Abstract
Our previous studies (1974. J. Clin. Invest.54: 753-762.) suggested that impaired metabolism of cyclic AMP (cAMP) may be involved in the renal unresponsiveness to vasopressin (VP) in mice with hereditary nephrogenic diabetes insipidus (NDI). To localize such a defect to specific segments of the nephron, we studied the activities of VP-sensitive adenylate cyclase, cAMP phosphodiesterase (cAMP-PDIE), as well as accumulation of cAMP in medullary collecting tubules (MCT) and in medullary thick ascending limbs of Henle's loop (MAL) microdissected from control mice with normal concentrating ability and from mice with hereditary NDI.
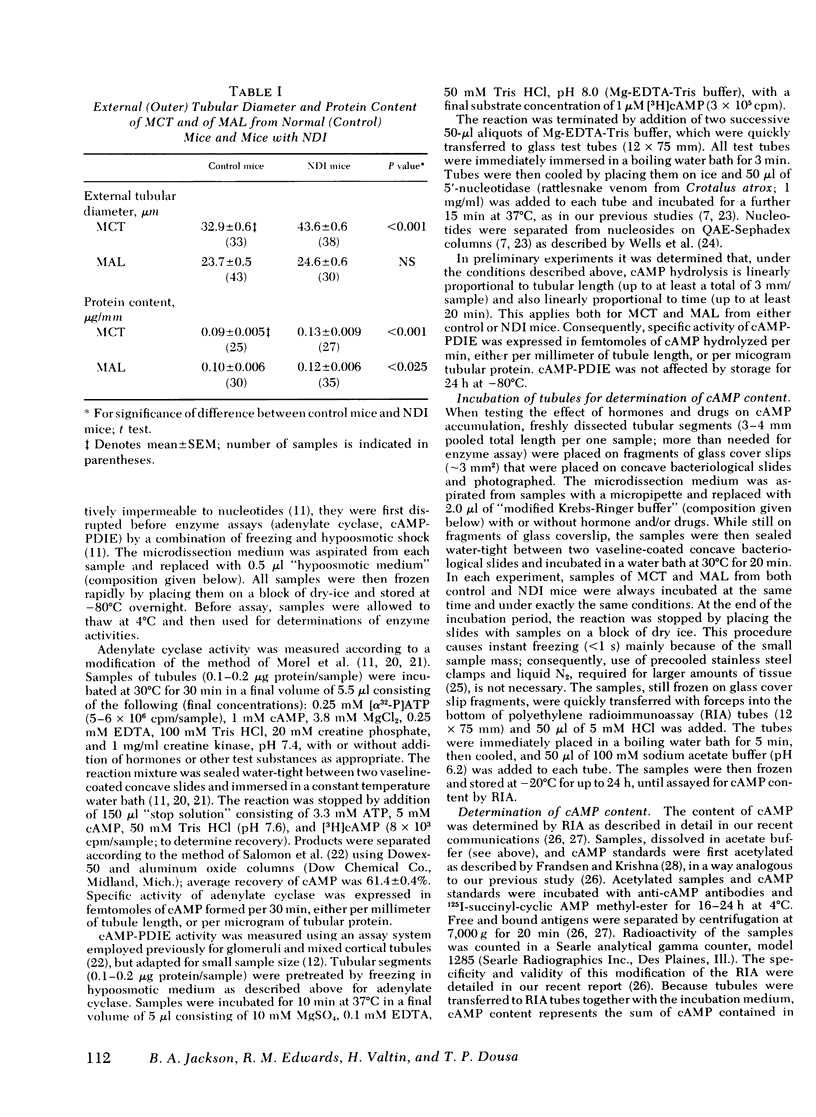
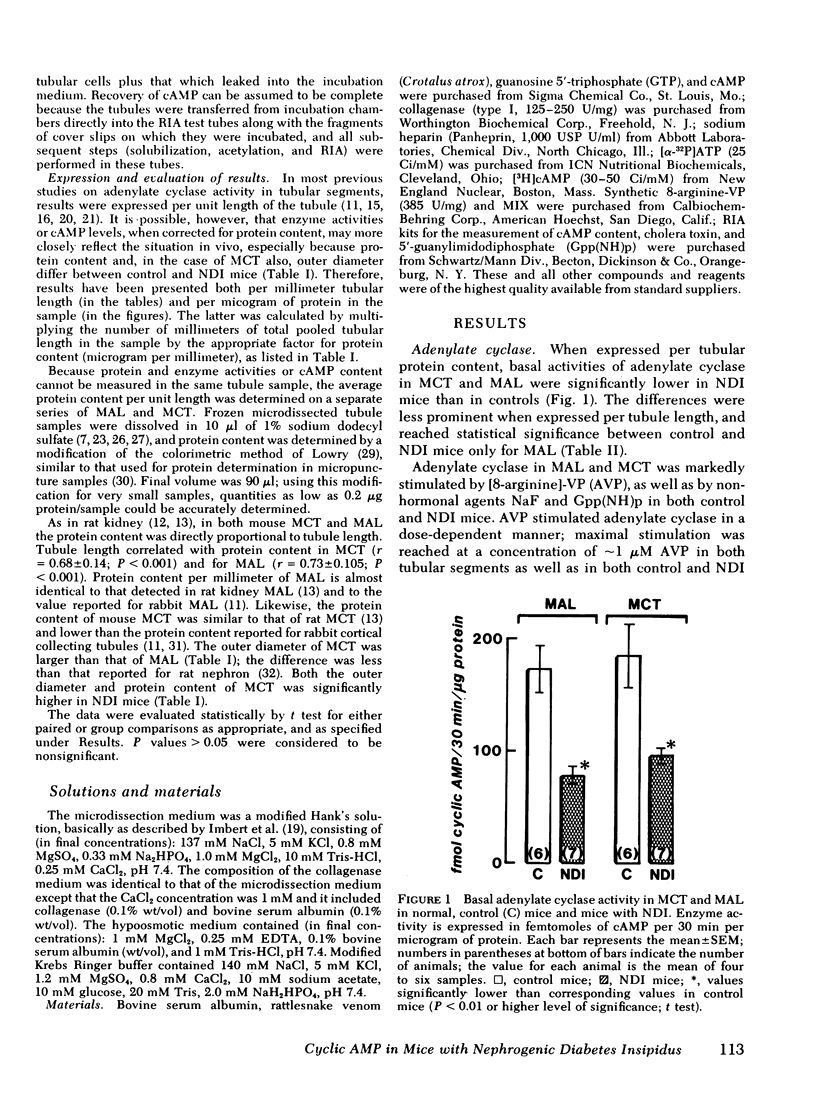
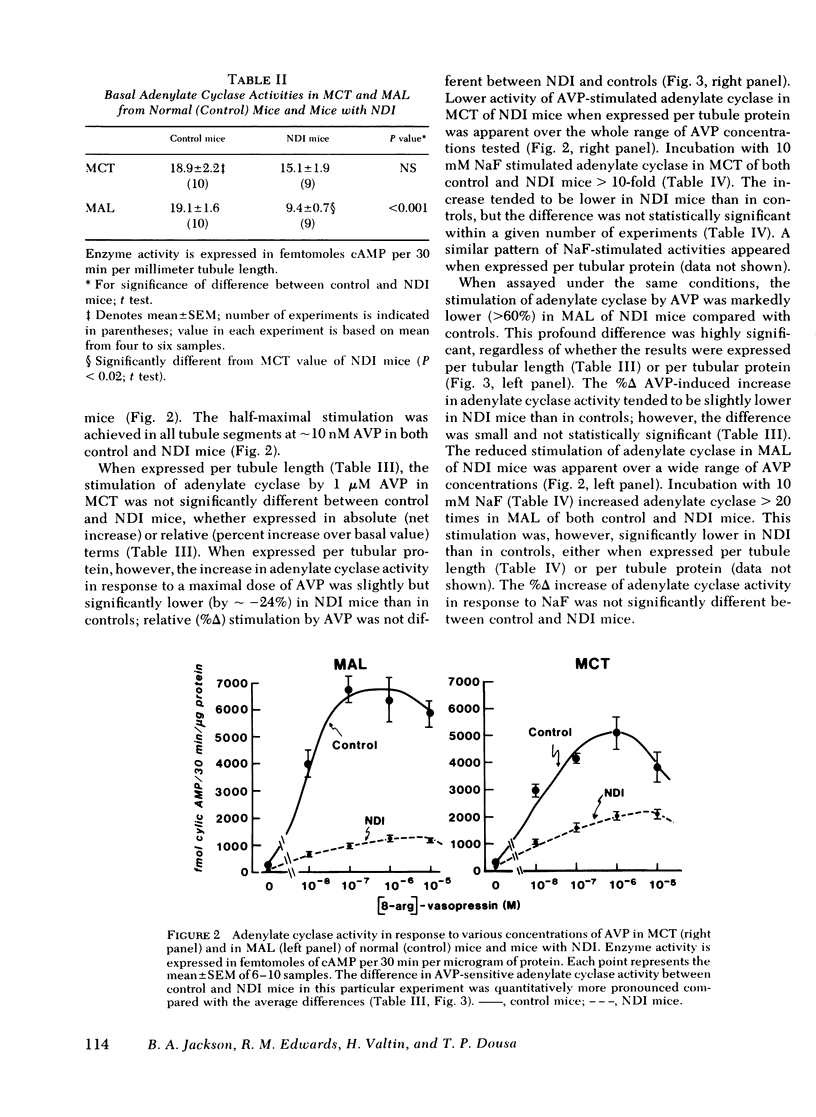
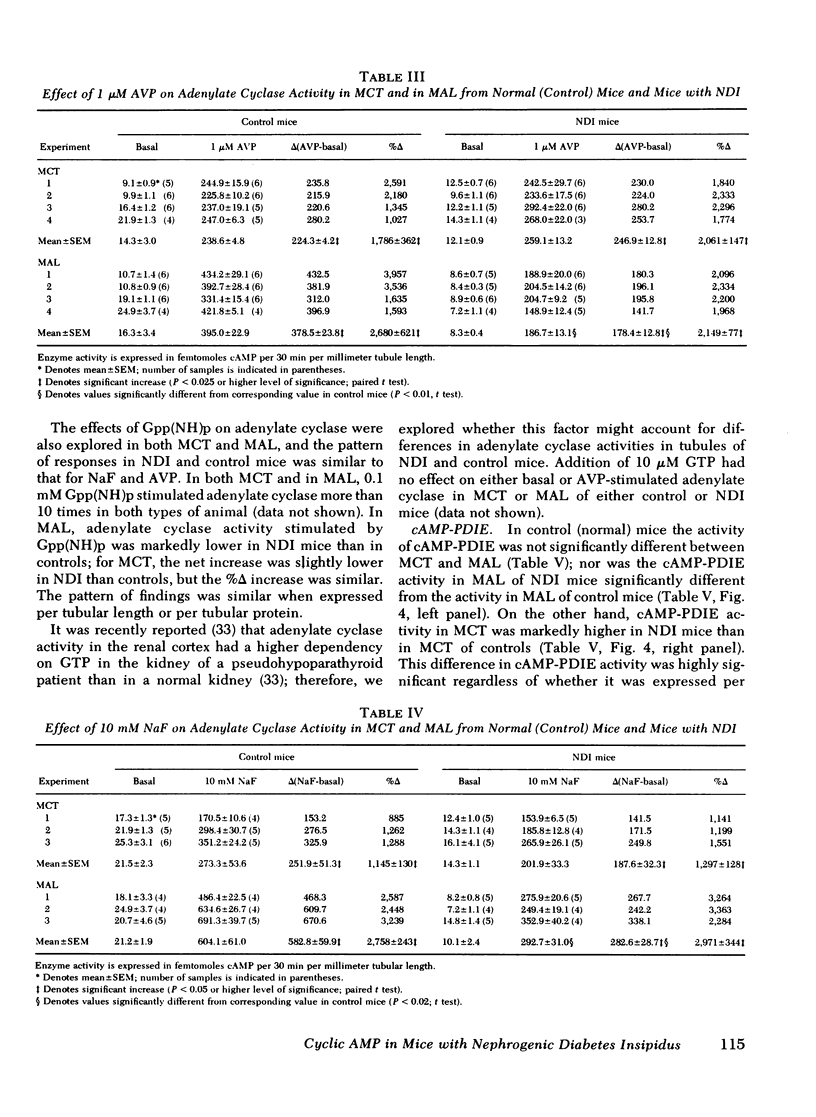
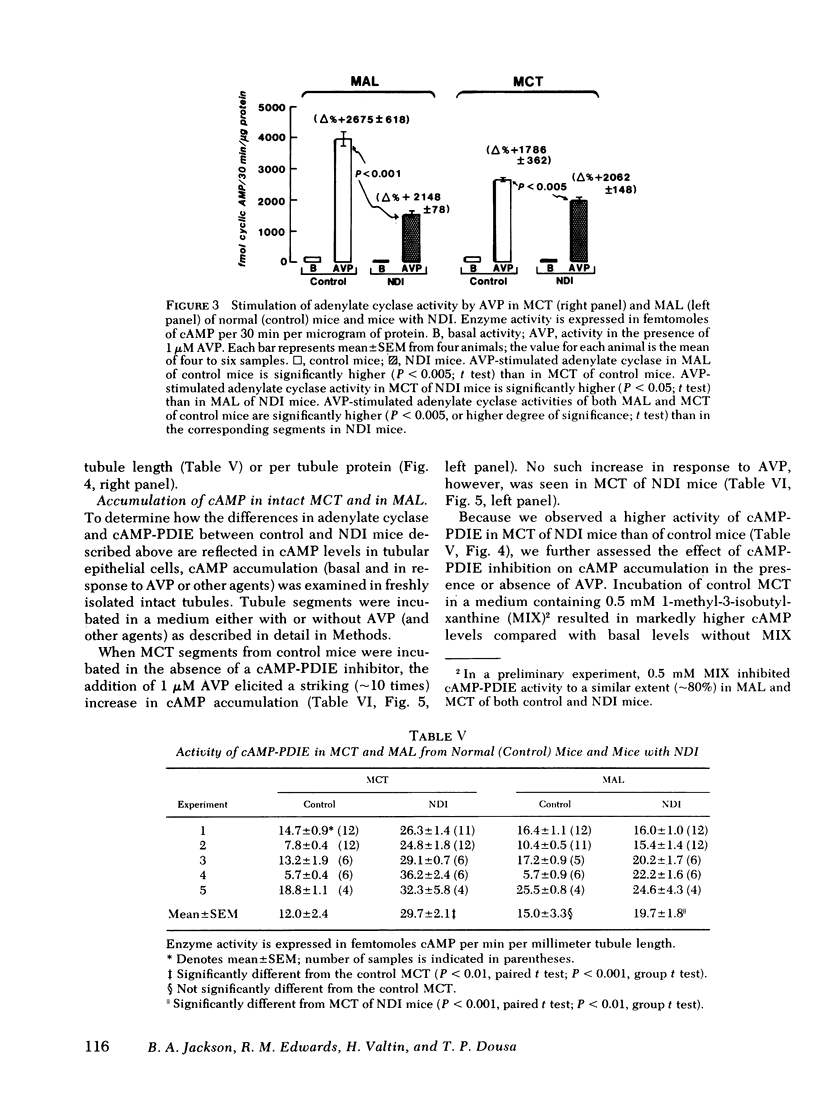
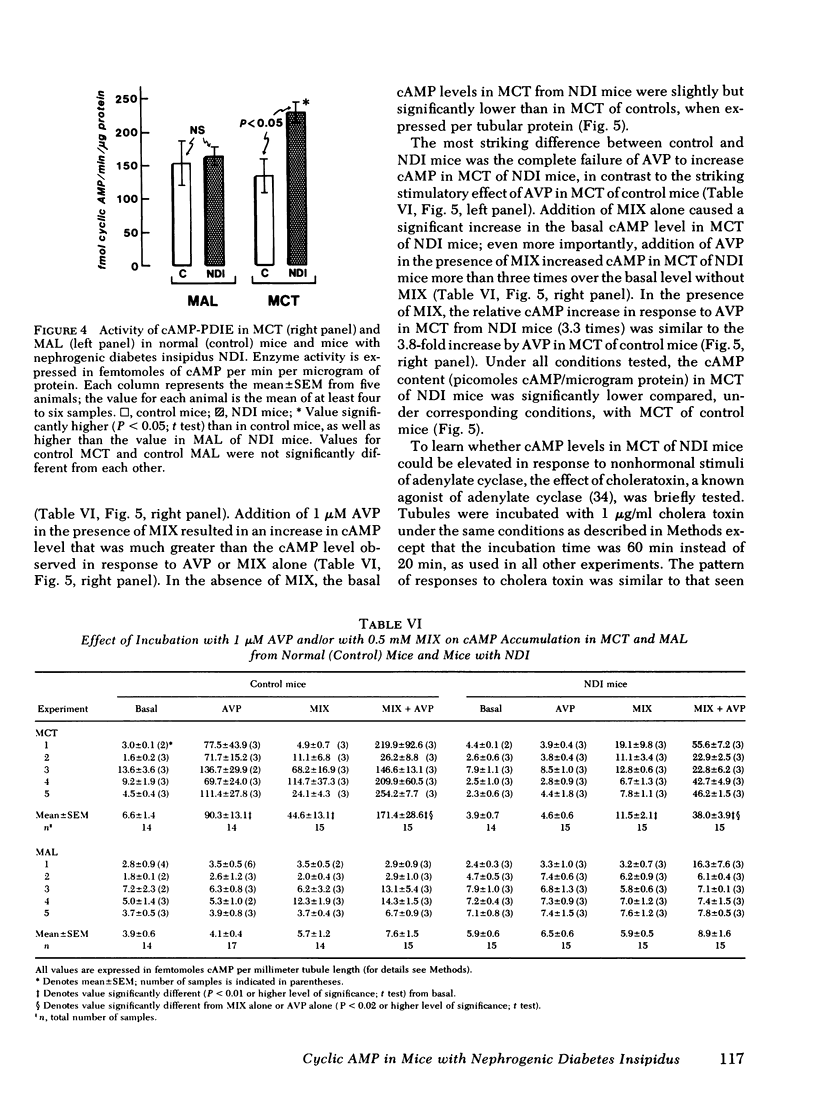
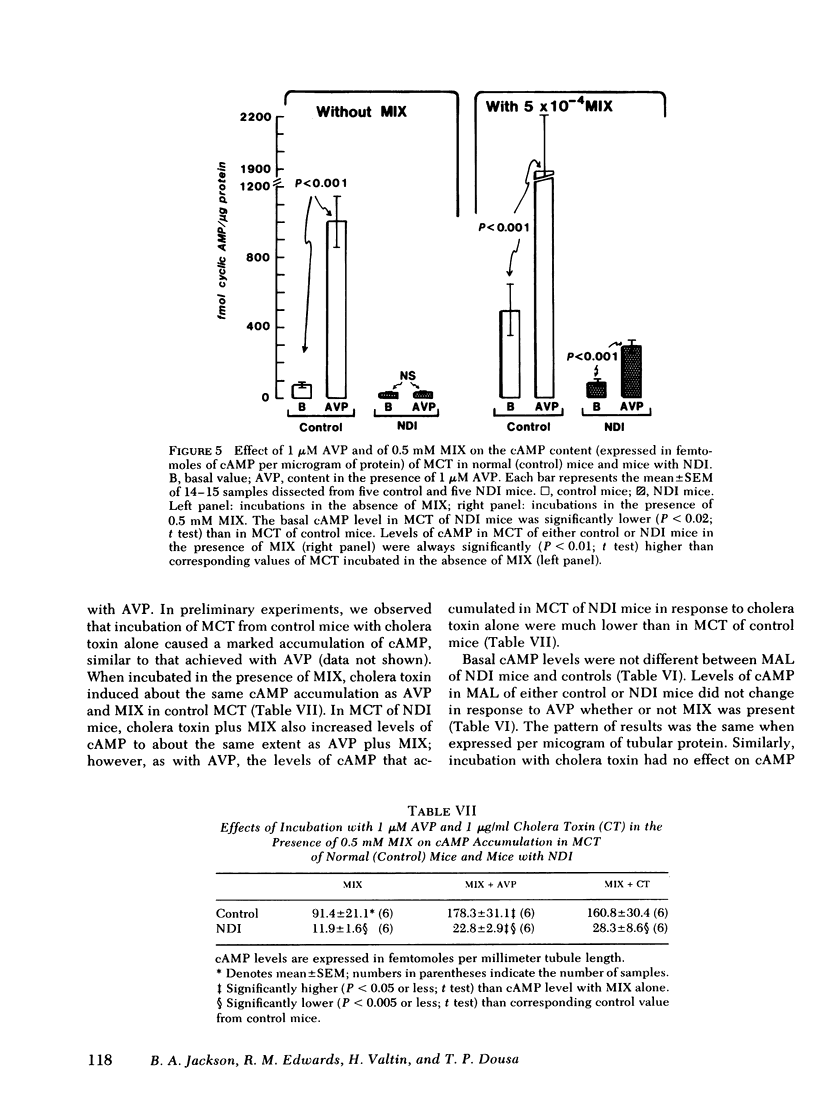
Adenylate cyclase activity stimulated by VP or by NaF was only slightly lower (−24%) in MCT from NDI mice, compared with controls. In MAL of NDI mice, basal, VP-sensitive, and NaF-sensitive adenylate cyclase was markedly (> −60%) lower compared with MAL of controls. The specific activity of cAMP-PDIE was markedly higher in MCT of NDI mice compared with controls, but was not different between MAL of control and NDI mice. Under present in vitro conditions, incubation of intact MCT from control mice with VP caused a striking increase in cAMP levels (>10), but VP failed to elicit a change in cAMP levels in MCT from NDI mice. When the cAMP-PDIE inhibitor 1-methyl-3-isobutyl xanthine (MIX) was added to the above incubation, VP caused a significant increase in cAMP levels in MCT from both NDI mice and control mice. Under all tested conditions, cAMP levels in MCT of NDI mice were lower than corresponding values in control MCT. Under the present experimental setting, VP and other stimulating factors (MIX, cholera toxin) did not change cAMP levels in MAL from either control mice or from NDI mice.
The results of the present in vitro experiments suggest that the functional unresponsiveness of NDI mice to VP is perhaps mainly the result of the inability of collecting tubules to increase intracellular cAMP levels in response to VP. In turn, this inability to increase cAMP in response to VP is at least partly the result of abnormally high activity of cAMP-PDIE, a somewhat lower activity of VP-sensitive adenylate cyclase in MCT of NDI mice, and perhaps to a deficiency of some other as yet unidentified factors. The possible contribution of low VP-sensitive adenylate cyclase activity in MAL of NDI mice to the renal resistance to VP remains to be defined.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramow M. Effects of ethacrynic acid on the isolated collecting tubule. J Clin Invest. 1974 Mar;53(3):796–804. doi: 10.1172/JCI107618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. M., Falchuk K. H., Keimowitz R. I., Berliner R. W. The relationship between peritubular capillary protein concentration and fluid reabsorption by the renal proximal tubule. J Clin Invest. 1969 Aug;48(8):1519–1531. doi: 10.1172/JCI106118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunette M. G., Chabardes D., Imbert-Teboul M., Clique A., Montégut M., Morel F. Hormone-sensitive adenylate cyclase along the nephron of genetically hypophosphatemic mice. Kidney Int. 1979 Apr;15(4):357–369. doi: 10.1038/ki.1979.47. [DOI] [PubMed] [Google Scholar]
- Chabardès D., Gagnan-Brunette M., Imbert-Teboul M., Gontcharevskaia O., Montégut M., Clique A., Morel F. Adenylate cyclase responsiveness to hormones in various portions of the human nephron. J Clin Invest. 1980 Feb;65(2):439–448. doi: 10.1172/JCI109687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabardés D., Imbert-Teboul M., Gagnan-Brunette M., Morel F. Different hormonal target sites along the mouse and rabbit nephrons. Curr Probl Clin Biochem. 1977 Oct 23;8:447–454. [PubMed] [Google Scholar]
- Dousa T. P., Valtin H. Cellular action of antidiuretic hormone in mice with inherited vasopressin-resistant urinary concentrating defects. J Clin Invest. 1974 Sep;54(3):753–762. doi: 10.1172/JCI107813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dousa T. P., Valtin H. Cellular actions of vasopressin in the mammalian kidney. Kidney Int. 1976 Jul;10(1):46–63. doi: 10.1038/ki.1976.78. [DOI] [PubMed] [Google Scholar]
- Drezner M. K., Burch W. M., Jr Altered activity of the nucleotide regulatory site in the parathyroid hormone-sensitive adenylate cyclase from the renal cortex of a patient with pseudohypoparathyroidism. J Clin Invest. 1978 Dec;62(6):1222–1227. doi: 10.1172/JCI109242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R. M., Jackson B. A., Dousa T. P. Protein kinase activity in isolated tubules of rat renal medulla. Am J Physiol. 1980 Apr;238(4):F269–F278. doi: 10.1152/ajprenal.1980.238.4.F269. [DOI] [PubMed] [Google Scholar]
- Fine L. G., Schlondorff D., Trizna W., Gilbert R. M., Bricker N. S. Functional profile of the isolated uremic nephron. Impaired water permeability and adenylate cyclase responsiveness of the cortical collecting tubule to vasopressin. J Clin Invest. 1978 Jun;61(6):1519–1527. doi: 10.1172/JCI109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandsen E. K., Krishna G. A simple ultrasensitive method for the assay of cyclic AMP and cyclic GMP in tissues. Life Sci. 1976 Mar 1;18(5):529–541. doi: 10.1016/0024-3205(76)90331-3. [DOI] [PubMed] [Google Scholar]
- Grantham J. J., Burg M. B. Effect of vasopressin and cyclic AMP on permeability of isolated collecting tubules. Am J Physiol. 1966 Jul;211(1):255–259. doi: 10.1152/ajplegacy.1966.211.1.255. [DOI] [PubMed] [Google Scholar]
- Imbert-Teboul M., Chabardès D., Montégut M., Clique A., Morel F. Vasopressin-dependent adenylate cyclase activities in the rat kidney medulla: evidence for two separate sites of action. Endocrinology. 1978 Apr;102(4):1254–1261. doi: 10.1210/endo-102-4-1254. [DOI] [PubMed] [Google Scholar]
- Jard S., Roy C., Barth T., Rajerison R., Bockaert J. Antidiuretic hormone-sensitive kidney adenylate cyclase. Adv Cyclic Nucleotide Res. 1975;5:31–52. [PubMed] [Google Scholar]
- Katz A. I., Doucet A., Morel F. Na-K-ATPase activity along the rabbit, rat, and mouse nephron. Am J Physiol. 1979 Aug;237(2):F114–F120. doi: 10.1152/ajprenal.1979.237.2.F114. [DOI] [PubMed] [Google Scholar]
- Kettyle W. M., Valtin H. Chemical and dimensional chracterization of the renal countercurrent system in mice. Kidney Int. 1972 Mar;1(3):135–144. doi: 10.1038/ki.1972.21. [DOI] [PubMed] [Google Scholar]
- Knepper M. A., Danielson R. A., Saidel G. M., Post R. S. Quantitative analysis of renal medullary anatomy in rats and rabbits. Kidney Int. 1977 Nov;12(5):313–323. doi: 10.1038/ki.1977.118. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mayer S. E., Stull J. T., Wastila W. B. Rapid tissue fixation and extraction techniques. Methods Enzymol. 1974;38:1–9. [PubMed] [Google Scholar]
- Moss J., Vaughan M. Activation of adenylate cyclase by choleragen. Annu Rev Biochem. 1979;48:581–600. doi: 10.1146/annurev.bi.48.070179.003053. [DOI] [PubMed] [Google Scholar]
- Naik D. V., Valtin H. Hereditary vasopressin-resistant urinary concentrating defects in mice. Am J Physiol. 1969 Oct;217(4):1183–1190. doi: 10.1152/ajplegacy.1969.217.4.1183. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Sasaki S., Imai M. Effects of vasopressin on water and NaCl transport across the in vitro perfused medullary thick ascending limb of Henle's loop of mouse, rat, and rabbit kidneys. Pflugers Arch. 1980 Feb;383(3):215–221. doi: 10.1007/BF00587521. [DOI] [PubMed] [Google Scholar]
- Shah S. V., Northrup T. E., Hui Y. S., Dousa T. P. Action of serotonin (5-hydroxytryptamine) on cyclic nucleotides in glomeruli of rat renal cortex. Kidney Int. 1979 May;15(5):463–472. doi: 10.1038/ki.1979.62. [DOI] [PubMed] [Google Scholar]
- Strada S. J., Thompson W. J. Multiple forms of cyclic nucleotide phosphodiesterases: anomalies or biologic regulators? Adv Cyclic Nucleotide Res. 1978;9:265–283. [PubMed] [Google Scholar]
- Torres V. E., Hui Y. S., Shah S. V., Northrup T. E., Dousa T. P. Cyclic nucleotide phosphodiesterases in glomeruli of rat renal cortex. Kidney Int. 1978 Nov;14(5):444–451. doi: 10.1038/ki.1978.149. [DOI] [PubMed] [Google Scholar]
- Torres V. E., Northrup T. E., Edwards R. M., Shah S. V., Dousa T. P. Modulation of cyclic nucleotides in islated rat glomeruli: role of histamine, carbamylcholine, parathyroid hormone, and angiotensin-II. J Clin Invest. 1978 Dec;62(6):1334–1343. doi: 10.1172/JCI109254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtin H., Sokol H. W., Sunde D. Genetic approaches to the study of the regulation and actions of vasopressin. Recent Prog Horm Res. 1975;31:447–486. doi: 10.1016/b978-0-12-571131-9.50016-5. [DOI] [PubMed] [Google Scholar]
- Wells J. N., Baird C. E., Hardman Y. J., Wu J. G. Cyclic nucleotide phosphodiesterase activities of pig coronary arteries. Biochim Biophys Acta. 1975 Apr 19;384(2):430–442. doi: 10.1016/0005-2744(75)90044-3. [DOI] [PubMed] [Google Scholar]


