Abstract
Gamma heavy-chain disease (gHCD) is defined as a lymphoplasmacytic neoplasm that produces an abnormally truncated immunoglobulin gamma heavy-chain protein that lacks associated light chains. There is scant information in the literature regarding the morphologic findings in this rare disorder, but cases have often been reported to resemble lymphoplasmacytic lymphoma (LPL). To clarify the spectrum of lymphoproliferative disorders that may be associated with gHCD, this study reports the clinical, morphologic, and phenotypic findings in 13 cases of gHCD involving lymph nodes (n = 7), spleen (n = 2), bone marrow (n = 8), or other extranodal tissue biopsies (n = 3). Clinically, patients showed a female predominance (85%) with frequent occurrence of autoimmune disease (69%). Histologically, 8 cases (61%) contained a morphologically similar neoplasm of small lymphocytes, plasmacytoid lymphocytes, and plasma cells that was difficult to classify with certainty, whereas the remaining 5 cases (39%) showed the typical features of one of several other well-defined entities in the 2008 WHO classification. This report demonstrates that gHCD is associated with a variety of underlying lymphoproliferative disorders but most often shows features that overlap with cases previously reported as “vaguely nodular, polymorphous” LPL. These findings also provide practical guidance for the routine evaluation of small B-cell neoplasms with plasmacytic differentiation that could represent a heavy-chain disease and give suggestions for an improved approach to the WHO classification of gHCD.
Keywords: gamma heavy-chain disease, lymphoplasmacytic lymphoma, plasmacytic differentiation, small B-cell lymphoma
Gamma heavy-chain disease (gHCD) is defined as a neoplasm of lymphocytes, plasmacytoid lymphocytes, and plasma cells characterized by the production and secretion of an abnormally truncated IgG heavy chain that is incapable of associating with immunoglobulin light chains.10,12,27,31,32 gHCD is a rare disorder, with fewer than 150 cases reported in the literature to date.12,20,31,32 The classification of the lymphoplasmacytic disorder underlying gHCD has been controversial. In general, it has been reported that most cases resemble lymphoplasmacytic lymphoma (LPL), although a wide variety of disorders have been reported in association with gHCD.9,32,33 However, interpretation of these data has been challenging, as most of the cases in the literature were diagnosed before 1980, were classified using now-outdated classification schemes, and were reported with little accompanying histopathologic information. For this reason, it has remained unclear whether gHCD should be considered a distinct clinical entity or perhaps a clinical variant of LPL.
In this report, we examine the clinical, histopathologic, and molecular features of 13 cases of gHCD, each of which has lymph node, spleen, bone marrow, and/or other extramedullary tissues available for examination. The findings in this series emphasize the spectrum of B-cell lymphoproliferative disorders that underlie gHCD, provide insights into the diagnostic workup of cases of lymphomas with plasmacytic differentiation that could represent a heavy-chain disease, and suggest the need for revisions to the current WHO classification of gHCD.
MATERIALS AND METHODS
Patients
Inclusion criteria consisted of serum and/or urine protein electrophoresis and immunofixation studies showing a monoclonal pattern of gamma heavy chain in the absence of corresponding light chains. Thirteen cases were identified from Cleveland Clinic (n = 6), University of Pittsburgh Medical Center (n = 2), Massachusetts General Hospital (n = 2), Medical College of Wisconsin (n = 1), University of New Mexico (n = 1), and Newcastle-upon-Tyne Hospitals (n = 1). One patient was previously reported,23 and 3 cases were presented at the 2009 Society for Hematopathology/European Association of Hematopathology Slide Workshop.21 Slides for lymph nodes (n = 7), spleen (n = 2), bone marrow (n = 8), or other extramedullary tissue biopsies (n = 3) and results of previously performed immunohistochemistry, flow cytometry, and cytogenetic studies were reviewed.
Statistics
Continuous variables were compared using the Student t test, and categorical variables were compared by the Fisher exact test using GraphPad Prism software (GraphPad Software, La Jolla, CA).
Immunohistochemistry
In all patients with available material, immunohistochemical stains for CD138 (BB4, 1:200 dilution; Biocare Medical, Concord, CA), IgG (polyclonal, 1:10,000 dilution; Dako, Carpinteria, CA), kappa (polyclonal, 1:32,000 dilution; Dako), and lambda (polyclonal, 1:64,000 dilution; Dako) and in situ hybridization studies for kappa (predilute; Ventana, Tucson, AZ) and lambda (predilute; Ventana) were conducted using a Ventana Benchmark automated immunostainer.
IGH and IGK Clonality Analysis
In all patients with appropriate available material, polymerase chain reaction (PCR) studies for IGH and IGK rearrangements were conducted using fluorescently labeled BIOMED-2 primers (IGH FW1, FW2, and FW3 and IGK V-J and V-Kde primer sets; Invivoscribe, San Diego, CA). PCR was performed according to the guidelines of the manufacturer and the BIOMED-2 consortium.7,30 PCR products were analyzed by capillary electrophoresis using an ABI 3730 genetic analyzer (Applied Biosystems, Carlsbad, CA). As per BIOMED-2 guidelines, DNA quality was assessed through amplification of a control gene size ladder.7,30 Amplification of at least a 100-bp fragment was required for interpretation of results. Cases with amplification of 100-bp but not 300-bp control fragments were accepted but were designated as having suboptimal DNA preservation, possibly limiting interpretation.
Fluorescence In Situ Hybridization
In all cases with appropriate available material, fluorescence in situ hybridization (FISH) studies were conducted using dual-color, break-apart probes for IGH, BCL6, and MALT1 translocations (Abbott Vysis, Abbott Park, IL). Probes for BCL6 and MALT1 were also used to evaluate for potential trisomy of chromosomes 3/3q and 18/18q, respectively. Tissue microarrays were created containing 2-mm tissue cores of representative material. FISH was performed on intact, 5-μm paraffin sections as described previously.24,35 Two hundred cells were evaluated by fluorescence microscopy, and cases with >10% abnormal nuclei were considered positive for the abnormality.
RESULTS
Thirteen patients with free gamma heavy chains detected in the serum by protein electrophoresis and immunofixation were identified. In 3 of the 5 patients tested, urine protein electrophoresis and immunofixation also demonstrated free gamma heavy chains; none showed free light chains in urine analysis. Initial histologic review showed that the 13 cases studied could be readily separated into 2 groups (Table 1). Group I cases (n = 8) showed a lymphoplasmacytic neoplasm with similar morphologic features that was difficult to classify by the 2008 WHO criteria. Group II cases (n = 5) lacked the typical morphology seen in group I cases and instead displayed features characteristic of one of several entities defined by the WHO classification. The features of these 2 groups are described separately below.
TABLE 1.
Clinical Features
| All Patients | Group I Cases | Group II Cases | PGroup (Group I vs. Group II) | |
|---|---|---|---|---|
| Age | 51 (39-77) | 59 (43-77) | 45 (39-56) | 0.10 |
| Gender | 11 F/2 M | 7 F/1 M | 4 F/1 M | 1.0 |
| Adenopathy | 8/13 | 7/8 | 1/5 | 0.03 |
| Splenomegaly | 6/13 | 3/8 | 3/5 | 0.59 |
| Autoimmune history | 9/13 | 8/8* | 1/5† | 0.007 |
Systemic lupus erythematosus (n = 5), Hashimoto’s thyroiditis (n = 2), rheumatoid arthritis (n = 1).
Hashimoto’s thyroiditis (n = 1).
Value in bold indicates significant P values.
Clinical Features
Group I Cases
Seven of the 8 (87%) patients were female with a median age of 59 (range, 43 to 77 y). Adenopathy was present in 7/8 and splenomegaly in 3/8 patients. Each patient had a reported history of autoimmune disease including systemic lupus erythematosus (n = 5), autoimmune thyroiditis (n = 2), and rheumatoid arthritis (n = 1).
Group II Cases
These patients consisted of 4 women and 1 man with a median age of 45 years (range, 39 to 56 y) (group I vs. group II, P = 0.10). One patient, with an extranodal marginal-zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) of the thyroid, had a history of Hashimoto’s thyroiditis. None of the remaining group II cases had a history of autoimmune disease (group I vs. group II, 100% vs. 20%, P = 0.007). Adenopathy was less frequent in group II than in group I cases [1/5 (20%) vs. 7/8 (87%), P = 0.03].
Histologic Findings in Lymph Node, Spleen, and/or Extramedullary Tissues
Group I Cases
Tissues biopsied included those from the lymph node (6 patients), spleen (1 patient), and salivary gland (1 patient). Each of the 6 nodal cases displayed a similar morphologic pattern, consisting of architectural effacement by an interfollicular and/or diffuse infiltrate of small lymphocytes, plasmacytoid cells, plasma cells, scattered large transformed cells, and histiocytes in varying proportions (Fig. 1). Germinal centers, present in 5 cases, appeared hyperplastic and intact, except in 1 case with a rare focus suggestive of possible follicular colonization. Overt monocytoid cytology was not seen in any case. In the patient with predominantly splenic disease, a splenectomy specimen contained similar infiltrate of small lymphocytes, plasmacytoid cells, plasma cells, and histiocytes within the white pulp and in red pulp regions (Fig. 2A). Similarly, the case with salivary gland involvement showed a diffuse architectural effacement by a similar proliferation without evidence of monocytoid cytology, follicular colonization, or lymphoepithelial lesions (Fig. 2B). The percentage of plasmacytic cells (plasma cells and plasmacytoid lymphocytes) as estimated on H&E stains ranged from 5% to 80% (median, 62%). Dutcher bodies were identified in 1 case.
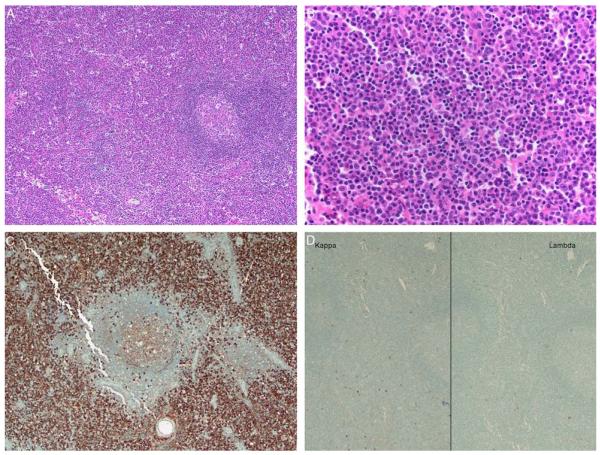
FIGURE 1.
This group I case (case 8) shows an interfollicular and diffuse infiltrate of small lymphocytes, plasmacytoid cells, and plasma cells (A, H&E; B, H&E). By immunohistochemistry, numerous IgG-positive cells (C) that lack kappa and lambda light-chain expression (D) are seen.
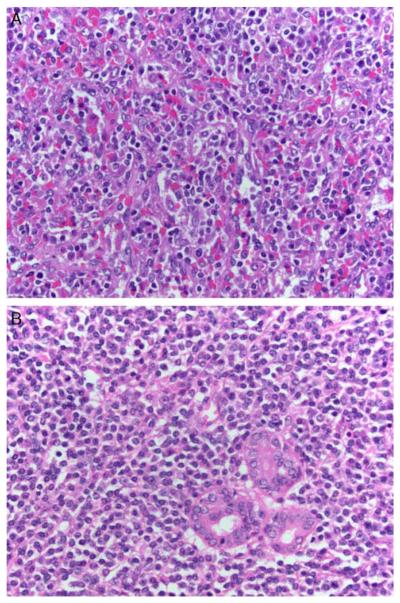
FIGURE 2.
A, This group I case (case 10) displays splenomegaly with a red pulp and white pulp infiltrate of small lymphocytes, plasmacytoid cells, and plasma cells. White pulp nodules suggestive of SMZL were not identified (H&E). B, In this group I case (case 9), a submandibular salivary gland mass contained a diffuse and interfollicular infiltrate of small lymphocytes, plasmacytoid cells, and plasma cells. Overt monocytoid morphology and lymphoepithelial lesions typical of salivary MALT lymphomas were not identified (H&E).
Group II Cases
Lymph node (n = 1), spleen (n = 2), or other extramedullary biopsy specimens (n = 2) were reviewed. Examination of the spleen in case 1 displayed a diffuse red pulp infiltrate of small lymphocytes admixed with occasional plasmacytoid cells, consistent with a splenic diffuse red pulp small B-cell lymphoma (SDRPSBCL). A splenectomy specimen from case 5 displayed expansion of the white pulp by nodules of small lymphocytes and marginal-zone–type cells, characteristic of a typical splenic marginal-zone lymphoma (SMZL) (Figs. 3A–C). In case 11, a thyroidectomy specimen displayed an infiltrate of small lymphocytes and numerous plasma cells. Numerous lymphoepithelial lesions and colonized germinal centers were also present, consistent with a MALT lymphoma with plasmacytic differentiation (Figs. 3D–F). In the remaining case (case 6), which was a patient with a history of typical IgM kappa-positive LPL/Waldenstrom macroglobulinemia involving the bone marrow, a conjunctiva biopsy specimen was seen to contain small lymphocytes admixed with plasma cells.
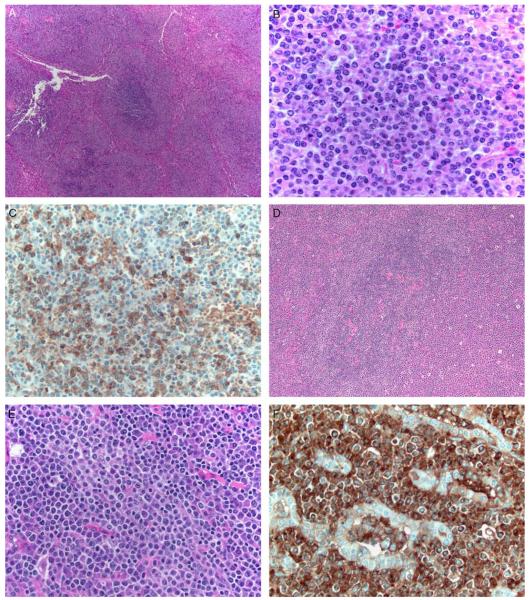
FIGURE 3.
A–C, This group II case (case 5) shows white pulp that is expanded (A, H&E) by nodules with dark cores of small lymphocytes and paler outer zones of marginal-zone cells and plasma cells (B, H&E). The pale rims of marginal-zone cells and plasma cells are positive for IgG (C) and lack detectable kappa and lambda staining by immunohistochemistry or in situ hybridization (not shown). D–F, In this group II case (case 11) a thyroid specimen shows colonized germinal centers surrounded by an interfollicular infiltrate of small lymphocytes and plasma cells (D, H&E; E, H&E). An IgG stain highlights lymphoepithelial lesions (F).
Immunophenotypic Findings
Group I Cases
As summarized in Table 2, the infiltrate in group I cases was positive for CD20 (8/8 cases) and lacked the expression of CD5 (0/7), CD10 (0/8), and CD23 (0/4). Numerous CD138-positive cells were present in 6 cases, whereas in 2 cases numerous plasmacytoid cells were present that lacked expression of CD138. Immunohistochemistry identified numerous IgG-positive cells in each case. Flow cytometry, performed in 6 cases, identified a population of surface immunoglobulin-negative cells in 3 cases, whereas in 2 cases only a small population of polytypic B cells was noted without an apparent surface immunoglobulin-negative B-cell population, and 1 case was interpreted as showing possible, weak cytoplasmic lambda light-chain restriction. By immunohistochemistry and in situ hybridization stains, 6 cases showed absence of light-chain staining, including the case with a possible, weak lambda light-chain stain reported by flow cytometry. In contrast, 1 case showed kappa monotypic plasma cells by in situ hybridization only, and 1 case showed weak kappa monotypic staining by both in situ hybridization stains and immunohistochemistry.
TABLE 2.
Pathologic Features of Group I Cases
| Phenotype | |
| CD20 | 8/8 (100%) |
| CD138 | 6/8 (75%) |
| CD5 | 0/7 (0%) |
| CD10 | 0/8 (0%) |
| CD23 | 0/4 (0%) |
| CD43 | 0/5 (0%) |
| κ/λ | IHC 1/8 (12%) weak kappa monotypic 7/8 (88%) negative |
| κ/λ ISH | 2/8 (25%) kappa monotypic 6/8 (75%) negative |
| Genotype | |
| PCR | IGH (FW1-3) 0/7 (0%) IGK 5/7 (71%) |
| FISH | t(IGH) 0/7 (0%) t(MALT1) 0/7 (0%) +18q 0/7 (0%) t(BCL6) 0/6 (0%) +3q FISH 0/6 (0%) |
Group II Cases
The phenotypic features of group II cases are summarized in Table 3. In cases 1, 5, and 11 (SDRPSBCL, SMZL, and MALT lymphoma, respectively), flow cytometric studies in each case showed the B-cell proliferation to be negative for surface immunoglobulin light chains, whereas immunohistochemical and in situ hybridization stains demonstrated subsets of tumor cells to be positive for IgG and to lack detectable kappa or lambda light chains. In case 6, an IgM kappa monotypic LPL also showed a few, admixed IgG-positive plasma cells that appeared to lack kappa and lambda light chains. This latter case showed both IgM kappa monoclonal paraprotein and free gamma heavy chains by immunofixation of the serum.
TABLE 3.
Pathologic Features of Group II Cases
| Case Designation |
1 | 2 | 5 | 6 | 11 |
|---|---|---|---|---|---|
| WHO diagnosis | SDRPSBCL and T- LGL |
MGUS and chronic NK-cell lymphocytosis |
SMZL | LPL | MALT lymphoma |
| Tissue biopsied | Spleen | Bone marrow | Spleen, lymph node, bone marrow |
Bone marrow, conjunctiva |
Thyroid |
| IHC and flow results |
Small CD20+ B cells negative for CD5, CD10, sIg. Few clusters of IgG+ cIg− plasma cells |
Rare scattered CD20+ small B cells and rare polytypic plasma cells |
Small CD20+ B cells negative for CD5, CD10, sIg. Marginalzone cells IgG+, cIg− |
Small CD20+ B cells negative for CD5, and CD10 with IgM+ Kappa+ plasma cells and scattered IgG+, cIg− plasma cells |
Small CD20+ B cells negative for CD5, CD10, sIg. Numerous IgG+ cIg− plasma cells |
| Genotype | |||||
| PCR | |||||
| IGH FW1-3 | Neg* | NP | Neg | NP | Neg |
| IGK FISH | Atypical, nondiagnostic |
NP | Neg | NP | Neg |
| t(IGH) | Neg | NP | NP | NP | Neg |
| t(MALT1) | Neg | NP | NP | NP | Neg |
| +18/18q | Neg | NP | NP | NP | Neg |
| t(BCL6) | Neg | NP | NP | NP | Neg |
| +3/3q | Neg | NP | NP | NP | Pos |
Southern blot positive for clonal IGH rearrangement.
Neg indicates negative; NP, not performed; Pos, positive.
Molecular and Cytogenetic Findings
Group I Cases
PCR studies for immunoglobulin heavy-chain or kappa light-chain clonality could be performed in 7 cases. IGH FW1-3 primers were negative for a clonal population in all cases. Clonal IGK rearrangements were detected in 5 cases and not detected in 2 cases (each of which showed suboptimal DNA preservation). Metaphase karyotyping was performed in 2 cases. One lymph node displayed a normal karyotype, whereas the case with predominantly splenic involvement displayed 2 abnormal metaphases containing inv(8)(p21q22). FISH studies were negative for IGH translocations (0/7), MALT1 translocations (0/7), + MALT1 consistent with trisomy 18/18q (0/7), BCL6 translocations (0/6), or + BCL6 consistent with trisomy 3/3q (0/6).
Group II Cases
PCR studies for immunoglobulin heavy-chain and/or kappa light-chain clonality were conducted in 3 cases. Case 1 (SDRPSBCL) was negative for an IGH rearrangement and showed an abnormal, nondiagnostic IGK peak, but Southern blot studies confirmed a clonal IGH rearrangement. Case 5 (SMZL) and case 11 (thyroid MALT lymphoma) lacked detectable IGH or IGK clonal rearrangements, although DNA preservation was suboptimal in case 5. Metaphase cytogenetic studies conducted in case 5 (SMZL) demonstrated an abnormal karyotype containing a der(19)t(1;19)(q23;p13); RT-PCR studies showed that this translocation did not represent the E2A/PBX1 translocation characteristic of precursor B-cell ALL. FISH studies could be conducted in 2 cases. Case 1 (SDRPSBCL) showed no evidence of translocations involving IGH, MALT1, or BCL6, and no evidence of trisomy of chromosome 3q or 18q. Case 11 (thyroid MALT) was negative for IGH, MALT1, and BCL6 translocations and also for trisomy of 18q. However, it showed trisomy of the BCL6 locus consistent with trisomy 3q.
Bone Marrow Findings
Group I Cases
Bone marrow biopsies were performed for all 8 patients. Bone marrow slides were reviewed for 5 cases, and outside reports were reviewed for 3 cases. Plasma cells were noted to range from <1% to 13% of the total cellularity. In 4 cases, involvement by gHCD was noted. Three of these cases showed mildly increased plasma cells (5% to 13%) that appeared to express IgG heavy chain without light chains by immunohistochemistry and/or in situ hybridization, whereas the fourth case showed multiple lymphoid aggregates containing admixed B cells and T cells and increased IgG-positive plasma cells lacking identifiable light chains (Fig. 4). In 4 cases, involvement by gHCD was not morphologically identified.
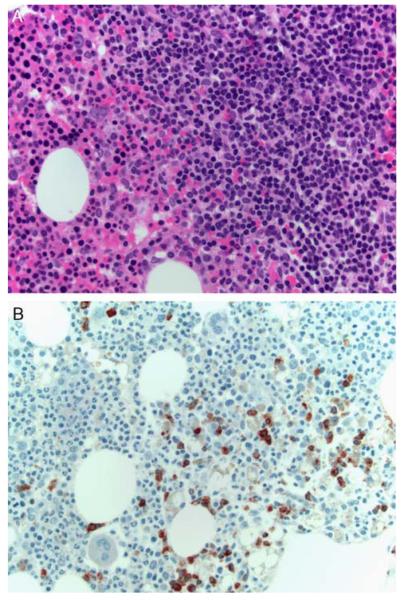
FIGURE 4.
A, A bone marrow core biopsy from a group I case (case 4) shows an aggregate of predominantly small lymphocytes (H&E). B, An IgG immunostain highlights positive cells within the aggregates that appeared to lack kappa or lambda light chains (not shown).
Group II Cases
Bone marrow biopsies were performed for 3 of the 5 patients [case 1 SDRPSBCL, case 2 monoclonal gammopathy of undetermined significance (MGUS), case 5 SMZL], and slides were reviewed in each case. The SDRPSBCL and SMZL cases demonstrated involvement by a B-cell lymphoproliferative disorder with surface immunoglobulin-negative B cells noted by flow cytometry and scattered plasma cells (5% to 10% each) that appeared polytypic by immunohistochemistry, although the possibility of a light-chain–negative population could not be entirely ruled out. In case 2 (MGUS), the bone marrow biopsy showed reactive-appearing predominantly T-cell lymphoid aggregates, with 4% plasma cells that appeared polytypic by immunohistochemistry. Flow cytometry in this case also showed a population of surface immunoglobulin-negative B cells, but an overt B-cell lymphoproliferative disorder was not identified by routine histology or immunohistochemistry.
Concurrent Large Granular Lymphocyte Disorders
Group I Cases
In the patient with predominantly splenic disease, the spleen also showed an increased red pulp infiltrate of CD57-positive T cells, suspicious for a coexisting large granular lymphocyte (T-LGL) leukemia. This patient had a history of neutropenia without lymphopenia, but a peripheral blood smear was not available to determine the number of circulating LGLs. PCR studies were negative for a clonal TCR β or TCR gamma population, and a definitive diagnosis of T-LGL was therefore not confirmed.
Group II Cases
Two of the group II cases had coexisting LGL disorders. In case 1, the patient had a history of neutropenia with increased peripheral blood LGLs. The LGLs exhibited a T-cell phenotype, and a clonal T-cell population was noted by PCR. In case 2, the patient had a history of anemia and neutropenia and increased peripheral blood LGLs. The LGLs showed a phenotype consistent with natural killer (NK) cells, and a diagnosis of chronic NK lymphocytosis was established. In both patients, the LGL leukemias were clinically identified first, with subsequent discovery of free gamma heavy chains during evaluation of the LGL disorders.
Clinical Course
Group I Cases
Clinical follow-up data were available for 6 patients. Two patients were treated (1 rituximab only, 1 with bortezomib/dexamethasone) with resolution of adenopathy (22- and 32-mo follow-up, respectively). One patient was not treated and was alive with stable disease after 134 months of follow-up. One patient was initially followed up for 1 year, but CHOP therapy was then begun because of increasing adenopathy with good response at 2 months’ follow-up. Another patient initially managed expectantly for 1 year received chlorambucil therapy because of increasing adenopathy with a very good partial response and no radiographic evidence of progressive disease at last follow-up 19 months later despite poor overall clinical status. One patient (with splenic disease and T-cell population suspicious for T-LGL) was refractory to therapy with fludarabine and died 25 months later because of complications of anemia and neutropenia.
Group II Cases
Clinical follow-up data were available for 3 cases. In case 2 (MGUS), methotrexate and cyclophosphamide therapy was initiated for the concurrent chronic NK-cell lymphocytosis with good response at 37 months’ follow-up. Case 1 (SDRPSBCL) was treated by splenectomy alone and was alive without clinically evident disease at 15 months’ follow-up. Case 5 (SMZL) was treated by splenectomy and CHOP chemotherapy and adjuvant therapy for a simultaneous pulmonary carcinoma. At 26-month follow-up, there was a new hilar adenopathy and lacrimal gland swelling, but the patient was then lost to further follow-up.
DISCUSSION
The heavy-chain diseases are B-cell lymphoproliferative disorders with plasmacytic differentiation characterized by the production of a monoclonal, abnormally truncated immunoglobulin heavy-chain protein that is incapable of assembling with immunoglobulin light chains.12,31,32,34 The heavy-chain diseases have been divided into 3 diagnostic categories on the basis of the heavy-chain isotype, each of which is associated with differing pathologic features. Alpha heavy-chain disease, also known as immunoproliferative small intestinal disease, is a variant of extranodal MALT lymphoma that involves the gastrointestinal tract, especially the small bowel, and may be related to an underlying chronic infection with Campylobacter jejuni.1,12,25 Mu heavy-chain disease is generally associated with a B-cell lymphoproliferative disorder that resembles chronic lymphocytic leukemia, although CD5 expression is reportedly absent, in contrast to typical chronic lymphocytic leukemia.8,12 gHCD, unlike alpha or mu heavy-chain diseases, has been reported to occur in a spectrum of lymphoproliferative disorders. Although most cases are said to contain a neoplasm of lymphocytes and plasma cells similar to LPL, gHCD has also been reported in association with marginal-zone lymphomas, plasmacytomas, and Hodgkin lymphoma.8,9,17,18,31–34 Interpretation of these reports by the current diagnostic criteria is challenging because the great majority of these cases were diagnosed and classified using outdated classification schemes, often with limited phenotypic studies, and because prior reports have included little detailed histologic information. The difficulties in the classification of gHCD are reflected in the 2008 WHO monograph, which discusses gHCD both as a variant of LPL (in the LPL chapter) and as a distinct disorder (in a separate heavy-chain disease chapter).28
In this study, we have examined the detailed histologic, phenotypic, and genotypic findings of 13 cases of gHCD. Within this series, the majority of cases (8/13, 62%) displayed very similar clinicopathologic features (group I cases). These cases showed a proliferation of small lymphocytes, plasma cells, and histiocytes that were difficult to classify according to the current WHO criteria. Features often seen in marginal-zone lymphomas (monocytoid morphology, overt follicular colonization, or lymphoepithelial lesions at extranodal sites) were not identified in these cases. Because LPL is currently defined as a diagnosis of exclusion (ie, a lymphoproliferative disorder with plasmacytic differentiation that does not meet criteria for one of the other, better-defined entities in the 2008 WHO classification), these cases could be appropriately classified as LPL.19,27 The detailed morphologic features of nodal LPL have been previously investigated.3,5,26,27 The most characteristic nodal pattern of LPL consists of a partially preserved architecture with patent sinuses and an expansion by small lymphocytes and plasma cells, wherein plasma cells usually represent a minority of the cellularity. Cases with this pattern are highly associated with bone marrow involvement and an IgM paraprotein. Other cases of LPL show diffuse effacement of the nodal architecture with a more heterogenous proliferation of small lymphocytes, plasma cells, and histiocytes. This latter pattern, which has been called “vaguely nodular, polymorphous” LPL, is less likely to display bone marrow involvement and is more likely to have a non-IgM monoclonal paraprotein (eg, IgG or IgA proteins). The group I gHCD cases in this series largely overlap with cases of “vaguely nodular, polymorphous” LPL.
Importantly, the remaining cases in this series (5/13, 38%) consisted of a variety of other, well-defined lymphoid neoplasms, emphasizing the heterogenous pathogenesis of gHCD. Specifically, 1 case each showed findings characteristic of SMZL, thyroid MALT lymphoma, and SDRPSBCL. At least 1 prior case of gHCD has been reported to be associated with SMZL,22 and numerous cases have been reported to be associated with extranodal plasmacytomas (especially skin and thyroid) that might be better classified as extranodal MALT lymphomas with plasmacytic differentiation using modern diagnostic criteria.31–34 One patient lacked adenopathy and had only mild splenomegaly attributed to his coexisting LGL disorder. Bone marrow biopsy displayed no evidence of a B-cell lymphoma or increase in plasma cells, although flow cytometric studies did demonstrate a small surface immunoglobulin-negative B-cell population. The findings in this latter case are therefore most consistent with a diagnosis of free gamma heavy-chain MGUS.31,32 Prior studies have also reported asymptomatic patients with incidentally found free gamma heavy-chain MGUS. These cases demonstrate that multiple types of lymphoproliferative disorders may give rise to gHCD and that gHCD can exist in the absence of clinically apparent neoplasms similar to other forms of MGUS.
The patients in this report display several notable clinical findings. First, this series shows a marked female predominance. This finding is similar to that noted in a relatively recent larger series32 but contrasts with the findings of older studies.8,9,31 These cases also show a strong association with autoimmune disease, particularly group I cases. An increased incidence of autoimmune disease has been previously reported in gHCD, with rheumatoid arthritis being most frequently identified.8,9,31,32,34 Prior reports have suggested that the abnormal secreted heavy chain may play a role in eliciting an autoimmune response, especially the production of rheumatoid factor.14,15 The patients in the current series displayed a particular association with a clinical diagnosis of systemic lupus erythematosus. The etiology of this association should also be further investigated. Interestingly, this series also showed cases with concurrent LGL disorders. The possibility that these proliferations represent, at least in part, a reaction to the secreted abnormal immunoglobulin could also be considered.
Of particular interest is a group II case that displayed both free gamma heavy chains and an IgM lambda monoclonal paraprotein by immunofixation and histologically showed IgG-only plasma cells admixed in an otherwise typical IgM lambda-restricted LPL. Prior case reports of gHCD have also included patients with coexisting intact (heavy chain and light chain) IgM paraproteins.31,32 This case and the prior reports raise the question of whether the gHCD-positive and IgM-positive LPLs represent 2 clonally unrelated neoplasms versus 1 IgM-positive neoplasm with a subclone that has undergone class switching and heavy-chain truncation. Resolving this question would require a detailed comparison of the IgM and IgG heavy chains at the amino acid or nucleotide level. Unfortunately, suitable material for such detailed analysis was not available in this case. Further studies will therefore be necessary to determine whether free gHCD can ever be seen as a secondary abnormality in a subclone of a B-cell neoplasm.
The molecular mechanism leading to abnormal truncation of the IgG heavy chain in gHCD is uncertain. Metaphase cytogenetic information has rarely been reported in cases of heavy-chain diseases, although 1 case reported as alpha heavy-chain disease contained a t(9;14)(p13;q32).16 This translocation, involving the PAX5 and IGH loci, was initially reported to be highly associated with LPL.2,16 More recent studies, however, have shown that t(9;14) has no association with LPL as defined by the current WHO criteria.5,11 FISH studies performed in this series showed no evidence of an IgH translocation in any case studied. This finding indicates that IGH translocations do not appear to play a role in the truncation of the immunoglobulin heavy chain in at least most cases of gHCD.
This study also highlights the challenge of correctly recognizing and diagnosing gHCD in routine practice. In at least most cases of gHCD, monoclonal light chains are not only absent in the serum or urine but are also not produced at detectable levels in the neoplastic cells.12,31,32 Prior studies have shown that coexisting light-chain mutations/deletions and/or transcriptional regulatory changes may be responsible for the lack of light-chain production in the setting of heavy-chain disease.4,6,29 As a consequence, flow cytometric studies yield surface immunoglobulin-negative B-cell populations that might be mistaken for a reactive finding or even missed entirely. By immunohistochemistry and in situ hybridization staining, most cases are seen to lack obvious light-chain staining in neoplastic cells, although background light-chain–positive plasma cells may be present. Suspicion for an underlying light-chain–negative population is raised by noting the presence of numerous plasmacytoid/plasmacytic cells not staining for kappa or lambda. Performing additional stains for CD138 and immunoglobulin heavy chains will assist in recognizing this abnormal light-chain–negative population. Interestingly, however, a minority of cases continue to produce detectable light-chain mRNA and/or protein even in the absence of light-chain secretion, and light-chain restriction may be identified by immunohistochemistry or in situ hybridization as seen in 2 cases in this series. Prior reports have also included cases of gHCD with detection of clonal plasma cells by flow cytometry or immunohistochemistry, or with secretion of monoclonal light-chain (Bence Jones) proteins.13,20,32,33 The finding of clonal plasmacytoid or plasmacytic cells by immunohistochemistry or by in situ hybridization therefore does not exclude the possibility of gHCD. Finally, PCR clonality studies may also be misleading. As seen in the majority of cases in this series, an IGH monoclonal peak may not be detected, consistent with the deletions of the IGH variable regions known to occur in gHCD.31,32,34 PCR studies for IGK rearrangements may therefore be particularly helpful in this setting. Correlation with electrophoresis and immunofixation studies confirm the diagnosis suspected on the basis of morphology and phenotype, emphasizing the importance of correlation with serologic studies whenever possible in any case of a B-cell lymphoproliferative disorder with plasmacytic differentiation.
In conclusion, this study has clarified the spectrum of lymphoproliferative disorders that underlie gHCD. As suggested by prior reports using outdated classification schemes, the majority of these patients have an underlying lymphoproliferative disorder that could be classified as LPL. Interestingly, these cases resemble those reported as “vaguely nodular, polymorphous” LPL rather than the classic histologic pattern that is more highly associated with an IgM paraprotein and bone marrow involvement (Waldenstrom’s macroglobulinemia). gHCD, however, is more heterogenous than alpha or mu heavy-chain diseases and can be seen with a variety of lymphoproliferative disorders, including typical examples of marginal-zone lymphomas or as an asymptomatic MGUS-like state. Therefore, gHCD should not be considered as a mere clinical variant of LPL. Despite this heterogeneity, gHCD patients show distinctive clinical features that are not seen in LPL and marginal-zone lymphoma, such as a marked female predominance and association with autoimmune disease. These latter observations suggest that gHCD is best recognized as a clinical syndrome that may be seen in association with one of several types of underlying lymphoproliferative disorders.
Footnotes
Conflicts of Interest and Source of Funding: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
REFERENCES
- 1.Al-Saleem T, Al-Mondhiry H. Immunoproliferative small intestinal disease (IPSID): a model for mature B-cell neoplasms. Blood. 2005;105:2274–2280. doi: 10.1182/blood-2004-07-2755. [DOI] [PubMed] [Google Scholar]
- 2.Amakawa R, Ohno H, Fukuhara S. t(9;14)(p13;q32) involving the PAX-5 gene: a unique subtype of 14q32 translocation in B cell non-Hodgkin’s lymphoma. Int J Hematol. 1999;69:65–69. [PubMed] [Google Scholar]
- 3.Andriko JA, Swerdlow SH, Aguilera NI, et al. Is lymphoplasmacytic lymphoma/immunocytoma a distinct entity? A clinicopathologic study of 20 cases. Am J Surg Pathol. 2001;25:742–751. doi: 10.1097/00000478-200106000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Cogne M, Bakhshi A, Korsmeyer SJ, et al. Gene mutations and alternate RNA splicing result in truncated Ig L chains in human gamma H chain disease. J Immunol. 1988;141:1738–1744. [PubMed] [Google Scholar]
- 5.Cook JR, Aguilera NI, Reshmi-Skarja S, et al. Lack of PAX5 rearrangements in lymphoplasmacytic lymphomas: reassessing the reported association with t(9;14) Hum Pathol. 2004;35:447–454. doi: 10.1016/j.humpath.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Corcos D, Osborn MJ, Matheson LS. B-cell receptors and heavy chain diseases: guilty by association? Blood. 2011;117:6991–6998. doi: 10.1182/blood-2011-02-336164. [DOI] [PubMed] [Google Scholar]
- 7.Evans PAS, Pott C, Groenen PJTA, et al. Significantly improved PCR-based clonality testing in B-cell malignancies by use of multiple immunoglobulin gene targets. Report of the BIOMED-2 Concerted Action BHM4-CT98-3936. Leukemia. 2007;21:207–214. doi: 10.1038/sj.leu.2404479. [DOI] [PubMed] [Google Scholar]
- 8.Fermand JP, Brouet JC. Heavy-chain diseases. Hematol/Oncol Clin N Am. 1999;13:1281–1294. doi: 10.1016/s0889-8588(05)70127-1. [DOI] [PubMed] [Google Scholar]
- 9.Fermand JP, Brouet JC, Danon F, et al. Gamma heavy chain “disease”: heterogeneity of the clinicopathologic features. Report of 16 cases and review of the literature. Medicine. 1989;68:321–335. [PubMed] [Google Scholar]
- 10.Franklin EC, Lowenstein J, Bigelow B, et al. Heavy chain disease—a new disorder of serum gamma-globulins : report of the first case. Am J Med. 1964;37:332–350. doi: 10.1016/0002-9343(64)90191-3. [DOI] [PubMed] [Google Scholar]
- 11.George TI, Wrede JE, Bangs CD, et al. Low-grade B-cell lymphomas with plasmacytic differentiation lack PAX5 gene rearrangements. J Mol Diagn. 2005;7:346–351. doi: 10.1016/S1525-1578(10)60563-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris NL, Isaacson PG, Grogan TM, et al. Heavy chain diseases. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon: 2008. pp. 196–199. [Google Scholar]
- 13.Hauke G, Schiltz E, Bross KJ, et al. Unusual sequence of immunoglobulin L-chain rearrangements in a gamma heavy chain disease patient. Scand J Immunol. 1992;36:463–468. doi: 10.1111/j.1365-3083.1992.tb02961.x. [DOI] [PubMed] [Google Scholar]
- 14.Husby G. Is there a pathogenic link between gamma heavy chain disease and chronic arthritis? Curr Opin Rheumatol. 2000;12:65–70. doi: 10.1097/00002281-200001000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Husby G, Blichfeldt P, Brinch L, et al. Chronic arthritis and gamma heavy chain disease: coincidence or pathogenic link? Scand J Rheumatol. 1998;27:257–264. [PubMed] [Google Scholar]
- 16.Iida S, Rao PH, Nallasivam P, et al. The t(9;14)(p13;q32) chromosomal translocation associated with lymphoplasmacytoid lymphoma involves the PAX-5 gene. Blood. 1996;88:4110–4117. [PubMed] [Google Scholar]
- 17.Kyle RA, Greipp PR, Banks PM. The diverse picture of gamma heavy-chain disease. Report of seven cases and review of literature. Mayo Clin Proc. 1981;56:439–451. [PubMed] [Google Scholar]
- 18.Lee MT, Parwani A, Humphrey R, et al. Gamma heavy chain disease in a patient with diabetes and chronic renal insufficiency: diagnostic assessment of the heavy chain fragment. J Clin Lab Anal. 2008;22:146–150. doi: 10.1002/jcla.20233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin P, Molina TJ, Cook JR, et al. Lymphoplasmacytic lymphoma and other non-marginal zone lymphomas with plasmacytic differentiation. Am J Clin Pathol. 2011;136:195–210. doi: 10.1309/AJCP8FOIVTB6LBER. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Anglada L, Puig N, Diez-Campelo M, et al. Monoclonal free light chains can be found in heavy chain diseases. Ann Clin Biochem. 2010;47:570–572. doi: 10.1258/acb.2010.010146. [DOI] [PubMed] [Google Scholar]
- 21.Molina TJ, Lin P, Swerdlow SH, et al. Marginal zone lymphomas with plasmacytic differentiation and related disorders. Am J Clin Pathol. 2011;136:211–225. doi: 10.1309/AJCP63OGXHXCSKSC. [DOI] [PubMed] [Google Scholar]
- 22.Morita K, Kawamoto H, Takada H, et al. Unusual gamma heavy chain disease protein in a patient with splenic marginal-zone lymphoma. Ann Clin Biochem. 2006;43:161–164. doi: 10.1258/000456306776021490. [DOI] [PubMed] [Google Scholar]
- 23.Munshi NC, Digumarthy S, Rahemtullah A. Case records of the Massachusetts General Hospital. Case 13-2008. A 46-year-old man with rheumatoid arthritis and lymphadenopathy. N Engl J Med. 2008;358:1838–1848. doi: 10.1056/NEJMcpc0800959. [DOI] [PubMed] [Google Scholar]
- 24.Ruiz A, Reischl U, Swerdlow SH, et al. Extranodal marginal zone B-cell lymphomas of the ocular adnexa: multiparameter analysis of 34 cases including interphase molecular cytogenetics and PCR for Chlamydia psittaci. Am J Surg Pathol. 2007;31:792–802. doi: 10.1097/01.pas.0000249445.28713.88. [DOI] [PubMed] [Google Scholar]
- 25.Salem PA, Estephan FF. Immunoproliferative small intestinal disease: current concepts. Cancer J. 2005;11:374–382. doi: 10.1097/00130404-200509000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Sargent RL, Cook JR, Aguilera NI, et al. Fluorescence immunophenotypic and interphase cytogenetic characterization of nodal lymphoplasmacytic lymphoma. Am J Surg Pathol. 2008;32:1643–1653. doi: 10.1097/PAS.0b013e3181758806. [DOI] [PubMed] [Google Scholar]
- 27.Swerdlow SH, Berger F, Pileri SA, et al. Lymphoplasmacytic lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon: 2008. pp. 194–195. [Google Scholar]
- 28.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon: 2008. [Google Scholar]
- 29.Teng MH, Rosen S, Gorny MK, et al. Gamma heavy chain disease in man: independent structural abnormalities and reduced transcription of a functionally rearranged lambda L-chain gene result in the absence of L-chains. Blood Cells Mol Dis. 2000;26:177–185. doi: 10.1006/bcmd.2000.0294. [DOI] [PubMed] [Google Scholar]
- 30.van Dongen JJM, Langerak AW, Bruggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17:2257–2317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- 31.Wahner-Roedler DL, Kyle RA. Heavy chain diseases. Best Pract Res. 2005;18:729–746. doi: 10.1016/j.beha.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 32.Wahner-Roedler DL, Witzig TE, Loehrer LL, et al. Gamma-heavy chain disease: review of 23 cases. Medicine. 2003;82:236–250. doi: 10.1097/01.md.0000085058.63483.7f. [DOI] [PubMed] [Google Scholar]
- 33.Wester SM, Banks PM, Li CY. The histopathology of gamma heavy-chain disease. Am J Clin Pathol. 1982;78:427–436. doi: 10.1093/ajcp/78.4.427. [DOI] [PubMed] [Google Scholar]
- 34.Witzig TE, Wahner-Roedler DL. Heavy chain disease. Curr Treat Options Oncol. 2002;3:247–254. doi: 10.1007/s11864-002-0014-3. [DOI] [PubMed] [Google Scholar]
- 35.Wongchaowart NT, Kim B, Hsi ED, et al. t(14;18)(q32;q21) involving IGH and MALT1 is uncommon in cutaneous MALT lymphomas and primary cutaneous diffuse large B-cell lymphomas. J Cutan Pathol. 2006;33:286–292. doi: 10.1111/j.0303-6987.2006.00459.x. [DOI] [PubMed] [Google Scholar]