Abstract
The safety, tolerability, and pharmacokinetics (PKs) of bapineuzumab (AAB–001), a humanized monoclonal antibody to amyloid β, were evaluated in patients with mild-to-moderate Alzheimer disease in a phase 1, randomized, third-party unblinded, placebo-controlled, single ascending dose trial. Thirty patients received bapineuzumab infusion of 0.5, 1.5, or 5mg/kg or placebo (6 active, 2 placebo for 0.5 and 1.5-mg/kg cohorts; 10 active, 4 placebo for 5.0-mg/kg cohort). Three patients in the highest dose cohort (5.0mg/kg) developed magnetic resonance imaging abnormalities consistent with vasogenic edema, predominantly high signal abnormalities on fluid-attenuated inversion recovery sequences, all of which resolved over time. Plasma amyloid β was elevated from baseline, peaking approximately 24 hours after infusion. PK analysis demonstrated a half-life of 21 to 26 days, supporting a 13-week dosing interval for bapineuzumab. This small, single-dose study demonstrated the safety profile and PK characteristics of bapineuzumab and was used to design later safety and efficacy trials.
Keywords: bapineuzumab, Alzheimer disease, humans, pharmacokinetics, monoclonal antibody
The amyloid β (Aβ) peptide is the product of the enzymatic cleavage of the amyloid precursor protein, a transmembrane protein found abundantly in neurons. Accumulation of Aβ is thought to play a central role in the neuropathogenesis of Alzheimer disease (AD).1 Anti-Aβ immunotherapy has been shown to reduce neuropathologic changes and reduce decline in cognitive and memory function in multiple transgenic mouse models of AD.2–4
Initial clinical studies of active immunization with synthetic full-length Aβ peptide (AN1792) were discontinued after reports of meningoencephalitis in 6% of patients that resulted in cognitive or neurologic sequelae in few of them.5 This adverse event (AE) was likely due to the induction of a proinflammatory T-cell response to full-length Aβ.6 Subsequent epitope mapping indicated that antibody responses to AN1792 were almost exclusively directed toward the N-terminal residues.7 Various studies in mouse models have demonstrated that immunotherapy with antibodies targeted to the N terminus of Aβ, which does not contain T-cell activating epitopes, inhibits neurocytotoxicity and fibrillogenesis.8 Evidence for the efficacy of N-terminal antibodies, combined with the desire to avoid harmful T-cell effects, have led to clinical trials using monoclonal antibodies (mAbs) targeted toward Aβ. Bapineuzumab (AAB-001) is a fully humanized mAb specific to the N-terminal region of Aβ.
The primary objective of this study was to determine the safety and tolerability of single ascending doses of bapineuzumab. The secondary objective was to characterize the pharmacokinetic (PK) profile of bapineuzumab and to provide a preliminary assessment for future multiple-dose regimens. An exploratory objective of the study was the assessment of efficacy using the Mini-Mental State Examination (MMSE).
METHODS
This was a multicenter, randomized, third-party unblinded, placebo-controlled, single dose study of 3 active ascending dose groups of intravenous bapineuzumab in male and female patients with mild-to-moderate AD.
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and was in compliance with Good Clinical Practice. Individual site institutional review boards provided the approval for the protocol as well as the informed consent forms.
Patients between the ages of 50 and 85 years with a diagnosis of probable AD according to National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association criteria were eligible for enrollment in the study. Other requirements included an MMSE score of 14 to 26, a Rosen Modified Hachinski Ischemic score ≤4, a magnetic resonance imaging (MRI) scan (performed between screening and baseline) consistent with the diagnosis of AD, and stable doses of medications for treatment of nonexcluded medical conditions for 4 weeks before screening. Patients receiving approved symptomatic treatments for AD were allowed in the study if they were on stable doses for 3 months.
Patients were randomly assigned in a blinded fashion (ie, third-party unblinded) to receive study drug at escalating doses of 0.5mg/kg (6 active, 2 placebo), 1.5 mg/kg (6 active, 2 placebo), and 5 mg/kg (10 active, 4 placebo). Dose escalation for the next cohort occurred every 10 weeks and was based on the safety data (including MRI) at 8 weeks after dosing from each patient in the cohort. The 5-mg/kg dose cohort was repeated in consultation with an independent safety monitoring committee, and the study was subsequently stopped at 5 mg/kg due to MRI abnormalities (vasogenic edema) occurring at that dose. One-year safety data were collected for patients who completed the study. The total duration of the study was approximately 2 years from the screening of first patient visit to the last one.
Safety
Safety assessments included AE reports; physical and neurologic examinations at screening and weeks 1, 4, 8, 10, 12, 16 and months 6, 9, and 12; 12-lead electrocardiogram at screening and weeks 4 and 16; vital signs at every visit; laboratory determinations at screening and weeks 1 to 16 and months 6, 9, and 12; and MRI brain scan including gradient echo sequences at baseline and week 8.
During and after the infusion of study drug, patients were observed for 6 hours. Sitting blood pressure and pulse were measured at 15-minute intervals during the infusion, for the first hour after infusion, and every 30 minutes thereafter.
PKs
Standard, model-independent PK methods were used to characterize the single-dose serum concentration-time profile of bapineuzumab over the dose range studied. PK parameters included peak concentration (Cmax), time to Cmax (tmax), total area under the concentration time curve (AUC), systemic clearance, apparent steady-state volume of distribution, mean residence time, and terminal-phase elimination half-life (t½). Blood samples for serum PK evaluations were collected from all patients at days 1, 2, and 3, and weeks 1, 2, 4, 6, 8, 10, 12, and 16.
Blood samples for exploratory analyses of plasma Aβ concentrations were collected from all patients at 0, 1, 6, and 24 hours after the start of bapineuzumab infusion, and at weeks 2, 6, and 16. Serum samples were assayed for concentrations of bapineuzumab and antibapineuzumab antibodies by Wyeth Research’s Drug Metabolism division or a designee using a validated enzyme-linked immunosorbent assay method.
Exploratory Efficacy
The MMSE was performed at screening, weeks 4 and 16, and months 6 and 12. As prespecified in the statistical analysis plan (SAP), week 16 was selected as the primary time point for a preliminary efficacy analysis, and a linear trend analysis was performed to explore a dose response based on MMSE change between the screening assessment and week 16. The SAP specified that if a statistically significant dose effect was observed, each dose group would be compared with placebo using Dunnett method to adjust the level of significance for multiple tests. The MMSE was assessed using an analysis of covariance with change from screening as the response, screening MMSE score as the covariate, and treatment as a main effect.
As specified in the SAP, in an exploratory analysis, t tests were performed on the mean MMSE change from screening scores, comparing placebo with each dose group. There was no control for multiplicity in these comparisons.
RESULTS
Patients
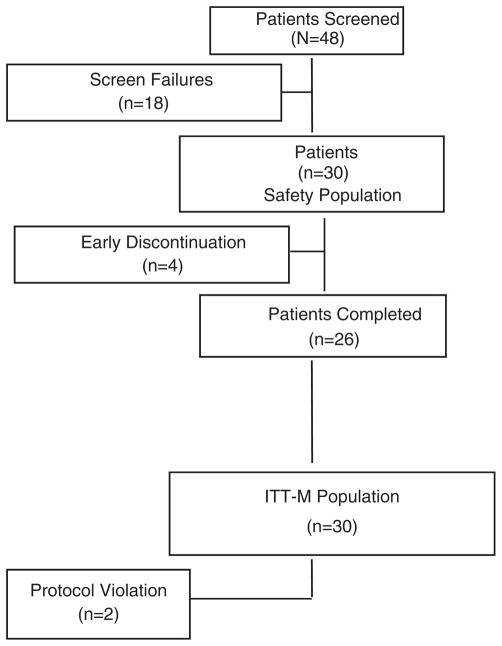
Forty-eight (48) patients were screened for participation in this study; 18 patients failed initial screening and were excluded. The remaining 30 patients were randomly assigned to a treatment group and comprised the safety population (Fig. 1). Patient demographics and baseline characteristics were comparable among groups (Table 1). Ages ranged from 56 to 88 years, and most patients (67%) were white. None of the participating patients were known to have any illness at baseline that might interfere with the activity of bapineuzumab or interpretation of the study results. Many enrolled patients had a history of chronic, stable medical conditions that did not influence or interfere with the conduct of this study. All enrolled patients received some concomitant medications, most often analgesics (73%), other central nervous system drugs, including parasympathomimetrics (63%), vitamins (53%), anti-inflammatory or antirheumatic products (40%), psychoanaleptics (37%), serum lipid-reducing agents (37%), and antihypertensives (33%).
FIGURE 1.
Patient disposition.
TABLE 1.
Demographic and Baseline Characteristics
| Characteristic | Treatment
|
|||
|---|---|---|---|---|
| Placebo (n = 8) | Bapineuzumab, 0.5 mg/kg (n = 6) | Bapineuzumab, 1.5 mg/kg (n = 6) | Bapineuzumab, 5 mg/kg (n = 10) | |
| Age, mean (SD) | 69.88 (10.71) | 74.67 (5.65) | 72.33 (9.85) | 74.70 (7.44) |
| Sex, n (%) | ||||
| Female | 7 (87.50) | 3 (50.00) | 1 (16.67) | 3 (30.00) |
| Male | 1 (12.50) | 3 (50.00) | 5 (83.33) | 7 (70.00) |
| Ethnic Origin, n (%) | ||||
| Black | 1 (12.50) | 0 | 1 (16.67) | 1 (10.00) |
| Hispanic | 2 (25.00) | 2 (33.33) | 0 | 3 (30.00) |
| White | 5 (62.50) | 4 (66.67) | 5 (83.33) | 6 (60.00) |
| Weight, kg | ||||
| Mean, (SD) | 71.79 (16.60) | 67.02 (12.61) | 86.47 (11.22) | 74.61 (15.50) |
| Baseline MMSE score | 20.8 | 21.8 | 21.3 | 22.3 |
MMSE indicates Mini-Mental State Examination.
Safety
Most patients (28/30, 93%) reported at least 1 AE (Table 2). One patient each in the bapineuzumab 1.5 and 5-mg/kg groups did not experience an AE. The most frequently reported treatment-emergent AEs (TEAEs) in all treatment groups, including placebo, were back pain (20%), accidental injury (17%), asthenia (13%), headache (13%), and infection (13%). Most TEAEs were mild to moderate in severity and were not related to treatment. TEAEs are summarized in Table 2. An episode of amaurosis fugax that was deemed possibly related to study drug by the investigator resolved without neurologic sequelae in 1 patient receiving 5-mg/kg bapineuzumab. One patient in the 1.5-mg/kg bapineuzumab dose group died of respiratory failure 7 months after treatment. This patient’s death was assessed as probably not related to bapineuzumab treatment by the investigator.
TABLE 2.
Treatment-emergent Adverse Events in the Safety Population, n (%)
| Adverse Event | Treatment
|
|||
|---|---|---|---|---|
| Placebo (n = 8) | Bapineuzumab, 0.5 mg/kg (n = 6) | Bapineuzumab, 1.5 mg/kg (n = 6) | Bapineuzumab, 5 mg/kg (n = 10) | |
| Any adverse event | 8 (100) | 6 (100) | 5 (83.3) | 9 (90.0) |
| Abdominal pain | 2 (25.0) | 1 (16.7) | 0 | 0 |
| Accidental injury | 2 (25.0) | 2 (33.3) | 0 | 1 (10.0) |
| Asthenia | 2 (25.0) | 1 (16.7) | 0 | 1 (10.0) |
| Back pain | 2 (25.0) | 2 (33.3) | 1 (16.7) | 1 (10.0) |
| Headache | 3 (37.5) | 0 | 0 | 1 (10.0) |
| Hernia | 0 | 1 (16.7) | 0 | 1 (10.0) |
| Infection | 1 (12.5) | 2 (33.3) | 0 | 1 (10.0) |
| Pain | 2 (25.0) | 0 | 0 | 0 |
| Hypertension | 1 (12.5) | 1 (16.7) | 0 | 1 (10.0) |
| Dyspepsia | 1 (12.5) | 0 | 1 (16.7) | 0 |
| Vomiting | 2 (25.0) | 0 | 0 | 0 |
| BUN increased | 1 (12.5) | 0 | 0 | 1 (10.0) |
| Hyperglycemia | 1 (12.5) | 0 | 0 | 1 (10.0) |
| Peripheral edema | 1 (12.5) | 0 | 1 (16.7) | 0 |
| Anxiety | 0 | 0 | 0 | 2 (20.0) |
| Confusion | 0 | 1 (16.7) | 0 | 1 (10.0) |
| Depression | 2 (25.0) | 0 | 0 | 0 |
| Dizziness | 0 | 1 (16.7) | 0 | 2 (20.0) |
| Insomnia | 1 (12.5) | 0 | 0 | 1 (10.0) |
| Vertigo | 1 (12.5) | 1 (16.7) | 0 | 0 |
| Hematuria | 1 (12.5) | 0 | 0 | 1 (10.0) |
| Local reaction to procedure | 0 | 0 | 0 | 2 (20.0) |
BUN indicates blood urea nitrogen.
MRI abnormalities consistent with vasogenic edema were reported in 3 patients receiving 5-mg/kg bapineuzumab. Two patients were asymptomatic, and the abnormalities were detected during the protocol MRI. One patient with mild, transient confusion received an unscheduled MRI scan at 4 weeks because of a decline in MMSE, but improved cognitively by the week 16 MMSE time point. The MRI abnormalities in the 3 patients predominantly consisted of high signal intensity on fluid-attenuated inversion recovery (FLAIR) sequences, which were not present at baseline. The gradient echo (T2*) image in the transiently symptomatic patient showed a small punctate magnetic susceptibility lesion in the brain, close to the area of FLAIR abnormality, which was not seen on the earlier scans. This was presumably a small focus of associated microhemorrhage. Repeat MRI scans performed several weeks to months after the initial MRI changes showed that observed FLAIR MRI abnormalities had resolved in all patients, although the small focus of microhemorrhage in 1 patient was still evident (Fig. 2). In the 2 patients who had lumbar puncture, the cerebrospinal fluid was acellular, with slight elevations of protein in both cases (58.5 and 59.8 mg/dL).
FIGURE 2.
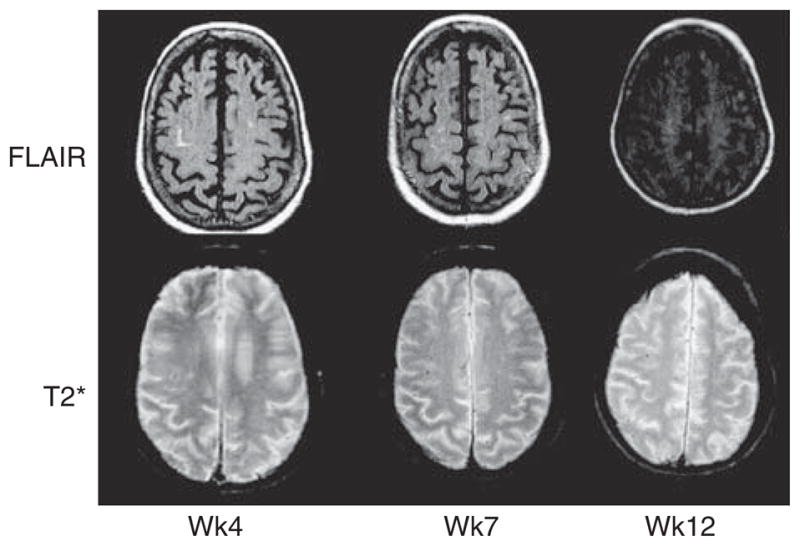
Transverse fluid-attenuated inversion recovery (FLAIR) and gradient-echo (T2*) magnetic resonance imaging scans showing emergence and resolution of left frontal abnormality on FLAIR 4, 7, and 12 weeks after bapineuzumab administration, with associated dot-like hypointense lesion on T2*, representing a focus of associated microhemorrhage.
There were no clinically important changes in laboratory tests, vital sign measurements, physical examination, neurologic examination, or electrocardiogram results.
PKs
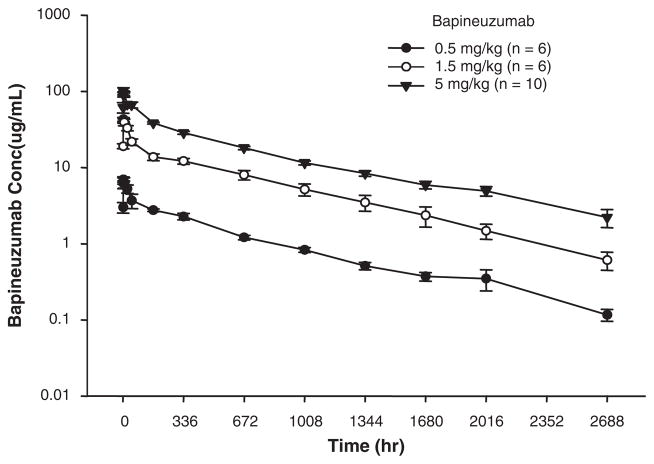
Mean bapineuzumab serum concentrations over time are shown for each dose group in Fig. 3. Exposure (Cmax and AUC) increased with increasing dose. A 3-fold increase in dose from 0.5 to 1.5 mg/kg resulted in an approximately 6-fold greater exposure to bapineuzumab (Table 3). Exposure for the 5-mg/kg dose group was approximately 2.6-fold greater (Table 3) than that for the 1.5-mg/kg group (a 3.3-fold increase in dose). Clearance was highest for the 0.5-mg/kg dose group and was lower for the 1.5 and 5-mg/kg dose groups. A median time to peak concentration between 1 and 2 hours after the start of bapineuzumab infusion was observed in all patients. Similar half-lives were observed for all 3 dose groups (mean=23.7 d).
FIGURE 3.
Mean (± SE) bapineuzumab serum concentrations for the 3 doses administered in this study.
TABLE 3.
Summary of Pharmacokinetic Parameters (Serum) of Bapineuzumab
| Bapineuzumab | Variables | Cmax (μg/mL) | tmax* (h) | AUC (μg×h/mL) | t½ (d) | Cl (mL/h/kg) | Vss (mL/kg) | MRT (d) |
|---|---|---|---|---|---|---|---|---|
| 0.5 mg/kg | Mean ± SD | 7.51 ± 1.41 | 1.75 (0.95–2.00) | 2716 ± 462 | 24.0 ± 4.0 | 0.190 ± 0.040 | 147 ± 27.4 | 32.8 ± 7.54 |
| n = 6 | % CV | 18.8 | 17 | 16.7 | 21.1 | 18.6 | 23.0 | |
| 1.5 mg/kg | Mean ± SD | 45.7 ± 6.59 | 1.53 (1.00–6.00) | 15,890 ± 3950 | 21.0 ± 1.53 | 0.099 ± 0.023 | 69.7 ± 10.1 | 30.2 ± 6.13 |
| n = 6 | % CV | 14.4 | 24.9 | 7.3 | 23.0 | 14.4 | 20.3 | |
| 5 mg/kg | Mean ± SD | 114 ± 29.9 | 1.31 (0.50–6.00) | 38,993 ± 5008 | 26.1 ± 4.96 | 0.130 ± 0.016 | 116 ± 17.1 | 33.8 ± 6.75 |
| n = 10 | % CV | 26.1 | 12.8 | 19.0 | 12.2 | 14.7 | 20.0 |
Median and range are provided for tmax.
AUC indicates area under the concentration-versus-time curve; Cl, systemic clearance; Cmax, peak concentration; CV, coefficient of variation; MRT, mean residence time; SD, standard deviation; t½, terminal phase elimination half-life; tmax, time to peak concentration; Vss, apparent steady-state volume of distribution.
Plasma total Aβ and Aβx–40 increased with the administration of bapineuzumab. Generally, plasma Aβ values peaked at the 24-hour or 2-week sampling time, returned close to baseline at 16 weeks for the 0.5-mg/kg cohort, and were above baseline at all time points for the 5-mg/kg cohort. Both plasma Aβ1–x and Aβx–40 displayed a positive relationship to bapineuzumab exposure (AUC).
No antibapineuzumab antibodies were found in any of the patients tested.
Exploratory Efficacy
The MMSE score increased from baseline at all time points for the 0.5-mg/kg dose and increased from baseline at all time points except month 6 for the 1.5-mg/kg dose. In contrast, the mean MMSE score decreased from baseline for all time points except month 6 for placebo and decreased from baseline for all time points for the 5-mg/kg dose of bapineuzumab.
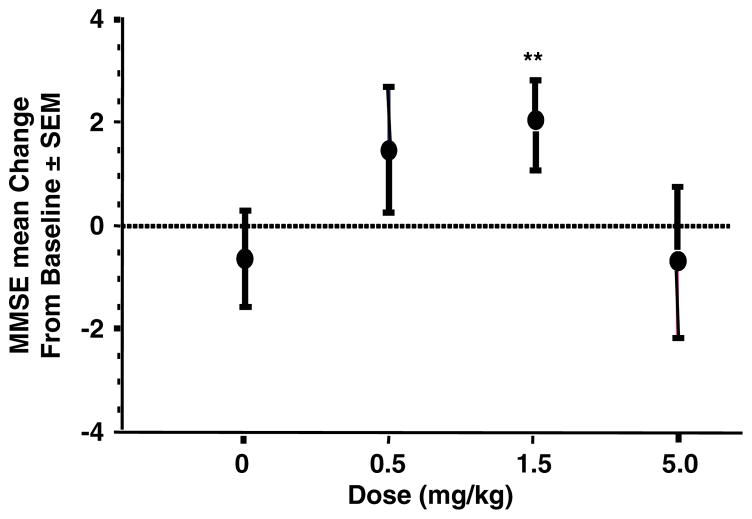
At week 16 (primary time point), the dose response linear trend was not significant, therefore no individual dose group can be declared statistically superior to placebo, based on the primary statistical procedure prespecified in the SAP. However, based on prespecified exploratory analyses (in which treatment groups were to be compared with pooled placebo without control for multiplicity), the treatment difference was higher in the 0.5-mg/kg group (treatment vs. pooled placebo difference=2.1, P=0.152) and the 1.5-mg/kg dose (treatment vs. placebo difference= 2.6, P=0.047) (Fig. 4).
FIGURE 4.
Mean change (± SE) from baseline in the mean MMSE scores at week 16 for the 3 dose groups in the study and the pooled placebo patients (zero dose). **1.5 mg/kg versus pooled placebo; P = 0.047. MMSE indicates Mini-Mental State Examination.
The final adjusted mean score on the MMSE increased to approximately 22.3 for the 0.5 and 1.5-mg/kg groups, but decreased to 20.7 for the 5-mg/kg group and 21.2 for the placebo group.
DISCUSSION
This study was conducted to determine the safety, tolerability, and PK of single ascending doses of bapineuzumab in patients with mild-to-moderate AD, and to provide a preliminary assessment for a future multiple-dose regimen study. We hypothesized that the administration of a humanized mAb directed against the N terminus of Aβ would reduce or eliminate the formation of plaque and/or mediate the removal of plaque in patients with AD, thus slowing disease progression.
The highest single infusion dose of 5 mg/kg was associated with MRI FLAIR abnormalities, the nature and pathophysiology of which remain the subject of investigation. The MRI findings were asymptomatic or resulted in transient side effects, such as mildly increased confusion. Repeat MRI scans performed over the subsequent weeks showed that observed MRI FLAIR changes had generally resolved. It is likely that these events represented vasogenic edema, indicative of altered vascular permeability induced by interaction of bapineuzumab with Aβ in blood vessel walls. It is also possible that some vessels (eg, those with preexisting cerebral amyloid angiopathy) may be susceptible to slowed drainage of interstitial fluid after amyloid is mobilized by immunotherapy and removed along perivascular pathways.9 N-terminal antibodies have been shown to clear amyloid deposits from the brain in studies of autopsy tissue derived from patients exposed to AN1792.10–12
Microhemorrhage was associated with the emergence of vasogenic edema in 1 patient. Cerebral hemorrhage has also been reported to occur in transgenic mice that expressed high levels of Aβ after administration of mAbs similar to m3D6, the murine parent of bapineuzumab, which are specific for the N terminus of Aβ.13,14 Immunized mice exhibited more than 2-fold increase in the frequency of cerebral amyloid angiopathy-associated cerebral hemorrhage. In these mice, microhemorrhages and intracellular accumulations of hemosiderin were found in proximity to areas of vascular amyloid deposition.14
To monitor microhemorrhage, patients in this study routinely underwent MRI scans using the T2* sequence, which is highly sensitive to the presence of microscopic heme deposits that might be sequelae of microhemorrhage in the brain. Microhemorrhages are reported to occur in up to 18% of AD patients using this MRI sequence.15 This finding warrants a continued follow-up in future bapineuzumab clinical studies.
After a single intravenous dose, plasma bapineuzumab levels increased, with median values peaking between 1 and 2 hours after the start of the infusion. The levels remained elevated, with half-lives ranging from 21 to 26 days depending on the dose, thus supporting a 13-week dosing interval in a subsequent multiple-dose study. Elevated plasma antibody concentrations were associated with markedly elevated plasma Aβ levels. Aβ in the plasma has generally been thought to have a rapid turnover under normal conditions,16 so it is unclear whether the observed elevation in plasma Aβ represents mobilization from sources outside the plasma, whether it is due to stabilization by antibody binding, reduced clearance, or both.
The MMSE is a simple test of memory, orientation, language, and constructional abilities, and was used in this study as an indicator of the general cognitive status of patients. A prespecified analysis of change in MMSE at week 16 was planned as an exploratory efficacy end point of this study, comparing the patients dosed with bapineuzumab in each dose group with the combined placebo group. Although there was evidence of efficacy at the 0.5 and 1.5- mg/kg doses, there was no effect on MMSE score in the highest dose group (5 mg/kg).
The results from this phase 1 study provide a preliminary assessment for designing a future multiple-dose regimen study. MMSE scores improved at the lower doses of bapineuzumab compared with placebo, but not with the highest dose (5 mg/kg). This higher dose was also associated with MRI FLAIR abnormalities that resolved over time. The safety and efficacy results, combined with the half-life of 21 to 26 days determined by PK analyses, suggest that it may be possible to administer bapineuzumab at quarterly dosing intervals throughout the year. On the basis of the findings in this phase 1 trial, a phase 2 trial has been conducted with quarterly dosing at a maximum dose of 2 mg/kg, and a phase 3 program to test this further is ongoing.
Acknowledgments
Sponsored by Wyeth Pharmaceuticals (Collegeville, PA) and Elan Pharmaceuticals (San Francisco, CA).
The authors thank Timothy Nicholas, PhD (Wyeth), for support with pharmacokinetic and pharmacodynamic analysis, and Susan A. Nastasee (Wyeth) for assistance in preparation of the manuscript.
Footnotes
Disclosures: Ronald S. Black, Allan Pallay, and Alice Nichols are employees of Wyeth Pharmaceuticals. Reisa A. Sperling has received grants from Wyeth and Elan for research activities, and honoraria for consulting services. Ruth N. Motter and Michael Grundman are employees of Elan Pharmaceuticals. The remaining authors have no disclosures.
References
- 1.Goñi F, Sigurdsson EM. New directions towards safer and effective vaccines for Alzheimer’s disease. Curr Opin Mol Ther. 2005;7:17–23. [PubMed] [Google Scholar]
- 2.Schenk D, Barbour R, Dunn W, et al. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 3.Morgan D, Diamond DM, Gottschall PE, et al. Aβ peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 4.Janus C, Pearson J, McLaurin J, et al. Aβ peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 5.Orgogozo J-M, Gilman S, Dartigues J-F, et al. Subacute meningoencephalitis in a subset of patients with AD after Aβ42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- 6.Pride M, Seubert P, Grundman M, et al. Progress in the active immunotherapeutic approach to Alzheimer’s disease: clinical investigations into AN1792-associated meningoencephalitis. Neurodegenerative Dis. 2008;5:194–196. doi: 10.1159/000113700. [DOI] [PubMed] [Google Scholar]
- 7.Lee M, Bard F, Johnson-Wood K, et al. Aβ42 immunization in Alzheimer’s disease generates Aβ N-terminal antibodies. Ann Neurol. 2005;58:430–435. doi: 10.1002/ana.20592. [DOI] [PubMed] [Google Scholar]
- 8.McLaurin J, Cecal R, Kierstead ME, et al. Therapeutically effective antibodies against amyloid-beta peptide target amyloid-beta residues 4–10 and inhibit cytotoxicity and fibrillogenesis. Nat Med. 2002;8:1263–1269. doi: 10.1038/nm790. [DOI] [PubMed] [Google Scholar]
- 9.Weller RO, Nicoll JAR. Cerebral amyloid angiopathy: both viper and maggot in the brain. Ann Neurol. 2005;58:348–350. doi: 10.1002/ana.20622. [DOI] [PubMed] [Google Scholar]
- 10.Ferrer I, Boada Rovira M, Sánchez Guerra ML, et al. Neuropathology and pathogenesis of encephalitis following amyloid-β immunization in Alzheimer’s disease. Brain Pathol. 2004;14:11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masliah E, Hansen L, Adame A, et al. Aβ vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology. 2005;64:129–131. doi: 10.1212/01.WNL.0000148590.39911.DF. [DOI] [PubMed] [Google Scholar]
- 12.Nicoll JAR, Wilkinson D, Holmes C, et al. Neuropathology of human Alzheimer disease after immunization with amyloid-β peptide: a case report. Nat Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 13.Pfeifer M, Boncristiano S, Bondolfi L, et al. Cerebral hemorrhage after passive anti-Aβ immunotherapy. Science. 2002;298:1379. doi: 10.1126/science.1078259. [DOI] [PubMed] [Google Scholar]
- 14.Racke MM, Boone LI, Hepburn DL, et al. Exacerbation of cerebral amyloid angiopathy-associated microhemorrhage in amyloid precursor protein transgenic mice by immunotherapy is dependent on antibody recognition of deposited forms of amyloid β. J Neurosci. 2005;25:629–636. doi: 10.1523/JNEUROSCI.4337-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cordonnier C, van der Flier WM, Sluimer JD, et al. Prevalence and severity of microbleeds in a memory clinic setting. Neurology. 2006;66:1356–1360. doi: 10.1212/01.wnl.0000210535.20297.ae. [DOI] [PubMed] [Google Scholar]
- 16.Bateman RJ, Munsell LY, Morris JC, et al. Human amyloid-β synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nature Med. 2006;12:856–861. doi: 10.1038/nm1438. [DOI] [PMC free article] [PubMed] [Google Scholar]