships between each hospital-level performance measure and patient-level mortality and cardiovascular readmission rates at 60 days and 1 year. The unadjusted models included only the hospital-level performance level as a predictor. The adjusted models controlled for baseline demographic characteristics, clinical characteristics, and hospital volume, as described above. We calculated robust standard errors to account for the clustering of patients within hospitals.14
A secondary analysis explored associations between patient-level performance measures and patient-level outcomes. We again used Cox proportional hazard models to estimate unadjusted and adjusted relationships between each patient-level performance measure and patient-level mortality during the year after discharge. We used SAS version 9.2 (SAS Institute Inc, Cary, North Carolina) for all analyses.
Results
The mean age of the study population was 79 years (SD, 7.7), 45% of the patients were men, and 82% were white. Approximately half of the patients had ischemic heart disease and a mean ejection fraction of 39.9% (SD, 15.4%). The most common comorbid conditions were diabetes mellitus (39%), atrial arrhythmias (39%), chronic obstructive pulmonary disease (28%), and chronic kidney disease (19%) (Table 1). The overall unadjusted mortality and cardiovascular readmission rates were 30.9% and 46.0%, respectively.
Table 1.
Baseline Characteristics of the Study Population
| Variable | Patients (N = 20,441) |
|---|---|
| Age, mean (SD), y | 79.0 (7.7) |
| Male sex, No. (%) | 9271 (45.4) |
| Race, No. (%) | |
| Caucasian | 16,819 (82.3) |
| African American | 2258 (11.0) |
| Other/unknown | 1364 (6.7) |
| Hispanic ethnicity | 533 (2.6) |
| Heart failure characteristics | |
| Ischemic etiology, No. (%) | 10,041 (49.1) |
| Ejection fraction, mean (SD), % | 39.9 (15.4) |
| Missing value for ejection fraction, No. (%) | 2942 (14.4) |
| Comorbid conditions and clinical characteristics | |
| Diabetes mellitus, No. (%) | 8053 (39.4) |
| Hyperlipidemia, No. (%) | 6957 (34.0) |
| Atrial arrhythmia, No. (%) | 7955 (38.9) |
| Chronic obstructive pulmonary disease, No. (%) | 5767 (28.2) |
| Peripheral vascular disease, No. (%) | 3012 (14.7) |
| Prior cerebrovascular accident or transient ischemic attack, No. (%) | 3461 (16.9) |
| Thyroid disorder, No. (%) | 3526 (17.2) |
| Depression, No. (%) | 2006 (9.8) |
| Smoking in the past year, No. (%) | 1927 (9.4) |
| Systolic blood pressure, mean (SD) | 125.4 (21.7) |
| Serum sodium at admission, mean (SD), mEq/L | 137.8 (4.7) |
| Serum creatinine at admission, mean (SD), mg/dL | 1.6 (1.2) |
| Hemoglobin at admission, mean (SD), g/dL | 12.1 (1.9) |
| In-hospital procedures, No. (%) | |
| Coronary angiography | 1508 (7.4) |
| Implantable cardioverter-defibrillator | 432 (2.1) |
| Mechanical ventilation | 383 (1.9) |
| Medications at discharge, No. (%) | |
| Angiotensin-converting enzyme inhibitor or angiotensin receptor blocker | 12,817 (62.7) |
| Aldosterone antagonist | 2391 (11.7) |
| Antiplatelet agent | 10,968 (53.7) |
| Beta-blocker | 13,218 (64.7) |
| Lipid-lowering agent | 7734 (37.8) |
| Digoxin | 5984 (29.3) |
| Diuretic | 16,831 (82.3) |
Definitions for comorbid conditions are based on ACC/AHA clinical data standards.24
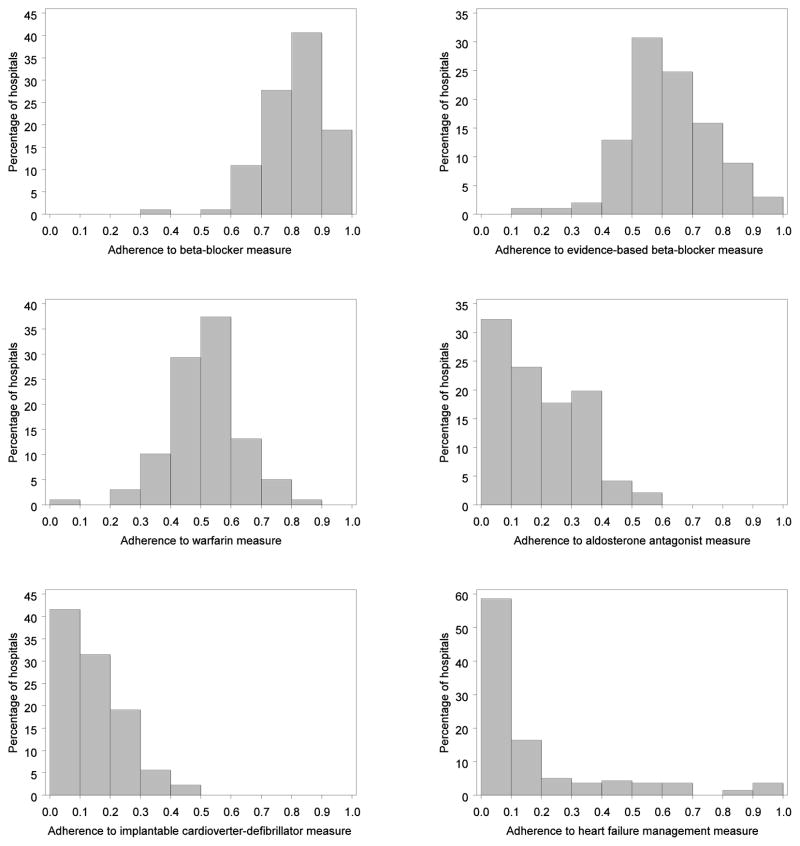
Median hospital-level adherence rates for the 6 performance measures varied significantly (Table 2); the highest adherence rate was for any beta-blocker (82%) and the lowest was for disease management (7%). The mean number of patients at each hospital who were eligible for a given process of care ranged from 60 for ICD to 130 for disease management. Figure 1 shows the distribution of adherence to the performance measures across hospitals. The greatest variation in adherence between hospitals was in the evidence-based beta-blocker and warfarin measures; the least variation was in the disease management, overall beta-blocker, and aldosterone antagonist measures.
Table 2.
Hospital-Level Adherence to Processes of Care
| Measure | No. of Eligible Hospitals | Adherence, Median (Interquartile Range), % |
|---|---|---|
| Any beta-blocker | 101 | 82 (74–89) |
| Evidence-based beta-blocker | 101 | 62 (53–71) |
| Warfarin | 99 | 51 (44–57) |
| Aldosterone antagonist | 96 | 16 (6–30) |
| Implantable cardioverter-defibrillator | 89 | 13 (6–20) |
| Disease management | 140 | 7 (0–19) |
Figure 1.
Histograms of Hospital-Level Adherence to Emerging Measures of Heart Failure Processes of Care
Note: The x-axes indicate hospital-level adherence to the given performance measure. The y-axes indicate the percentage of hospitals.
Table 3 shows relationships between the hospital-level performance measures and patient-level outcomes. Before adjustment, the hospital-level performance measures for any beta-blocker, evidence-based beta-blocker, aldosterone antagonist, and ICD were significantly associated with hospital-level 1-year mortality, whereas warfarin and disease management were not. After adjustment for other patient and hospital characteristics, 4 of the 6 performance measures were significantly associated with lower 1-year mortality. For each absolute 10% increase in the performance measure, any beta-blocker, evidence-based beta-blocker, aldosterone, and ICD were associated with a 5% to 8% lower risk of 1-year mortality. Relationships between the performance measures and 60-day mortality were significant for the aldosterone antagonist and ICD measures only. With the exception of the evidence-based beta-blocker measure, none of the hospital-level performance measures were significantly associated with lower risk of 60-day or 1-year cardiovascular readmission.
Table 3.
Relationships Between Hospital-Level Process Measures and Patient-Level Outcomes*
| Measure | Unadjusted | Adjusted | ||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Mortality at 1 Year | ||||
| Any beta-blocker | 0.95 (0.90–1.00) | .03 | 0.94 (0.90–0.98) | .004 |
| Evidence-based beta-blocker | 0.95 (0.92–0.99) | .01 | 0.95 (0.92–0.98) | .004 |
| Warfarin | 0.99 (0.93–1.04) | .62 | 0.97 (0.92–1.03) | .33 |
| Aldosterone antagonist | 0.94 (0.91–0.98) | .006 | 0.94 (0.91–0.98) | .006 |
| ICD | 0.93 (0.87–0.99) | .02 | 0.92 (0.87–0.98) | .007 |
| Disease management | 1.01 (0.99–1.03) | .24 | 1.01 (0.99–1.03) | .21 |
| Mortality at 60 Days | ||||
| Any beta-blocker | 0.95 (0.89–1.03) | .20 | 0.95 (0.89–1.02) | .20 |
| Evidence-based beta-blocker | 0.95 (0.89–1.02) | .14 | 0.95 (0.88–1.02) | .13 |
| Warfarin | 0.94 (0.87–1.03) | .18 | 0.93 (0.86–1.01) | .07 |
| Aldosterone antagonist | 0.93 (0.86–0.99) | .03 | 0.92 (0.86–1.00) | .04 |
| ICD | 0.92 (0.82–1.03) | .14 | 0.90 (0.81–1.00) | .04 |
| Disease management | 1.01 (0.97–1.05) | .62 | 1.01 (0.97–1.05) | .55 |
| Cardiovascular Readmission at 1 Year | ||||
| Any beta-blocker | 0.98 (0.93–1.03) | .47 | 0.97 (0.92–1.02) | .21 |
| Evidence-based beta-blocker | 0.96 (0.92–1.00) | .03 | 0.95 (0.91–0.99) | .008 |
| Warfarin | 1.00 (0.96–1.04) | .87 | 1.00 (0.97–1.04) | .88 |
| Aldosterone antagonist | 0.99 (0.96–1.03) | .73 | 0.99 (0.95–1.02) | .48 |
| ICD | 1.06 (1.01–1.12) | .01 | 1.07 (1.02–1.13) | .005 |
| Disease management | 1.01 (1.00–1.03) | .13 | 1.01 (1.00–1.03) | .06 |
| Cardiovascular Readmission at 60 Days | ||||
| Any beta-blocker | 0.99 (0.93–1.05) | .64 | 0.98 (0.93–1.04) | .56 |
| Evidence-based beta-blocker | 0.94 (0.90–0.99) | .009 | 0.93 (0.89–0.98) | .005 |
| Warfarin | 1.01 (0.96–1.05) | .82 | 1.02 (0.97–1.06) | .47 |
| Aldosterone antagonist | 0.97 (0.92–1.01) | .16 | 0.97 (0.91–1.03) | .30 |
| ICD | 1.02 (0.95–1.09) | .53 | 1.02 (0.95–1.10) | .62 |
| Disease management | 1.01 (0.99–1.03) | .17 | 1.01 (1.00–1.03) | .15 |
Abbreviations: HR, hazard ratio; CI, confidence interval; ICD, implantable cardioverter-defibrillator.
Hazard ratios estimate the risk of the outcome dependent upon a 10% increase in hospital-level adherence.
Table 4 shows relationships between patient-level performance measures and patient-level mortality. Before risk adjustment, a number of measures were significantly related to 1-year mortality. After risk adjustment, the measures for any beta-blocker (OR, 0.75; 95% confidence interval [CI], 0.69–0.83), evidence-based beta-blocker (OR, 0.82; 95% CI, 0.76–0.89), and warfarin (OR, 0.79; 95% CI, 0.72–0.86) were significantly associated with 1-year mortality. Patient-level performance measures for aldosterone antagonist, ICD, and disease management were not associated with 1-year mortality.
Table 4.
Relationship Between Patient-Level Performance on Emerging Measures and 1-Year Mortality
| Measure | Received | One-Year Mortality | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| No. of Events | Incidence, % | P Value | Unadjusted HR (95% CI) | Adjusted HR (95% CI)* | ||
| Any beta-blocker | Yes | 1875 | 30.7 | < .001 | 0.67 (0.61–0.74) | 0.75 (0.69–0.83) |
| No | 597 | 41.8 | ||||
| Evidence-based beta-blocker | Yes | 1493 | 30.8 | < .001 | 0.80 (0.74–0.86) | 0.82 (0.76–0.89) |
| No | 979 | 36.6 | ||||
| Warfarin | Yes | 1272 | 31.2 | < .001 | 0.70 (0.64–0.76) | 0.79 (0.72–0.86) |
| No | 1657 | 41.2 | ||||
| Aldosterone antagonist | Yes | 362 | 28.7 | .06 | 0.90 (0.79–1.03) | 0.91 (0.80–1.04) |
| No | 1759 | 31.2 | ||||
| Implantable cardioverter-defibrillator | Yes | 266 | 28.4 | .30 | 0.93 (0.79–1.11) | 0.97 (0.83–1.12) |
| No | 1652 | 30.3 | ||||
| Disease management | Yes | 748 | 28.8 | .39 | 1.03 (0.95–1.12) | 1.07 (0.98–1.16) |
| No | 4546 | 27.7 | ||||
Abbreviations: HR, hazard ratio; CI, confidence interval.
Discussion
This study is the first to examine associations between emerging measures of processes of care for patients hospitalized with heart failure with early and long-term postdischarge clinical outcomes. The principal findings are threefold. First, hospital-level performance measures for any beta-blocker, evidence-based beta-blocker, aldosterone antagonist, and ICD were significantly associated with 1-year patient-level mortality. Second, fewer hospital-level performance measures were associated with early outcomes or 1-year cardiovascular readmission. Third, in a secondary analysis, associations for some but not all of the emerging performance measures at the patient level were similar to those at the hospital level. Although existing Hospital Quality Alliance heart failure performance measures have been shown not to be associated with patient-level or hospital-level mortality, alternative measures have meaningful associations with patient-centered outcomes. These findings have important implications for quality-improvement efforts, public reporting of adherence to performance measures, and heart failure pay-for-performance programs.
The hospital discharge period has been a focus of heart failure guidelines and performance measures because of the ease of access to patients; patients’ receptivity to health care recommendations; and the opportunity to implement, manage, and measure intervention strategies.16 Existing criteria for development of performance measures include quantifying numerators and denominators and evaluating the interpretability, applicability, and feasibility of proposed measures so they accurately reflect quality of care.13 Performance measures are meant to measure structural aspects or processes of care for which evidence is so strong that failure to perform them reduces the likelihood of optimal patient outcomes.17 Although there are a number of evidence-based therapies for patients with heart failure with LVSD, current Joint Commission and CMS heart failure measures include 3 measures—use of discharge instructions, smoking cessation counseling, and assessment of left ventricular function—that no controlled trial has specifically addressed in patients hospitalized with heart failure. Guideline recommendations for these measure are based on expert opinion.13 In previous studies, none of the 4 current performance measures for heart failure in inpatient settings was associated with patient-level or hospital-level mortality, and only the measure for angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) in patients with LVSD was associated with mortality or readmission.
This study provides evidence to support expansion of heart failure performance measures to include 4 measures associated with clinical outcomes. After adjustment for case mix, an absolute 10% increase in hospital adherence to these measures was significantly associated with a 5% to 8% lower risk of 1-year mortality. Associations for these measures were stronger than for current Hospital Quality Alliance and ACC/AHA performance measures. Moreover, use of current heart failure performance measures for public reporting and CMS pay-for-performance programs may not be the most efficacious way to assess quality, given the lack of association between most current performance measures and early and long-term outcomes.
Attention has increasingly focused on improvements in short-term outcomes, given the high mortality and readmission rates observed. Hospitals are profiled on these early outcomes, but it is unclear what can be done to improve outcomes. We found that the hospital-level aldosterone antagonist and ICD measures were associated with lower risk of 60-day mortality, after adjustment for case mix and other therapies. Moreover, the measures for any beta-blocker and evidence-based beta-blocker were associated with a trend toward lower 60-day mortality. A clinical trial of aldosterone antagonists in patients hospitalized with LVSD and heart failure after myocardial infarction found significantly lower all-cause mortality in the first 30 days after randomization. Although clinical trials for ICD therapy have focused on long-term outcomes in ambulatory patients with chronic heart failure, there may be effects on early sudden cardiac death after hospitalization.18,19 Alternatively, there may be other unmeasured processes of care or clinical characteristics associated with ICDs and early outcomes. The significant associations of the beta-blockers, aldosterone antagonists, and ICD measures with mortality provide a rationale for incorporation into standard performance measures of quality of care for heart failure.
A more difficult challenge is the selection of process measures for readmission, now a focus of public reporting by CMS and national initiatives.20 Use of evidence-based beta-blockers was associated with lower short-term and long-term cardiovascular readmission. Coupled with the association with lower long-term mortality, evidence-based beta-blocker use could also be considered for a standard performance measure. Other processes of care, such as referral to disease management, were not associated with lower cardiovascular readmission. In previous studies, disease management has been associated with lower readmission rates.21 This discrepancy may be attributable to the fact that the processes of care studied here were at the point of discharge. Referral at time of discharge does not necessarily indicate that the patient enrolled or adhered to the program. Furthermore, heart failure disease management programs can be heterogeneous, and recent studies have suggested limited to no impact on clinical outcomes with certain programs.22
This study also reveals potential discrepancies between analysis of hospital-level performance measures and analysis of patient-level performance measures. For example, although hospital-level conformity to the ICD performance measure was associated with improved long-term outcomes, conformity at the patient level was not significantly associated with outcomes. The registry records whether a patient was discharged with an ICD but not when the ICD was placed. As a consequence, the ICD group may include patients who were healthy enough to receive an ICD during the registry hospitalization as well as sicker-than-average patients who received an ICD months or years earlier. When the relationship between exposure and outcome is measured at the patient level, this heterogeneity biases the results toward the null. Relationships between hospital-level adherence and patient-level outcomes are likely to be less affected by such heterogeneity.
Other studies have examined the link between performance measures evaluated at the hospital level and postdischarge outcomes. Werner et al6 found that the Hospital Compare performance measures for myocardial infarction, heart failure, and pneumonia predicted small or no differences in risk-adjusted in-hospital mortality at the hospital level. They concluded that efforts to develop performance measures “tightly linked” to patient outcomes would be worthwhile. In an previous OPTIMIZE-HF study, none of the Hospital Quality Alliance performance measures were significantly associated with lower 60-day to 90-day postdischarge mortality; only the ACE inhibitor/ARB measure was associated with lower mortality or readmission at the patient level.8 However, a beta-blocker measure was associated with lower mortality and a combined end point of mortality or rehospitalization at 60 to 90 days. Jha et al23 found a modest relationship between heart failure performance measures and inpatient outcomes.
Limitations
This study has several limitations. First, eligibility for processes of care was based on medical record documentation and thus depended on the accuracy of the documentation. Patients may have had undocumented contraindications or intolerances, which may have led to overestimation of the number of patients eligible for each performance measure. Second, despite the size of the study, the analysis may not have had sufficient statistical power to detect small but clinically important differences in postdischarge outcomes. Third, we were not able to adjust for socioeconomic factors or adherence. Inclusion of these variables would have diminished our ability to detect process–outcome links, making the finding that some measures did have process–outcome links more remarkable. There may also be other measured or unmeasured confounding variables that, had they been included, would have strengthened or weakened the process–outcome link for some measures. We did not assess health-related quality of life, functional capacity, patient satisfaction, or other clinical outcomes that may be of interest. Fourth, the study population included Medicare fee-for-service beneficiaries enrolled in OPTIMIZE-HF and may not be representative of all patients hospitalized with heart failure, though recent data suggest that patients in OPTIMIZE-HF are reasonably representative of Medicare beneficiaries. Finally, we were not able to determine whether a therapy was initiated or discontinued after discharge, whether patients referred to disease management actually participated, or the intensity and procedures of the disease management program after referral, which limits the assessment of improved conformity to a process measure after discharge.
Conclusion
Adherence to heart failure process measures for any beta-blocker, evidence-based beta-blocker, aldosterone antagonist, and ICD are significantly associated with postdischarge clinical outcomes and can be used to effectively discriminate quality of care at the hospital level. These measures could be considered for inclusion in heart failure performance measure sets. Given the moderate associations between individual process measures and clinical outcomes, it may be appropriate to include multiple new measures in hospital profiling efforts and incentive programs aimed at improving the quality of care for patients with heart failure.
Acknowledgments
We thank Damon M. Seils, MA, Duke University, for editorial assistance and manuscript preparation.
Funding/Support: This study was supported by grant U18HS10548 from the Agency for Healthcare Research and Quality; and by a research agreement between GlaxoSmithKline and Duke University. Dr Hernandez is a recipient of an American Heart Association Pharmaceutical Roundtable grant (0675060N). Drs Curtis and Schulman were supported in part by grants U01HL066461 from the National Heart, Lung, and Blood Institute and R01AG026038 from the National Institute on Aging. Dr Fonarow is supported by the Ahmanson Foundation and the Corday Family Foundation.
Footnotes
Trial Registration: clinicaltrials.gov Identifier: NCT00344513
Disclaimer: The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality, the National Heart, Lung, and Blood Institute, the National Institute on Aging, or the National Institutes of Health.
References
- 1.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–28. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 2.Curtis LH, Greiner MA, Hammill BG, et al. Early and long-term outcomes of heart failure in elderly persons, 2001–2005. Arch Intern Med. 2008;168:2481–8. doi: 10.1001/archinte.168.22.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fonarow GC, Yancy CW, Heywood JT. Adherence to heart failure quality-of-care indicators in US hospitals: analysis of the ADHERE Registry. Arch Intern Med. 2005;165:1469–77. doi: 10.1001/archinte.165.13.1469. [DOI] [PubMed] [Google Scholar]
- 4.Krumholz HM, Normand SL. Public reporting of 30-day mortality for patients hospitalized with acute myocardial infarction and heart failure. Circulation. 2008;118:1394–7. doi: 10.1161/CIRCULATIONAHA.108.804880. [DOI] [PubMed] [Google Scholar]
- 5.Rosenthal MB, Landon BE, Normand SL, Frank RG, Epstein AM. Pay for performance in commercial HMOs. N Engl J Med. 2006;355:1895–1902. doi: 10.1056/NEJMsa063682. [DOI] [PubMed] [Google Scholar]
- 6.Werner RM, Bradlow ET. Relationship between Medicare’s hospital compare performance measures and mortality rates. JAMA. 2006;296:2694–702. doi: 10.1001/jama.296.22.2694. [DOI] [PubMed] [Google Scholar]
- 7.Patterson ME, Hernandez AF, Hammill BG, et al. Process of care performance measures and long-term outcomes in patients hospitalized with heart failure. Med Care. doi: 10.1097/MLR.0b013e3181ca3eb4. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fonarow GC, Abraham WT, et al. Association between performance measures and clinical outcomes for patients hospitalized with heart failure. JAMA. 2007;297:61–70. doi: 10.1001/jama.297.1.61. [DOI] [PubMed] [Google Scholar]
- 9.Curtis LH, Greiner MA, Hammill BG, et al. Representativeness of a national heart failure quality-of-care registry: comparison of OPTIMIZE-HF and non-OPTIMIZE-HF Medicare patients. Cirulation: Cardiovascular Quality and Outcomes. 2009;2:377–84. doi: 10.1161/CIRCOUTCOMES.108.822692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fonarow GC, Abraham WT, Albert NM, et al. Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF): rationale and design. Am Heart J. 2004;148:43–51. doi: 10.1016/j.ahj.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Joint Commission on Accreditation of Healthcare Organizations. Specification Manual for National Implementation of Hospital Core Measures. Oak Terrace, IL: Joint Commission on Accreditation of Healthcare Organizations; 2004. [Google Scholar]
- 12.Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157:995–1000. doi: 10.1016/j.ahj.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonow RO, Bennett S, Casey DE, Jr, et al. ACC/AHA clinical performance measures for adults with chronic heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Heart Failure Clinical Performance Measures) endorsed by the Heart Failure Society of America. J Am Coll Cardiol. 2005;46:1144–78. doi: 10.1016/j.jacc.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–8. [Google Scholar]
- 15.Shahian DM, Torchiana DF, Shemin RJ, Rawn JD, Normand S-LT. The Massachusetts cardiac surgery report card: implications of statistical methodology. Ann Thorac Surg. 2005;80:2106–13. doi: 10.1016/j.athoracsur.2005.06.078. [DOI] [PubMed] [Google Scholar]
- 16.Fonarow GC, Yancy CW, Heywood JT. Adherence to heart failure quality-of-care indicators in US hospitals: analysis of the ADHERE Registry. Arch Intern Med. 2005;165:1469–77. doi: 10.1001/archinte.165.13.1469. [DOI] [PubMed] [Google Scholar]
- 17.Spertus JA, Eagle KA, Krumholz HM, Mitchell KR, Normand SL. American College of Cardiology and American Heart Association methodology for the selection and creation of performance measures for quantifying the quality of cardiovascular care. Circulation. 2005;111:1703–12. doi: 10.1161/01.CIR.0000157096.95223.D7. [DOI] [PubMed] [Google Scholar]
- 18.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 19.Solomon SD, Dobson J, Pocock S, et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116:1482–7. doi: 10.1161/CIRCULATIONAHA.107.696906. [DOI] [PubMed] [Google Scholar]
- 20.Keenan PS, Normand ST, Lin Z, et al. An administrative claims measure suitable for profiling hospital performance on the basis of 30-day all-cause readmission rates among patients with heart failure. Circulation: Cardiovascular Quality and Outcomes. 2008;1:29–37. doi: 10.1161/CIRCOUTCOMES.108.802686. [DOI] [PubMed] [Google Scholar]
- 21.Whellan DJ, Hasselblad V, Peterson E, O’Connor CM, Schulman KA. Metaanalysis and review of heart failure disease management randomized controlled clinical trials. Am Heart J. 2005;149:722–9. doi: 10.1016/j.ahj.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 22.Fonarow GC. Heart failure disease management programs: not a class effect. Circulation. 2004;110:3506–8. doi: 10.1161/01.CIR.0000151101.17629.20. [DOI] [PubMed] [Google Scholar]
- 23.Jha AK, Orav EJ, Li Z, Epstein AM. The inverse relationship between mortality rates and performance in the Hospital Quality Alliance measures. Health Aff (Millwood) 2007;26:1104–10. doi: 10.1377/hlthaff.26.4.1104. [DOI] [PubMed] [Google Scholar]
- 24.Radford MJ, Arnold JM, Bennett SJ, et al. ACC/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with chronic heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Heart Failure Clinical Data Standards) J Am Coll Cardiol. 2005;46:1179–1207. [Google Scholar]