Abstract
Neuronal activity, especially of the excitatory glutamatergic type, is highly dependent on energy from the oxidative pathway. We hypothesized that the coupling existed at the transcriptional level by having the same transcription factor to regulate a marker of energy metabolism, cytochrome c oxidase (COX) and an important subunit of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) glutamate receptors, GluR2 (Gria2). Nuclear respiratory factor 1 (NRF-1) was a viable candidate because it regulates all COX subunits and potentially activates Gria2. By means of in silico analysis, electrophoretic mobility shift and supershift, chromatin immunoprecipitation, and promoter mutational assays, we found that NRF-1 functionally bound to Gria2 promoter. Silencing of NRF-1 with small interference RNA prevented the depolarization-stimulated up-regulation of Gria2 and COX, and over-expression of NRF-1 rescued neurons from TTX-induced down-regulation of Gria2 and COX transcripts. Thus, neuronal activity and energy metabolism are tightly coupled at the molecular level, and NRF-1 is a critical agent in this process.
Keywords: AMPA receptor, energy metabolism, excitatory neurotransmission, gene regulation, KCl depolarization, siRNA silencing
Introduction
AMPA-type glutamate receptors represent one of the major classes of receptors by which glutamate, the dominant excitatory neurotransmitter in the nervous system, regulates neuronal functions (Nakanishi, 1992; Seeburg, 1993). AMPA receptors also play a crucial role in synaptogenesis, formation of neuronal circuitry, as well as in synaptic plasticity (Tanaka et al., 2000; Palmer et al., 2005). These receptors mediate fast synaptic transmission, are ionotropic, ligand-gated, and are made up of GluR1, GluR2, GluR3, and GluR4 (GluR A–D) subunits in various combinations (Keinänen et al., 1990; Borges and Dingledine, 1998). Subunit 2 (GluR2) is of special interest, because it determines the Ca2+ permeability of AMPA receptors. Receptors that contain this subunit inhibit Ca2+ entry because the glutamine residue in its transmembrane segment 2 has been mRNA-edited to a positively-charged arginine residue (Hollman et al., 1991; Verdoorn et al., 1991; Tanaka et al., 2000). The expression of GluR2 has been shown to be governed by neuronal activity (Wong-Riley and Jacobs, 2002; Bai and Wong-Riley, 2003). Neuronal activity is tightly coupled to energy metabolism, and repolarization subsequent to depolarizing excitation constitutes the bulk of energy demand in neurons and requires ATP-dependent Na+/K+ ATPase to actively pump cations against their concentration and electrical gradients (Wong-Riley, 1989). Most of the ATP in neurons is derived from oxidative metabolism, and cytochrome c oxidase (COX) is a critical energy-generating enzyme (Wong-Riley, 1989). It is the terminal enzyme of the electron transport chain, catalyzing the final step of oxidative metabolism (Wikström et al., 1981). Regions and neurons rich in COX are dominated by excitatory glutamatergic input (Wong-Riley et al., 1998). When excitatory transmission is suppressed, such as with tetrodotoxin (TTX)-induced impulse blockade, both COX and AMPA receptor levels are reduced (Wong-Riley and Jacobs, 2002; Bai and Wong-Riley, 2003). Such parallel regulation in the expression of COX and AMPA receptors begs the question: Is it possible that the same transcription factor co-regulates the expression of both? The goal of the present study is to test our hypothesis that nuclear respiratory factor 1 (NRF-1), recently found to functionally regulate all 13 subunits of COX (Dhar et al., 2008), plays such a dual role. Remarkably, NRF-1 also regulates specific subunit genes of NMDA receptors, a mediator of glutamatergic synaptic transmission (Dhar and Wong-Riley, 2008). The GC-rich proximal region of the rat Gria2 promoter contains consensus recognition sequence for NRF-1 (Myers et al., 1998). However, its functional role in the regulation of AMPA receptor subunit genes in neurons has not been rigorously tested. It is also not known if NRF-1 regulates the other AMPA subunit genes. Using a variety of approaches, the present study sought to determine the potential role of NRF-1 in dually regulating the expressions of both COX and AMPA receptor subunit genes in neurons.
Materials and methods
All experiments were carried out in accordance with the US National Institutes of Health Guide for the care and use of laboratory animals and the Medical College of Wisconsin regulations. All efforts were made to minimize the number of animals and their suffering.
Cell culture
Murine neuroblastoma (N2a) cells (ATCC, Manassas, VA) were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 50 units/ml penicillin, and 100 µg/ml streptomycin (Invitrogen, Carlsbad, CA) at 37°C in a humidified atmosphere with 5% CO2.
Sprague Dawley rats (Harlan, Indianapolis, IN) at 1 day of age were used for primary cultures. Rat primary visual cortical neurons were cultured as described previously (Ongwijitwat and Wong-Riley, 2005). Briefly, 1-day-old neonatal rat pups were sacrificed by decapitation. Brains were removed from the skull and the meninges were removed. Visual cortical tissue was dissected, trypsinized, and triturated to release individual neurons. Neurons were plated in 35 mm poly-l-lysine-coated dishes at a density of 50,000 cells/dish. Cells were maintained in Neurobasal-A media supplemented with B27 (Invitrogen). Ara-C (Sigma, St. Louis, MO) was added to the media to suppress the proliferation of glial cells.
In silico analysis of promoters of murine AMPA receptor subunit genes
DNA sequences surrounding the transcription start points (TSPs) of AMPA receptor subunit genes (Gria1-4) were derived from the mouse genome database in GenBank™ (NCBI GenBank accession numbers for Gria1: NM_008165, Gria2: NM_013540, Gria3: NM_016886, and Gria4: NM_019691), using Genomatix Gene2promoter software. These promoter sequences encompassed 1 kb upstream and up to 200 bps downstream (excluding protein-coding sequence) of the TSP of each gene analyzed. Computer-assisted search for putative NRF-1 core binding sequences “GCGCAT/CGC” or “GCGCAG/CGC” was conducted on each promoter sequence. Alignment of human, mouse, and rat promoter sequences were done as previously described, using the Genome VISTA genome alignment tool (Ongwijitwat and Wong-Riley, 2005). Mouse Gria1-4 promoter sequences were compared with rat and human genomic sequences using a 5-bp calculation window. Regions of high homology and/or containing known NRF-1 binding sites were compared for the conservation of NRF-1 binding.
Electrophoretic mobility shift (EMSA) and supershift assays
EMSAs to assay NRF-1 interactions with putative binding elements on all AMPA receptor subunit promoters were carried out with methods as previously described (Dhar et al., 2008). Briefly, oligonucleotide probes with putative NRF-1 binding site on each promoter (Gria1-4 subunit) (Table I, based on in silico analysis) were synthesized, annealed, and labeled by a Klenow fragment (Invitrogen) fill-in reaction with [α-32P] dATP (50 µCi/200 ng; Perkin-Elmer, Shelton, CT). Each labeled probe was incubated with 2 µg of calf thymus DNA and 5 µg of HeLa nuclear extract (Promega, Madison, WI) and processed for EMSA. Supershift assays were also performed and, in each reaction, 1–1.5 µg of NRF-1-specific antibodies (polyclonal goat antibodies, gift of Dr. Richard Scarpulla, Northwestern University, Chicago, IL) were added to the probe/nuclear extract mixture and incubated for 20 min at room temperature. For competition, 100-fold excess of unlabeled oligonucleotides were incubated with nuclear extract before adding labeled oligonucleotides. Shift reactions were loaded onto 4% polyacrylamide gel and run at 200 V for 2.5 h in 0.25X TBE buffer. Results were visualized by autoradiography. Rat cytochrome c with NRF-1 binding site at position −172/−147 was designed as previously described (Evans and Scarpulla, 1990) and used as a positive control. NRF-1 mutants with mutated sequences as shown in Table IA were used as negative controls.
Table I. A EMSA Probes.
Positions of probes are given relative to TSP. Putative NRF-1 binding sites are in boldface. Mutated nucleotide sequences are underlined.
| Gene Promoter | Position | Sequence |
|---|---|---|
| Gria1 | −218/−185 | F 5′TTTTGAGCGAGCTAGCGCTCCAAGCATGAGGACGGGC 3′ R 3′CTCGCTCGATCGCGAGGTTCGTACTCCTGCCCGTTTT 5′ |
| Gria2 | −89/−57 | F 5′TTTTGCCTCGAGTCGCGCACGCGCGCCCGGGACTGC 3′ R 3′CGGAGCTCAGCGCGTGCGCGCGGGCCCTGACGTTTT 5′ |
| Gria3 | −161/−136 | F 5′TTTTACATACGCCGGCGCCTAGGCTTTGC 3′ R3′TGTATGCGGCCGCGGATCCGAAACGTTTT 5′ |
| Gria4 | −137/−102 | F 5′TTTTCTCGCGTGCGCGCGCTCGCTCGCTCGCGCGGGCGC 3′ R 3′GAGCGCACGCGCGCGAGCGAGCGAGCGCGCCCGCGTTTT 5′ |
| Gria2 with NRF-1 site mutated | −89/−57 | F 5′TTTTGCCTCGAGTCGCTTTCGCGTTTCCGGGACTGC 3′ R 3′CGGAGCTCAGCGAAAGCGCAAAGGCCCTGACGTTTT 5′ |
| RatCyt C | −172/−147 | F 5′TTTTCTGCTAGCCCGCATGCGCGCGCACCTTA3′ R 3′GACGATCGGGCGTACGCGCGCGTGGAATTTTT5′ |
| B Chip Primers | |||
|---|---|---|---|
| Positions of amplicons are given relative to TSP. | |||
| Gene Promoter | Position | Sequence | Amplicon length |
| Gria1 | −272 to −81 | F 5′GAGAGAGGCTGGCTGCTAGA 3′ R 5′TTCCTCCCTTCCTTCTGTGA 3′ |
191 bps |
| Gria2 | −218 to +45 | F 5′AGAAGCTGCCAGGGCTGT 3′ R 5′CCGGTGTCTGGGAATATCAG 3′ |
263 bps |
| Gria3 | −208 to +21 | F 5′TCCACGTGAGGATTGGTGTA 3′ R 5′GTGGGGAAGAAGAAAAGAGC 3′ |
229 bps |
| Gria4 | −191 to +23 | F 5′CTGGTTGTTTGCTGGTGAGA 3′ R 5′ACCTCCCCATGGTCTCAGTC 3′ |
214 bps |
| TFB2M promoter | −64 to +115 | F 5′GAAGCGAGTGAGCAAAGGAC 3′ R 5′GGTCCCCTCATCCTCCTCTA 3′ |
179 bps |
| β-Actin exon 5 | −134 to +53 | F 5′GCTCTTTTCCAGCCTTCCTT 3′ R 5′CGGATGTCAACGTCACACTT 3′ |
187 bps |
| C PCR cloning primers | |
|---|---|
| NRF-1 mutated nucleotide sequences are in boldface. | |
| Cloning Primers | Primer sequence |
| Gria2 | F 5′AAACGCGTTTTGCTTTTTATCTGGGTCAGC 3′ R 5′AAAGATCTTCCCTCTCTCCTCTCTCACA 3′ |
|
MCOX5b |
F 5′AAGGTACCCAACACTGAGCAATGGAGGA 3′ R 5′AAAAGCTTGGAACAGGCTGAGCAAGATG 3′ |
| Mutagenesis Primers | |
| Gria2Mut | F 5′ AGTCGTTTACGTTTGCCCGGGACTGCCTGCCCCTCTCTGTGACTTT 3′ R 5′ AAAGTCACAGAGAGGGGCAGGCAGTCCCGGGCAAACGTAAACGACT 3′ |
| MCOX5bMut | F 5′CCACGAAGAGGCAGAACTGCTTTTGTGCGGCGTCTACT 3′ R 5′AGTAGACGCCGCACAAAAGCAGTTCTGCCTCTTCGTGG 3′ |
Chromatin immunoprecipitation (ChIP) assays
ChIP assays were performed similar to those previously described (Dhar et al., 2008). Briefly, ~750,000 N2a cells were used for each immunoprecipitation and were fixed with 1% formaldehyde for 10 min at room temperature. ChIP assay kit (Upstate, Charlottesville, VA) was used with minor modifications. Following formaldehyde fixation, cells were resuspended in a swelling buffer (5 mM PIPES, pH 8.0, 85 mM KCl, and 1% Nonidet P-40, and protease inhibitors added right before use) and homogenized 10 times in small pestle Dounce tissue homogenizer (7 ml). Nuclei were then isolated by centrifugation before being subjected to sonication. The sonicated lysate was immunoprecipitated with either 0.2 µg of NRF-1 polyclonal rabbit antibodies (gift of Dr. Scarpulla) or 2 µg of anti-nerve growth factor receptor (NGFR) p75 polyclonal goat antibodies (C20, sc-6188, Santa Cruz Biotechnology, Santa Cruz, CA). Semi-quantitative PCR was performed using 1/20th of precipitated chromatin. Primers targeting promoter sequences near TSP of AMPA receptor subunit genes were designed (Table I) as previously described (Ongwijitwat and Wong-Riley, 2005). Transcription factor B2 of mitochondria (TFB2M) promoter, previously found to be activated by NRF1 (Gleyzer et al, 2005; Ongwijitwat et al., 2006), was used as a positive control, and exon 5 of β-actin gene was used as a negative control (Table I). PCR reactions were carried out with the EX Taq hot-start polymerase (Takara Mirus Bio, Madison, WI) with the following cycling parameters: 30-s denaturation at 94°C, 30-s annealing at 59.5°C, and 20-s extension at 72°C (32–36 cycles per reaction). All reactions were hot-started by heating to 94°C for 120 s. Use of hot-start polymerase and PCR additives significantly improved the quality and reproducibility of ChIP. PCR products were visualized on 2% agarose gels stained with ethidium bromide.
Construction and transfection of luciferase reporter vectors for promoter mutagenesis study
Luciferase reporter constructs of Gria2 promoters were made by PCR cloning the proximal promoter sequences using genomic DNA prepared from mouse N2a cells as template, digesting with restriction enzymes MluI and BglII endonucleases, and ligating the product directionally into pGL3 basic (Promega).
Sequences of primers used for PCR cloning and mutagenesis are provided in Table I. Subunit COX5b clone was used from our previous study (Dhar et al., 2008). Site-directed mutations of putative NRF-1 binding site on each promoter was generated using QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). All constructs were verified by sequencing.
Each promoter construct was transfected into N2a cells in a 24-well plate using Lipofectamine 2000 (Invitrogen). Each well received 0.6 µg of reporter construct and 0.03 µg of pCMVβgal, a vector with CMV promoter and constitutively expressed β-galactosidase. Transfected neurons were stimulated with KCl at a final concentration of 20 mM in the culture media for 5 h as previously described (Yang et al., 2006). After five hours of treatment, cell lysates were harvested and measured for luciferase activity as described previously (Dhar et al., 2008). Data from 6 independent transfections were averaged for each promoter construct.
Plasmid construction of NRF-1 shRNA
NRF-1 silencing was carried out using two different vectors with comparable results. Small hairpin RNAs (shRNA) against murine NRF-1 (GenBankTM accession no. for NRF-1: NM_010938) were cloned either into pLL3.7/U6 promoter vector with puromycin resistance (Addgene, Cambridge, MA) or pLVTHM with H1 promoter and green fluorescent protein reporter (gift of Dr. P. Aebischer, Swiss Federal Institute of Technology). Four shRNA sequences were selected: 5’-GAAAGCTGCAAGCCTATCT-3’; 5’-GCCACAGGAGGTTAATTCA-3’; 5’-GCATTACGGACCATAGTTA-3’; and 5’-AGAGCATGATCCTGGAAGA-3’. Multiple shRNA sequences enhance the efficiency of gene silencing (Sun et al., 2006; Tomlison et al., 2007). When expressing shRNA in one of the vectors, the other vector (without the insert) was used concurrently for puromycin selection (pLL3.7) or GFP identification. These empty vectors by themselves or with scrambled shRNA also served as negative controls. The basic gene cloning method was followed as described previously (Ongwijitwat et al., 2006, Dhar et al., 2008). N2a cells or primary neurons were plated in 35-mm dishes at a density of 5 to 8 × 106 cells/dish. They were co-transfected 3 days post-plating with shRNA-containing plasmids (four sequences at equal amounts; 4 µg total for N2a cells and 1 µg total for primary neurons) and the other vector for identification (1.5 µg for N2a cells and 1 μg for primary neurons) via Lipofectamine 2000. Empty vectors or scrambled shRNA vectors alone were used at the same concentrations as vectors with shRNA insert. Puromycin at a final concentration of 0.5 µg/ml was added to the culture medium on the second day after transfection to select for purely transfected cells. Green fluorescence was observed to monitor transfection efficiency. Transfection efficiency for N2a cells ranged from 40% to 75%, while that for primary cortical neurons was from 40% to 60%. However, puromycin selection effectively yielded 100% of transfected cells. N2a cells transfected with shRNA against NRF-1 were further stimulated with KCl at a final concentration of 20 mM in the culture media for 5 h as previously described (Yang et al., 2006). After five hours of treatment, cells were harvested for RNA isolation.
RNA isolation and cDNA synthesis
Total RNA was isolated by RNeasy kits (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Three micrograms of total RNA was treated with DNase I and purified by phenol-chloroform. cDNA was synthesized using random hexamer primers and SuperScript™ II RNase H-Reverse Transcriptase (Invitrogen) according to the manufacturer’s instructions.
Real-time quantitative PCR
Real-time quantitative PCRs were carried out in a Cepheid Smart Cycler Detection system (Cepheid, Sunnyvale, CA). SyBr Green (BioWhittaker Molecular Application) and EX Taq real-time quantitative PCR hotstart polymerase were used following the manufacturer’s protocols and as described previously (Dhar et al., 2008). Primer sequences are shown in Table II. PCR runs: hot start 2 min at 95 °C, denaturation 10 s at 95 °C, annealing 15 s according to the Tm of each primer, and extension 10 s at 72°C for 15–30 cycles. Melt curve analyses verified the formation of single desired PCR product. Mouse β-actin for N2a cells and rat 18s for primary neurons was used as the internal control and the ΔΔCT method (Livak and Schmittgen, 2001) was used for the relative amount of transcripts.
Table II.
Primers for real-time PCR
| Gene | Sequence | Amplicon length | T m |
|---|---|---|---|
| Gria1 | F 5′CAACAATCACAGGAACATGCGGCT 3′ R 5′TGGAGAACTGGGAACAGAAACGGT 3′ |
139 | 60º |
| Gria2 | F 5′ GGAGCAAATGTCTCTGGATTTC 3′ R 5′ATCACTTGGACAGCATCATACG 3′ |
161 | 60º |
| Gria3 | F 5′ACACCAACCAGAACACCACTGAGA 3′ R 5′TGGAGAACTGGGAGCAGAAAGCAT 3′ |
111 | 60º |
| Gria4 | F 5′TGGTGTCAGTGTGGTCTTGTTCCT3′ R 5′ ATGCCAAATTCATTGGGAGGCTGG 3′ |
117 | 60º |
| NRF-1 | F 5′GGCACAGGCTGAGCTGATG 3′ R 5′CTAGTTCCAGGTCAGCCACCTTT 3′ |
90 | 59.5º |
| NRF-2α | F 5′CTCCCGCTACACCGACTAC 3′ R 5′TCTGACCATTGTTTCCTGTTCTG 3′ |
145 | 59.5º |
| COX2 | F 5′TGGCTTACAAGACGCTACATC 3′ R 5′GGAGGGAAGGGCAATTAGAA 3′ |
201 | 59.5º |
| COX6c | F 5′AGCGTCTGCGGGTTCATA 3′ R 5′GCCTGCCTCATCTCTTCAAA 3′ |
154 | 60º |
| β-Actin | F 5′GGCTGTATTCCCTCCATCG 3′ R 5′CCAGTTGGTAACAATGCCATGT 3′ |
154 | 59.5 |
| 18S | F 5′CGCGGTTCTATTTTGTTGGT 3′ R 5′AGTCGGCATCGTTTATGGTC 3′ |
219 | 59.5º |
Western blot assay
Control and NRF-1 shRNA samples were loaded onto 10% SDS-PAGE gel and electrophoretically transferred onto polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA). Subsequent to blocking, blots were incubated in primary antibodies (polyclonal antibodies against NRF-1 (1:500; gift of Dr. Scarpulla,), GluR1 (1:300; AB1504, Chemicon, Temecula, CA), GluR2 (1:300; AB1768, Chemicon), GluR3 (1:200; C20, sc-7612, Santa Cruz Biotechnology), GluR4 (1:200; C20, sc-7614, Santa Cruz Biotechnology), or our polyclonal antibodies against COX that recognize primarily subunit IV Hevner and Wong-Riley 1989 R.F. Hevner and M.T. Wong-Riley, Brain cytochrome oxidase purification, antibody production and immunohistochemical/histochemical correlations in the CNS, J Neurosci 9 (1989), pp. 3884–3898. View Record in Scopus | Cited By in Scopus (35) (Hevner and Wong-Riley, 1989; 1:500). Monoclonal antibodies against β-actin (A5316, Sigma) at 1:3,000 dilution were used as loading controls. Blots were then incubated in secondary antibodies (goat-anti-rabbit, goat-anti-mouse, or rabbit-anti-goat; Chemicon), reacted with ECL, and exposed to autoradiographic film (Santa Cruz Biotechnology). Quantitative analyses of relative changes were done with an Alpha Imager (Alpha Innotech, San Leandro, CA).
NRF-1 over-expression and TTX treatment
pSG5NRF-1 plasmid (gift of Dr. Richard Scarpulla) was used for NRF-1 over-expression. N2A cells and primary neurons were each plated in 35 mm dish at a density of 2 to 5 × 105 cells/dish. N2a cells were co-transfected 3 days post-plating with either 2.5 µg of the pSG5NRF-1 plasmid or an empty vector plus 0.5 µg of pLL3.7 Puro vector, using Lipofectamine 2000 (Invitrogen) at a 1:3 ratio. Procedures were identical for the transfection of primary neurons, except that 2 µg pSG5NRF-1 plasmids were used. Puromycin at a final concentration of 0.5 µg/ml was added on the second day after transfection to select for purely transfected cells. After one day of over-expression, TTX at a final concentration of 0.4 µM was added to the culture media for 3 days. N2A cells and primary neurons were harvested on the 4th day for RNA isolation.
Statistical analysis
Significance among group means was determined by analysis of variance (ANOVA). Significance between two groups was analyzed by Student’s t test. P-values of 0.05 or less were considered significant.
Results
In silico promoter analysis of AMPA receptor subunit genes
In silico analysis of the proximal promoters of murine Gria 1, 2, 3, and 4 subunit genes with DNA sequence 1 kb 5’ upstream and 100 bps beyond 3’ of TSP revealed typical sequence G/TGCGCAT/CC/TG/C/T for NRF-1 binding in Gria2 promoter (Table IA), whereas Gria1, Gria3 and Gria4 promoters had atypical NRF-1 sites which lacked the GCA core found previously to be invariant in COX subunit promoters (Dhar et al, 2008).
In vitro binding of NRF-1 to AMPA receptor subunit promoters
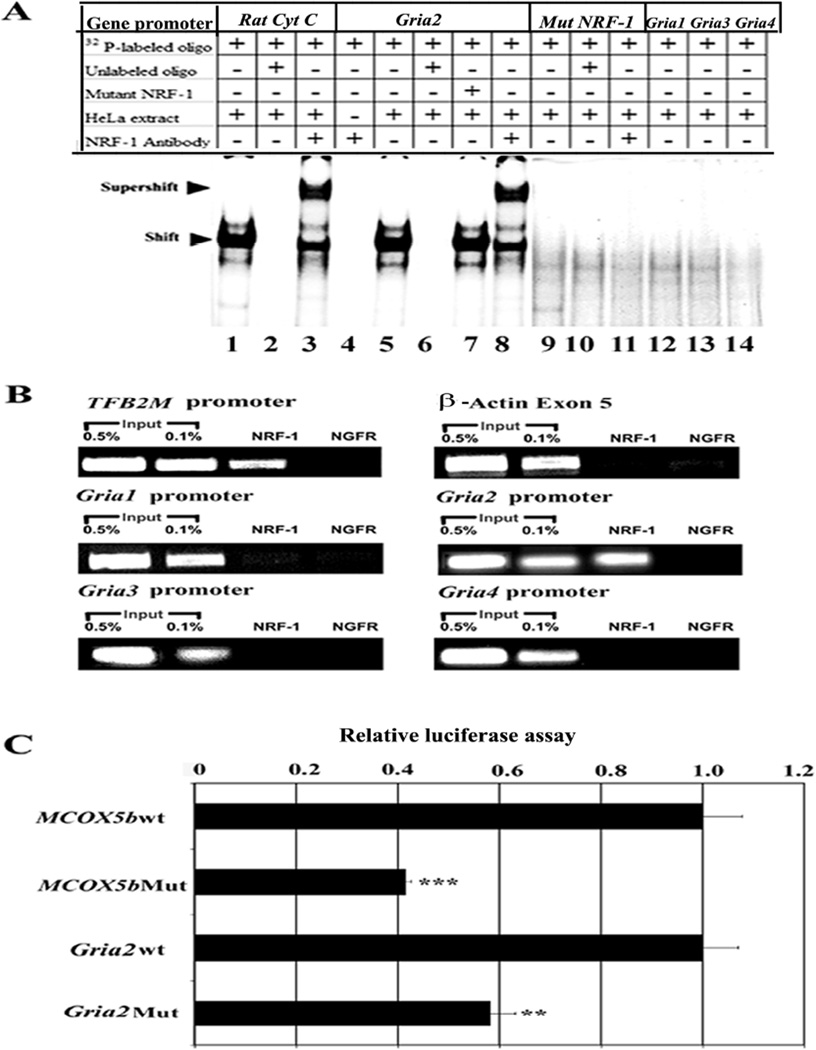
Electrophoretic mobility shift assays (EMSAs) for NRF-1 interactions were carried out in vitro using 32P-labeled oligonucleotide probes that encompassed putative NRF-1 binding sites on each of 4 murine AMPA receptor subunit promoters (Gria1, Gria2, Gria3, and Gria4) (Fig 1A). Rat cytochrome c promoter with NRF-1 site on positions −172/−147 served as a positive control and it formed specific DNA/NRF-1 shift and supershift complexes (Fig. 1A, lanes 1 and 3, respectively). When an excess of unlabelled cytochrome c probe was added as a competitor, no shift band was formed (Fig. 1A, lane 2). Gria2 promoter formed specific DNA-protein shift complex and DNA-protein-antibody supershift complex when incubated with HeLa nuclear extract (Fig. 1A, lanes 5 and 8, respectively). Competition with excess unlabelled Gria2 probe eliminated the shift complex (Fig. 1A, lanes 6 and l0, respectively), whereas the addition of unlabeled mutated NRF-1 probes had no effect (Fig. 1A, lane 7). To rule out any non-specific antibody and oligonucleotide interaction, labeled probes were incubated with NRF-1 antibody without HeLa nuclear extract, and no shift bands were observed. (Fig. 1A, lane 4). Gria2 probe with mutant NRF-1 binding site showed neither shift nor supershift complexes (Fig. 1A, lanes 9 and 11, respectively). Gria1, Gria3, and Gria4 lacked NRF-1 binding and did not produce any shift bands (Fig. 1A, lanes 12–14).
Fig. 1.
In-vitro and in-vivo interactions between NRF-1 and AMPA receptor subunit genes and promoter mutational analysis. (A) EMSAs for NRF-1. 32P- labeled oligonucleotides, excess unlabeled oligos specific for each promoter as competitors, excess unlabeled mutant NRF-1 as competitors, HeLa extract, and NRF-1 antibodies are indicated by a + or a – sign. Arrowheads indicate NRF-1 shift and supershift complexes. The positive control, cytochrome c, shows a shift (A, lane 1) and a supershift (A, lane 3) band. When excess unlabeled competitor was added, it did not yield any band (A, lane 2). Gria2 subunit showed specific shift and supershift bands that were eliminated by excess unlabeled competitors (A, lanes 5, 8 and 6 respectively), whereas Gria1, Gria3, and Gria4 had no bands (A, lanes 12–14). Labeled mutated NRF-1 site on Gria2 was used as a negative control, and it did not yield any band (A, lanes 9–11). Excess unlabeled but mutated NRF-1 site could not compete (A, lane 7). Labeled oligos with NRF-1 antibodies alone with no HeLa extract did not yield any band (A, lane 4). (B) ChIP assays. Input lanes represent 0.5% and 0.1% of chromatin. TFB2M promoter was the positive control and β-actin was the negative control. Gria2 promoters co-immunoprecipitated with NRF-1, while Gria1, Gria3, and Gria4 did not. Anti-nerve growth factor receptor p75 antibodies (NGFR) represent a negative control. (C) Site-directed mutations of NRF-1 binding sites on Gria2 and COX5b promoters resulted in significant reductions in luciferase activity as compared to wild type (wt). N = 6 for each construct. **, P < 0.05, ***, P < 0.001 as compared to the wild type.
In vivo interaction of NRF-1 with AMPA receptor subunit genes
To verify NRF-1 interactions with AMPA receptor subunit genes in vivo, chromatin immunoprecipitation assays (ChIP) were performed. β-actin served as a negative control, whereas transcription factor B2 of mitochondria (TFB2M) with a known NRF-1 binding site (Gleyzer et al., 2005; Dhar et al., 2008) served as a positive control. As a negative control, another immunoprecipitation from the same stock of cell lysate was done using anti-nerve growth factor receptor p75 antibodies (NGFR). PCRs targeting regions of AMPA receptor subunit promoters encompassing putative NRF-1 binding sites were carried out in parallel on chromatin immunoprecipitated from N2a cells. A 0.5 and 0.1 % dilution of input chromatin (i.e. prior to immunoprecipitation) was used as a standard to indicate the efficiency of the PCRs.
The proximal promoters of Gria1 - 4 subunits were immunoprecipitated with NRF-1 antibodies. Gria2 and TFB2M each produced a band from DNA immunoprecipitated with anti-NRF-1 antibodies at a position identical to that from the genomic DNA control (input) (Fig. 1B). On the other hand, Gria1, Gria3, Gria4 and β-actin yielded no bands (Fig.1B). In all cases, immunoprecipitation with NGFR antibodies did not yield any PCR product, confirming the specificity of the ChIP reaction.
Investigation of mutated NRF-1 binding sites for Gria2 and COX5b promoters
Based on EMSA probes (Table IA) that formed NRF-1 specific complexes (Fig. 1A), site-directed mutations of these same putative NRF-1 binding sites on Gria2 and murine COX5b promoters were constructed (Table IC), generated in luciferase reporter plasmids, and analyzed by gene transfection. As shown in Fig. 1C, mutation of NRF-1 binding sites led to ~ 42 – 60% reduction in promoter activity of Gria2 and COX5b genes (P < 0.05–0.001). COX5b subunit promoter served as a positive control and confirmed our previous report (Dhar et al., 2008).
NRF-1 silencing by RNA interference
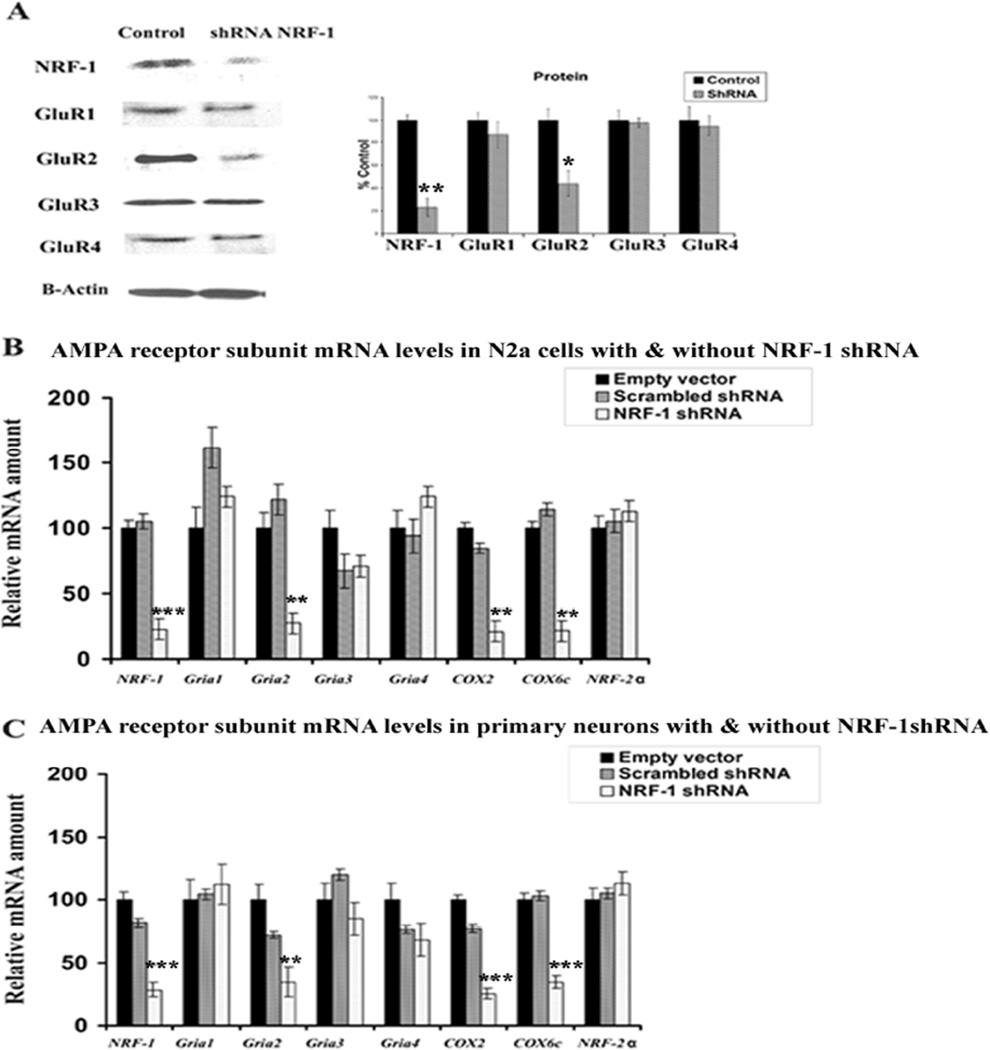
To determine the effect of silencing NRF-1 transcripts on the expression of AMPA receptors, plasmid vectors expressing small hairpin RNA (shRNA) against four target sequences of NRF-1 mRNA were used. These vectors were previously found to silence NRF-1 expression in N2a cells (Dhar et al., 2008). Transfection of neurons with shRNA vectors resulted in ~ 60–85% decrease in levels of NRF-1 and GluR2 proteins as measured by western blots (P < 0.05–0.01) (Fig. 2A). However, GluR1, GluR3, and GluR4 showed no alterations in protein levels (Fig. 2A). cDNAs from N2a cells (Fig. 2B) and primary neurons (Fig. 2C) transfected with NRF-1 shRNA vectors, scrambled shRNA vectors, or empty vectors were analyzed with quantitative real-time PCRs. As shown in Figs. 2B and 2C, mRNA levels of NRF-1, Gria2, COX2 (mitochondrial-encoded) and COX6c (nuclear-encoded) were significantly reduced in both N2a cells and primary neurons transfected with shRNA as compared to those transfected with empty vectors. The extent of reduction ranged between 70 to 80% (P < 0.01–0.001). On the other hand, the expressions of Gria1, Gria3, Gria4, and nuclear respiratory factor 2α (NRF-2α, a negative control) remained unchanged. The scrambled shRNA also did not have any effect on any mRNA level tested (Fig. 2B–C).
Fig. 2.
RNA interference-mediated silencing of NRF-1 suppresses mRNAs in AMPAR subunit genes and COX genes. (A) Western blot reveals a down-regulation of NRF-1 and GluR2 protein levels in NRF-1 shRNA-transfected neuron, whereas GluR1, GluR3 and GluR4 protein levels were not affected. β-Actin served as a loading control. *, P < 0.05; **, P < 0.01 as compared to controls. (B–C) N2a cells and primary neurons were transfected with shRNA against NRF-1 (clear bars) or with empty vectors (black bars) or with scrambled shRNA (gray bars). NRF-2α{PRIVATE “TYPE=PICT;ALT={alpha}”} served as a negative control. NRF-1, Gria2, COX2, and COX6c subunit mRNAs show significant decreases in shRNA-treated samples as compared to those with empty vectors, whereas Gria1, Gria3,Gria4, and NRF-2α{PRIVATE “TYPE=PICT;ALT={alpha}”} mRNA remained unchanged. N = 6 for each data point and 3 sets of independent experiments were done for each. **, P < 0.01; ***, P < 0.001 as compared to empty vectors for both B and C.
Response of Gria2 and COX subunit mRNAs to KCl depolarizing stimulation
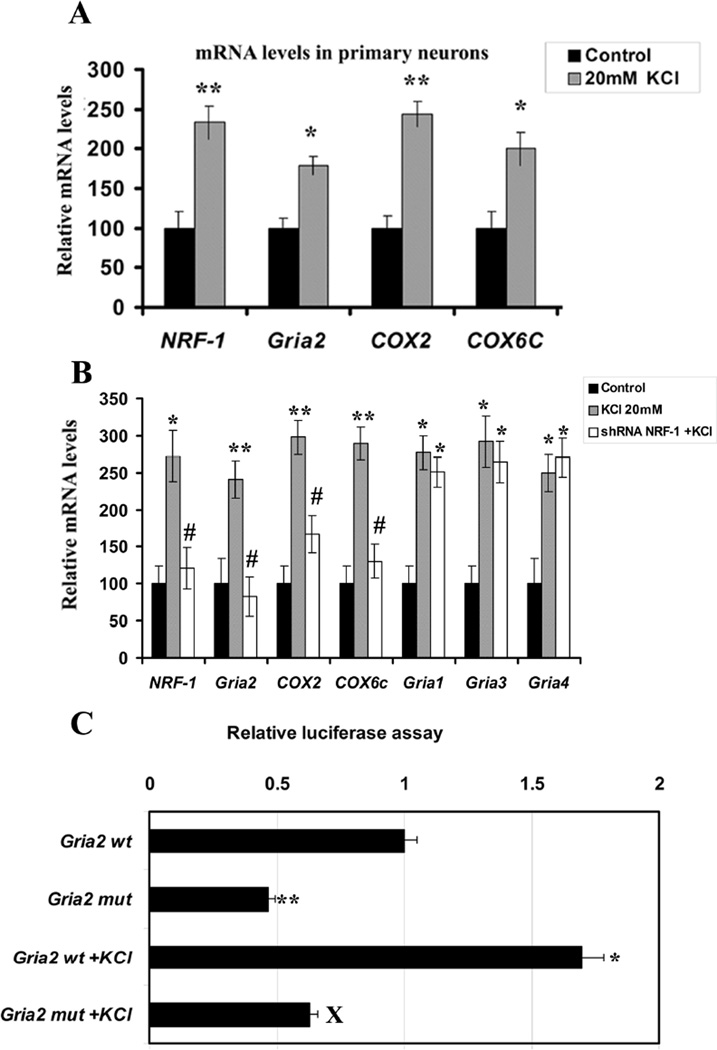
To investigate if the expression of Gria2 gene in primary neurons responded directly to depolarizing stimulation, cells were subjected to 20 mM of potassium chloride (KCl) for 5 hours, a regimen previously found to activate NRF-1 and COX gene expressions in primary neurons (Liang and Wong-Riley, 2006; Liang et al., 2006; Yang et al., 2006). As shown in Fig. 3A (for primary neurons), depolarizing stimulation resulted in a significant increase in the expressions of NRF-1, Gria2, COX2, and COX6c genes, as monitored by real time quantitative PCR (P < 0.05 – 0.01). The increase ranged from 79% to 145%.
Fig. 3.
Depolarization-induced up-regulation of mRNA levels and promoter gene expressions of AMPA and COX subunit genes and the effects of NRF-1 silencing or mutation of its binding site in neurons. (A) KCl depolarization induced an up-regulation of NRF-1, Gria2, COX2, and COX6c in primary neurons analyzed with real-time quantitative PCR. * P < 0.05, ** P < 0.01 as compared to controls. (B) Comparable results as in A were obtained in N2a cells. NRF-1 silencing with shRNA prevented the up-regulation of these transcripts by KCl. Gria1, Gria3, and Gria4 were also up-regulated by KCl, indicating a general stimulatory effect of KCl on neurons. However, NRF-1 silencing did not prohibit these transcripts from being induced by KCl, as their levels are significantly different from controls, but are not different from those of KCl alone. * P < 0.05, ** P < 0.01 as compared to controls. All #P values (< 0.05) were compared to 20 mM KCl alone. Values in A and B each represents mean ± S.E.M of combined data from 3 independent experiments. (C) Site-directed mutations of NRF-1 binding sites on Gria2 promoter resulted in a significant reduction in luciferase activity as compared to their wild type (wt) controls. KCl depolarization increased promoter activity in the wild type but not in mutated Gria2. N = 6 for each construct. ** P < 0.01 as compared to Gria2 wild type. X = P < 0.05 as compared to Gria2 wt with KCl depolarization.
Effect of NRF-1 silencing on COX and Gria2 mRNAs in the presence of KCl stimulation
To determine the response of AMPA receptor and COX subunit genes to KCl after NRF-1 silencing, N2a cells transfected with shRNA against NRF-1 were subjected to 20 mM of KCl for five hours. As shown in Fig. 3B, depolarizing stimulation without gene silencing resulted in a significant increase in the expressions of NRF-1, Gria1, Gria2, Gria3, Gria4, COX2, and COX6c genes, as monitored by real-time quantitative PCR (P < 0.05 – 0.01). The increase ranged from 140% to 198%, indicating that KCl has a general stimulatory effect on transcription (at least of the genes tested). However, in the presence of NRF-1 silencing, mRNA levels of NRF-1, Gria2, COX2, and COX6c were not up-regulated by KCl. On the other hand, NRF-1 silencing did not prevent Gria1, Gria3, and Gria4 from being up-regulated by KCl, and their levels remained higher than those of controls (P < 0.05) but not different from those with KCl alone. These results confirmed the specificity of NRF-1 in the regulation of Gria2 and COX subunits in response to increased neuronal activity.
Effect of mutating NRF-1 binding site on the response of Gria2 promoter to KCl stimulation
To further verify that NRF-1 binding is necessary for the up-regulation of Gria2 transcript by KCl, this binding site was mutated in the Gria2 promoter (Table IC). As shown in Fig. 3C for N2a cells, depolarizing stimulation resulted in a significant increase (~65%) in the expression of Gria2 gene, as monitored by luciferase assays (P < 0.05). This increase was abolished by the mutation of NRF-1 site, confirming a link between KCl–induced depolarization, NRF-1, and Gria2.
NRF-1 over-expression increased Gria2 and COX subunit mRNA levels and rescued neurons from tetrodotoxin-induced transcript reduction
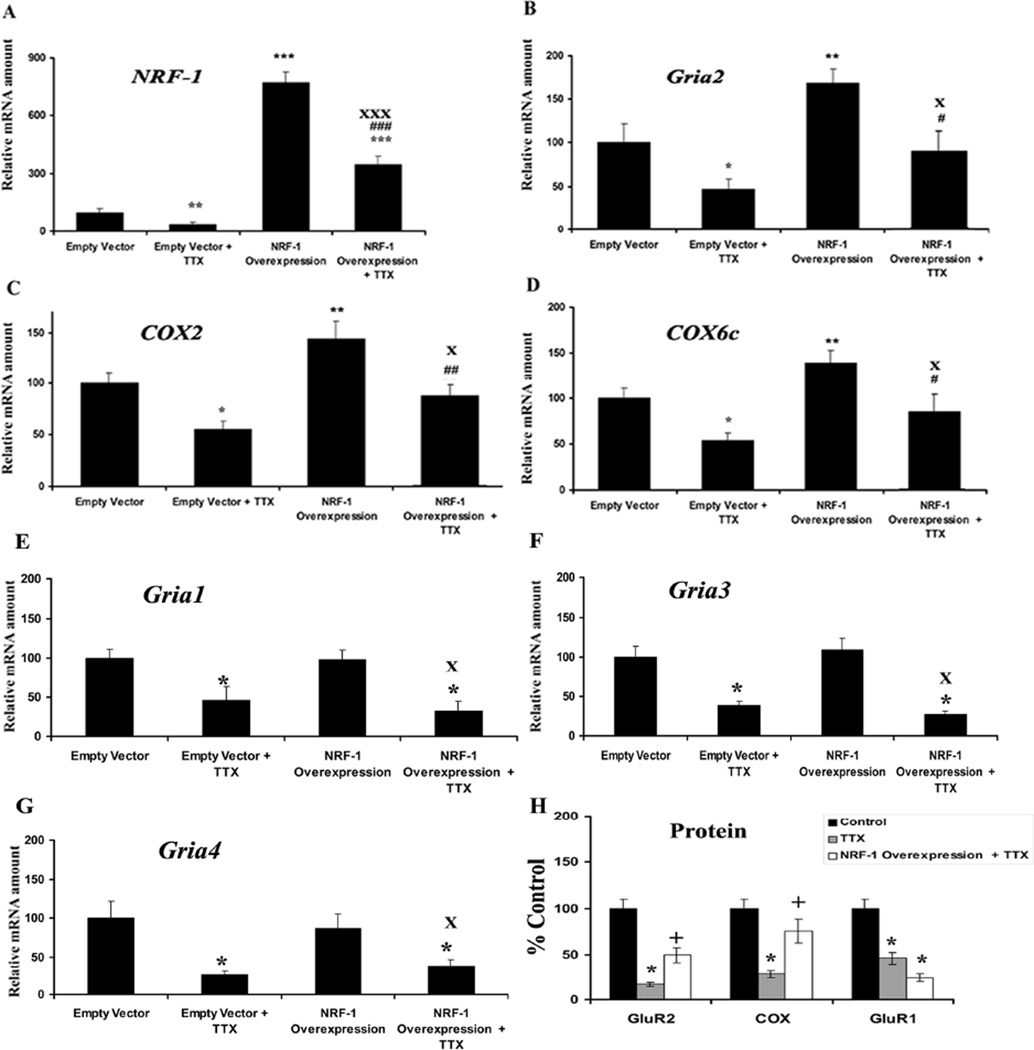
A low concentration of TTX (0.4 µM) has been shown to decrease the level of COX subunit mRNAs as well as COX enzyme activity in vivo and in primary neurons (Wong-Riley et al., 1998; Liang et al., 2006). To determine if over-expression of NRF-1 could rescue not only COX but also Gria2 transcripts, a pSG5NRF-1 construct (gift of Dr. Richard Scarpulla) for NRF-1 over-expression was transfected into primary neurons that were then exposed to TTX (0.4 µM) for 3 days. When neurons were transfected with empty vectors, exposure to TTX led to a 45–75% reduction in mRNA levels of NRF-1, Gria2, COX2, COX6c, Gria1, Gria3, and Gria4 (Figs. 4A–G), indicating a general suppressive effect of TTX on transcriptional activity in neurons. Neurons transfected with the pSG5NRF-1 construct had an 719% increase in NRF-1 mRNAs (P < 0.001) (Fig. 4A) and a 35 – 55% increase in message levels of Gria2, COX2, and COX6c (Fig. 4B–D) as compared to empty vector controls (P < 0.05–0.01). However, there was no change in Gria1, Gria3, and Gria4 mRNA levels with NRF-1 over-expression (Fig. 4E–G). When exposed to TTX, neurons transfected with NRF-1 expressed 30–50% more Gria2, COX2, and COX6c transcripts as compared to those with empty vectors plus TTX (P < 0.05–0.01). Likewise, NRF-1 mRNA levels in neurons over-expressing NRF-1 were 290% greater than those with empty vectors in the presence of TTX (P < 0.001) and 249% more as compared to empty vectors only (P < 0.001) but the levels of Gria1, Gria3 and Gria4 remained low and not different from those transfected with empty vectors and exposed to TTX. These results confirmed that NRF-1 could rescue Gria2 as well as COX subunits, but not Gria1, Gria3, and Gria4 mRNA levels after TTX exposure. Neurons exposed to TTX had ~ 70–90% decrease in levels of COX, GluR2, and GluR1 proteins as compared to controls when measured by western blots (P < 0.05) (Fig. 4H). Neurons transfected with NRF-1 and exposed to TTX expressed 25 –55% more COX and GluR2 proteins as compared to TTX alone (P < 0.05). However, NRF-1 could not rescue GluR1 protein levels after TTX exposure, confirming the specificity of NRF-1 (Fig. 4H).
Fig. 4.
NRF-1 over-expression in primary neurons significantly increased mRNA levels for Gria2, COX2, and COX6c genes and rescued them from TTX-induced reduction. NRF-1,Gria2, COX2, and COX6c mRNA levels (A–D) are significantly higher in NRF-1 over-expressing neurons but not Gria1, Gria3, and Gria4, (E–G) as compared to empty vectors. NRF-1, Gria2, COX2, COX6c, Gria1, Gria3, andGria4, mRNA levels (A–G) were all reduced by TTX as compared to controls, indicating a generalized depressive effect of TTX on neurons. Over-expression of NRF-1 was able to rescue NRF-1, Gria2, COX2, and COX6c, but not Gria1, Gria3, and Gria4 transcripts, from being down-regulated by TTX. Group means were analyzed for overall statistical significance using the Student's t-test (N = 6 for each group). All * P values were compared to empty vector (* P < 0.05, ** P < 0.01, *** P < 0.001). All #P values were compared to empty vector + TTX (#P < 0.05, ##P < 0.01, ###P < 0.001), and all X P values were compared to NRF-1 over-expression (XP < 0.05, XXXP < 0.001). (H) Protein levels for GluR2, COX and GluR1 were all reduced with TTX blockade as compared to controls in neurons. However, NRF-1 over-expression rescued protein levels for GluR2 and COX, but not for GluR1, from being down-regulated by TTX. All * P values were compared to controls (* P < 0.05). All + P values were compared to TTX (+P < 0.05).
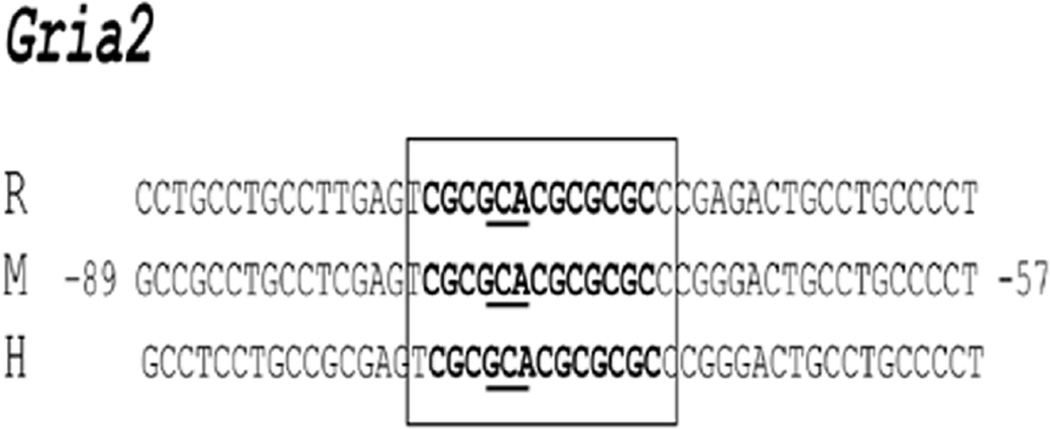
Conservation of NRF-1 binding sites among mouse, rat, and human Gria2 promoters
The sequence of murine Gria2 subunit gene was aligned with homologous regions in the rat and human genomes to determine if NRF-1 binding sites tested in the present study were conserved in other species. Results showed a high degree of homology (95–100%) among the three species (Fig. 5). Thus, the typical NRF-1 binding site with an invariant GCA core (Dhar et al., 2008) is highly conserved among mice, rats, and humans.
Fig. 5.
Aligned partial sequences of Gria2 promoters from rat (R), mouse (M), and human (H) genomes show conservation of typical NRF-1 binding sites. Conserved binding site sequences are in boldface. Invariant GCA core sequences are underlined. Solid box highlight NRF-1 sites that are highly conserved in all three species.
Discussion
Using multiple approaches, the present study documents that Gria2 and COX subunit genes are functionally regulated by the same transcription factor, NRF-1, thus linking glutamatergic synaptic transmission and energy metabolism at the molecular level of regulation in neurons. Furthermore, the NRF-1 binding site is conserved among mice, rats, and humans for Gria2 (the present study) as well as for COX subunit promoters (Dhar et al., 2008), emphasizing the conservation of such co-regulation through evolution. NRF-1 protein and mRNA levels are up-regulated by depolarizing neuronal activity (Yang et al., 2006), and NRF-1, in turn, coordinates transcriptional activation of both AMPA receptor and COX subunit genes (the present study). Such tight coupling is highly efficient, as energy consumed by AMPA receptor-mediated synaptic transmission can be readily met by energy generated via oxidative metabolism, when the two processes are regulated by the same molecular mechanism.
AMPA receptors are widely expressed in the central nervous system, with GluR2 present in the majority of them, and GluR3 and GluR4 occur at much lower levels (reviewed in Isaac et al., 2007). GluR2 subunit is critical in governing Ca2+ and Zn2+ permeability, receptor kinetics, single channel conductance, and voltage-dependent channel block by cytosolic polyamines (Bowie and Mayer, 1995; Swanson et al., 1997; Tanaka et al., 2000). It is the most tightly regulated of the AMPA subunits, and it plays a critical role in normal brain functions as well as in synaptic plasticity (Isaac et al., 2007). Previously, we found that GluR2 immunoreactivity and gene expression were up-regulated by KCl-stimulated depolarization and down-regulated by TTX-induced impulse blockade, in concert with the up- and down-regulation of cytochrome c oxidase (Bai and Wong-Riley, 2003). This positive relationship is greatly strengthened at the molecular level by the present finding of a common regulatory factor for transcription. Moreover, silencing of NRF-1 prohibited both Gria2 and COX from being up-regulated by KCl, and NRF-1 over-expression rescued both Gria2 and COX transcripts from being down-regulated by TTX.
Gria2 promoter shares common features with Gria1 and Gria4 subunits, such as multiple transcription start sites, GC-rich, lack of a TATA box, and presence of Sp1 binding site in vitro (Myers et al., 1998, 1999; Borges and Dingledine, 2001; Borges et al., 2003). These features are also common to genes of the electron transport chain, such as cytochrome c oxidase (Grossman and Lomax, 1997). The binding of NRF-1, however, appears to be unique to Gria2. The regulation of the other Gria subunits awaits further investigation, but preliminary in silico analysis (our unpublished observation) suggests that nuclear respiratory factor 2 (NRF-2), which often binds to the same promoters as NRF-1 (Scarpulla, 2008), may be involved.
NRF-1 is known to be a key transcriptional activator of nuclear genes encoding a number of mitochondrial respiratory enzymes, including subunits of the five respiratory chain complexes (Scarpulla, 2006, 2008). Recently, we found that NRF-1 regulates all ten nuclear-encoded COX subunit genes in neurons (Dhar et al., 2008). In addition, extensive proof supports a potential integrative function for NRF-1 in coordinating respiratory subunit expression with that of the mitochondrial transcriptional machinery. NRF-1 binds and activates the promoters of mitochondrial transcriptional factor A (TFAM), transcription specificity factors (TFB1M and TFB2M), and RNA-processing proteins required for mtDNA transcription and replication (Virbasius and Scarpulla, 1994, Gleyzer et al., 2005, Scarpulla, 2008). The polycistronic mitochondrial transcript gives rise to 13 polypeptides, 3 of which form the catalytic core of cytochrome c oxidase enzyme (Wong-Riley et al., 1998). Thus, NRF-1 regulates all 13 subunits of COX from the two genomes in neurons. In heart and skeletal muscles, NRF-1 does not regulate tissue-specific isoforms of COX (COX 6A and 7A) directly, but it controls the transcription of myocyte enhancer factor 2A (MEF2A), which, in turn, regulates these two subunits (Ramachandran et al., 2008). NRF-1 has also been linked to the expression of 5-aminolevulinate synthase (Braidotti et al., 1993) and uroporphyrinogen III synthase (Aizencang et al., 2000), key enzymes of the heme biosynthetic pathway. The former is the rate-limiting enzyme in the biosynthesis of heme, a cofactor essential to the function of electron carriers encoded by both genomes. Thus, NRF-1 is vital for mitochondrial biogenesis, for normal cell growth, and cellular functioning (Scarpulla, 2006, 2008). NRF-1 knockout is embryonically lethal (Huo and Scarpulla, 2001). Silencing of NRF-1 down-regulates both mitochondrial-encoded (COX2) and nuclear-encoded (COX6c) subunit transcripts, and over-expression of NRF-1 rescues both transcripts from being severely decreased by TTX (the present study). These findings reaffirm the significant role that NRF-1 plays in the regulation of COX gene expression in neurons (Dhar et al., 2008).
In conclusion, the present study documents the tight coupling between neuronal activity and energy metabolism beyond the cellular level (Wong-Riley, 1989) to the molecular level. The regulation of both processes can be instigated and/or retained by the same transcription factor, NRF-1. A steady and harmonious interaction between energy generation and synaptic transmission is organized by NRF-1 by effectively synchronizing the expressions of both COX and Gria2. The fact that NRF-1 regulates critical subunit genes of NMDA receptors (Dhar and Wong-Riley, 2008) and AMPA receptor subunit 2 (present study) strongly indicates that NRF-1 is intimately associated with the regulation of glutamatergic synaptic transmission in neurons. This coordinated regulation may require other factors along with NRF-1, such as nuclear respiratory factor 2 (NRF-2) that has also been shown to regulate all 13 COX subunits from the two genomes (Ongwijitwat and Wong-Riley, 2005; Ongwijitwat et al., 2006). In addition to transcription factors, a transcriptional coactivator, peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α), has been shown to induce mitochondrial biogenesis by stimulating a powerful induction of NRF-1 and NRF-2 (Wu et al., 1999). PGC1α also responds promptly to external signals in non-neuronal cells (Puigserver et al., 2001) as well as to changing activity in primary neurons (Liang and Wong-Riley, 2006; Meng et al., 2007). Thus, transcriptional mechanism may involve all of these important factors in co-regulating neuronal activity and energy metabolism.
Acknowledgments
It gives us great pleasure to thank Dr. Richard Scarpulla for his generous gift of NRF-1 antibodies and pSG5NRF-1 plasmid, and Dr. P. Aebischer for his gift of PLVTHM. We thank Dr. H. Meng and James Clay for assisting in the construction of shRNA vectors and western blot assays. Supported by NIH Grant EY018441.
Glossary
Abbreviations
- AMPA
alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- ChIP
chromatin immunoprecipitation
- CMV
cytomegalovirus
- COX
cytochrome c oxidase
- EMSA
electrophoretic mobility shift assay
- NGFR
nerve growth factor receptor
- NRF-1
nuclear respiratory factor-1
- NRF-2
nuclear respiratory factor 2
- PGC-1α
peroxisome proliferator-activated receptor-γ coactivator 1α
- shRNA
short hairpin RNA
- Sp1
specificity protein 1
- TFAM
transcription factor A of mitochondria
- TFB1M
transcription factor B1 of mitochondria
- TFB2M
transcription factor B2 of mitochondria
- TSP
transcription start point
- TTX
tetrodotoxin.
References
- Aizencang GI, Bishop DF, Forrest D, Astrin KH, Desnick RJ. Uroporphyrinogen III synthase. An alternative promoter controls erythroid-specific expression in the murine gene. J. Biol Chem. 2000;275:2295–2304. doi: 10.1074/jbc.275.4.2295. [DOI] [PubMed] [Google Scholar]
- Bai X, Wong-Riley MTT. Neuronal activity regulates protein and gene expressions of GluR2 in postnatal rat visual cortical neurons in culture. J. Neurocytol. 2003;32:71–78. doi: 10.1023/a:1027380315902. [DOI] [PubMed] [Google Scholar]
- Borges K, Dingledine R. AMPA receptors: molecular and functional diversity. Prog. Brain Res. 1998;116:153–170. doi: 10.1016/s0079-6123(08)60436-7. [DOI] [PubMed] [Google Scholar]
- Borges K, Dingledine R. Functional organization of the GluR1 glutamate receptor promoter. J Biol Chem. 2001;276:25929–25938. doi: 10.1074/jbc.M009105200. [DOI] [PubMed] [Google Scholar]
- Borges K, Myers SJ, Zhang S, Dingledine R. Activity of the rat GluR4 promoter in transfected cortical neurons and glia. J. Neurochem. 2003;86:1162–1173. doi: 10.1046/j.1471-4159.2003.01926.x. [DOI] [PubMed] [Google Scholar]
- Bowie D, Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine mediated ion channel block. Neuron. 1995;15:453–462. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]
- Braidotti G, Borthwick IA, May BK. Identification of regulatory sequences in the gene for 5-aminolevulinate synthase from rat. J. Biol Chem. 1993;268:1109–1117. [PubMed] [Google Scholar]
- Dhar SS, Wong-Riley MTT. J. Neurosci. 2008. Coupling of energy metabolism and synaptic transmission at the transcriptional level: Role of nuclear respiratory factor 1 in regulating both cytochrome c oxidase and NMDA glutamate receptor subunit genes. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar SS, Ongwijitwat S, Wong-Riley MTT. Nuclear respiratory factor 1 regulates all ten nuclear-encoded subunits of cytochrome c oxidase in neurons. J. Biol. Chem. 2008;283:3120–3129. doi: 10.1074/jbc.M707587200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MJ, Scarpulla RC. NRF-1: a trans-activator of nuclear-encoded respiratory genes in animal cells. Genes Dev. 1990;4:1023–1034. doi: 10.1101/gad.4.6.1023. [DOI] [PubMed] [Google Scholar]
- Gleyzer N, Vercauteren K, Scarpulla RC. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators.Mol. Cell Biol. 2005;25:1354–1366. doi: 10.1128/MCB.25.4.1354-1366.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman LI, Lomax MI. Nuclear genes for cytochrome c oxidase. Review. Biochem. Biophys Acta. 1997;1352:174–192. doi: 10.1016/s0167-4781(97)00025-0. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Wong-Riley MTT. Brain cytochrome oxidase: Purification, antibody production and immunohistochemical/histochemical correlations in the CNS. J. Neurosci. 1989;9:3884–3898. doi: 10.1523/JNEUROSCI.09-11-03884.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollman M, Hartley M, Heinemann S. Ca2+ permeability of KA-AMPA glutamate receptor channels depends on subunit composition. Science. 1991;252:851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- Huo L, Scarpulla RC. Mitochondrial DNA instability and peri-implantation lethality associated with targeted disruption of nuclear respiratory factor 1 in mice. Mol. Cell Biol. 2001;21:644–654. doi: 10.1128/MCB.21.2.644-654.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac JT, Ashby M, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54:859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Keinänen K, Wisden W, Sommer B, Werner P, Herb A, Verdoorn TA, Sakmann B, Seeburg PH. A family of AMPA-selective glutamate receptors. Science. 1990;249:556–560. doi: 10.1126/science.2166337. [DOI] [PubMed] [Google Scholar]
- Liang HL, Wong-Riley MTT. Activity-dependent regulation of nuclear respiratory factor-1, nuclear respiratory factor-2, and peroxisome proliferator-activated receptor gamma coactivator-1 in neurons. Neuroreport. 2006;17:401–405. doi: 10.1097/01.wnr.0000204980.98876.11. [DOI] [PubMed] [Google Scholar]
- Liang HL, Ongwijitwat S, Wong-Riley MTT. Bigenomic functional regulation of all 13 cytochrome c oxidase subunit transcripts in rat neurons in vitro and in vivo. Neurosci. 2006;140:177–190. doi: 10.1016/j.neuroscience.2006.01.056. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Meng H, Liang HL, Wong-Riley M. Quantitative immuno-electron microscopic analysis of depolarization-induced expression of PGC-1alpha in cultured rat visual cortical neurons. Brain Res. 2007;1175:10–16. doi: 10.1016/j.brainres.2007.07.063. [DOI] [PubMed] [Google Scholar]
- Myers SJ, Peters J, Huang Y, Comer MB, Barthel F, Dingledine R. Transcriptional regulation of the GluR2 gene: Neural-specific expression, multiple promoters, and regulatory elements. J. Neurosci. 1998;18:6723–6739. doi: 10.1523/JNEUROSCI.18-17-06723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers SJ, Dingledine R, Borges K. Genetic regulation of glutamate receptor ion channels. Annu. Rev. Pharmacol. Toxicol. 1999;39:221–241. doi: 10.1146/annurev.pharmtox.39.1.221. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Ongwijitwat S, Wong-Riley MTT. Is nuclear respiratory factor 2 a master transcriptional coordinator for all ten nuclear-encoded cytochrome c oxidase subunits in neurons? Gene. 2005;360:65–77. doi: 10.1016/j.gene.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Ongwijitwat S, Liang HL, Graboyes EM, Wong-Riley MTT. Nuclear respiratory factor 2 senses changing cellular energy demands and its silencing down-regulates cytochrome oxidase and other target gene mRNAs. Gene. 2006;374:39–49. doi: 10.1016/j.gene.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Palmer CL, Lim W, Hastie PG, et al. Hippocalcin functions as a calcium sensor in hippocampal LTD. Neuron. 2005;47:487–494. doi: 10.1016/j.neuron.2005.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver P, Rhee J, Lin J, et al. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1Mol. Cell. 2001;8:971–982. doi: 10.1016/s1097-2765(01)00390-2. [DOI] [PubMed] [Google Scholar]
- Ramachandran B, Yu G, Gulick T. Nuclear respiratory factor 1 controls myocyte enhancer factor 2A transcription to provide a mechanism for coordinate expression of respiratory chain subunits. J Biol Chem. 2008;283:11935–11946. doi: 10.1074/jbc.M707389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpulla RC. Nuclear control of respiratory gene expression in mammalian cells. J. Cell Biochem. 2006;97:673–683. doi: 10.1002/jcb.20743. [DOI] [PubMed] [Google Scholar]
- Scarpulla RC. Nuclear control of respiratory chain expression by nuclear respiratory factors and PGC-1-related coactivator. Ann N Y Acad Sci. 2008;1147:321–334. doi: 10.1196/annals.1427.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg PH. The TIPS/TINS lecture: the molecular biology of mammalian glutamate receptor channels. Trends Pharmacol Sci. 1993;14:297–303. doi: 10.1016/0165-6147(93)90047-N. [DOI] [PubMed] [Google Scholar]
- Sun D, Melegari M, Sridhar S, Rogler CE, Zhu L. Multi-miRNA hairpin method that improves gene knockdown efficiency and provides linked multi gene knockdown. Bio Techniques. 2006;41:59–63. doi: 10.2144/000112203. [DOI] [PubMed] [Google Scholar]
- Swanson GT, Kamboj SK, Cull-Candy SG. Single-channel properties of recombinant AMPA receptors depend on RNA editing, splice variation, and subunit composition. J Neurosci. 1997;17:58–69. doi: 10.1523/JNEUROSCI.17-01-00058.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Grooms SY, Bennett MVL, Zukin RS. The AMPAR subunit GluR2: still front and center-stage. Brain Res. 2000;886:190–207. doi: 10.1016/s0006-8993(00)02951-6. [DOI] [PubMed] [Google Scholar]
- Tomlinson DC, Hurst CD, Knowles MA. Knockdown by shRNA identifies S249C mutant FGFR3 as a potential therapeutic target in bladder cancer. Oncogene. 2007;26:5889–5899. doi: 10.1038/sj.onc.1210399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoorn TA, Burnashev N, Monyer H, Seeburg PH, Sakmann B. Structural determinants of ion flow through recombinant glutamate receptor channels. Science. 1991;252:1715–1718. doi: 10.1126/science.1710829. [DOI] [PubMed] [Google Scholar]
- Virbasius JV, Scarpulla RC. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc. Natl. Acad. Sci USA. 1994;91:1309–1313. doi: 10.1073/pnas.91.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikström M, Krab K, Saraste M. A Synthesis. New York: Academic Press; 1981. Cytochrome Oxidase. [Google Scholar]
- Wong-Riley MTT. Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Trends Neurosci. 1989;3:94–101. doi: 10.1016/0166-2236(89)90165-3. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MTT, Jacobs P. AMPA glutamate receptor subunit 2 in normal and visually deprived macaque visual cortex. Vis. Neurosci. 2002;19:563–573. doi: 10.1017/s0952523802195022. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MTT, Nie F, Hevner RF, Liu S. Brain cytochrome oxidase: functional significance and bigenomic regulation in the CNS, in. In: Gonzalez-Lima F, editor. Cytochrome oxidase in Neuronal Metabolism and Alzheimer’s Disease. New York: Plenum Press; 1998. pp. 1–53. [Google Scholar]
- Wu Z, Puigserver P, Andersson U, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Yang SJ, Liang HL, Wong-Riley MT. Activity-dependent transcriptional regulation of nuclear respiratory factor-1 in cultured rat visual cortical neurons. Neurosci. 2006;141:1181–1192. doi: 10.1016/j.neuroscience.2006.04.063. [DOI] [PubMed] [Google Scholar]