Abstract
Objective
To assess glaucoma medication adherence in children, hypothesizing that poor parental health literacy and eye drop instillation by the child are associated with worse adherence.
Methods
This prospective, observational study enrolled pediatric patients with glaucoma who were prescribed eye drops. Parent(s) reported who was responsible for eye drop instillation (parent vs child), took the Rapid Estimate of Adult Literacy in Medicine, and were instructed on the use and purpose of the Medication Event Monitoring System. Calculations included average adherence (proportion of prescribed doses taken), dosing errors (number of overdosing or underdosing events in 24 hours), and proportion of doses taken on schedule (doses taken within 2 hours of prescribed dosing interval). Results are reported as mean (SD) or median.
Results
The study included 46 of the 50 enrolled children who used the Medication Event Monitoring System for 30 days or more. Adherence ranged from 43% to 107% (93%[12%]) and was not associated with age (slope, 0.09 [0.52]; P = .86) but decreased with the parent’s lower health literacy (slope, 0.62 [0.24]; P = .01).The mean number of dosing errors for medications prescribed daily vs twice daily was similar (3.3 vs 2.9; P = .66). The proportion of doses taken on schedule (within 2 hours of prescribed dosing interval) ranged from 3% to 97% (median, 34%; mean, 41% [24%]) and was better when the parent vs the child instilled eye drops (46% [26%] vs 23% [19%]; P < .001).
Conclusions
Time-dependent glaucoma medication adherence was better when the parent was responsible for eye drop instillation. Overall decreased adherence was associated with decreased parental health literacy. Children of parents with poor health literacy are vulnerable to poor medication adherence; efforts to address poor health literacy may improve outcomes.
Poor medication adherence is a problem prevalent in many realms of health care and especially in the management of asymptomatic, chronic disease.1 Children with chronic diseases are not immune to this phenomenon. In a study2 of children with atopic dermatitis, electronic medication monitors revealed that the mean proportion of the prescribed dose of topical cream that was actually administered was only 32%.
Recently, much attention has been paid to the problem of poor medication adherence in adults with open-angle glaucoma. Adherence rates vary widely, depending on the definition of adherence and the methods of measurement used.3–6 Methods of measuring adherence include self-report, pharmacy refill records, electronic monitors, and direct observation of eye drop instillation.7–9 Although no one measure captures every aspect of adherence to the prescribed treatment regimen, electronic medication monitors record the date and time that the medication is accessed by the patient, allowing for estimates of time-appropriate dosing and overall medication use.6 Previously, electronic medication monitors have been used to evaluate adherence to ophthalmic medications for children undergoing treatment for myopia10,11 but not for management of glaucoma.
The study of medication adherence in adults with glaucoma has elucidated factors associated with nonadherence. In structured interviews and self-report questionnaires, patients with glaucoma report barriers to adherence, including complex dosing regimens12,13 and situational factors, such as competing activities.12 Electronic monitoring of medication use reveals worse adherence to medications prescribed to be taken more frequently,6,14 and pharmacy claims reviews demonstrate worse adherence for some classes of glaucoma medications compared with others.15 Pharmacy claims reviews also indicate that patients who are unaware of the potential blinding consequences of glaucoma16 and patients with poor health literacy skills17 are less adherent to their medications.
Health literacy is related to, but not synonymous with, education and represents a person’s ability to comprehend and act on written and verbal information in a health care setting.18 In adults, inadequate health literacy skills are linked to poor self-management of chronic diseases, such as diabetes mellitus19 and asthma.20 Previous studies21 have shown that children of parents with lower levels of educational attainment are less adherent with amblyopia therapy; these studies led us to question whether poor parental health literacy may be associated with poor glaucoma medication adherence for the child. We also considered that adherence in childhood glaucoma might be affected by factors not relevant to the treatment of adult glaucoma, such as whether the parent or the child instills the eye drops.
Medication adherence to intraocular pressure (IOP)–lowering medications in children with glaucoma has not been explored. We therefore designed this study to address the following: glaucoma medication adherence in children with glaucoma, the effect of child vs parental responsibility for eye drop instillation, and the effect of parental health literacy skills on the child’s adherence.
METHODS
This prospective, observational study was conducted with approval from the Duke institutional review board. Consecutive patients aged 5 through 17 years under the care of one pediatric ophthalmologist were approached regarding study participation during a regularly scheduled clinic visit. A sample size of 50 patients was chosen empirically. Patients were recruited if their medical record indicated a diagnosis of glaucoma for which topical ocular hypotensive medications were prescribed. Patients were excluded if they had a history of intraocular surgery in the past month or plans for intraocular surgery in the following month.
After obtaining informed consent, study participants and their parents were given a short survey (Figure 1) that questioned who (child or parent) was responsible for giving the prescribed eye drops. Parents of the study participants were also given an assessment of the parent’s health literacy skills, the Rapid Assessment of Adult Literacy in Medicine (REALM). REALM is a word recognition test that involves words commonly used in the health care setting and can be administered in 2 to 3 minutes.22
Figure 1.
Survey tools administered to study participants and their parents that questioned who was responsible for giving the prescribed eye drops. A, Child/patient survey. B, Parent/caregiver survey.
As part of standard care in this pediatric glaucoma practice, patients were instructed orally by the physician to use once-daily medications every 24 hours, twice-daily medications every 12 hours, and 3-times-daily medications every 8 hours (as opposed to being told to use medications once a day, twice a day, or 3 times a day). Children and their parents were given and instructed on the use and purpose of the Medication Event Monitoring System (MEMS 6 SmartCap; Aardex) by a member of the research team (S.K.J.). The MEMS SmartCap is a bottle cap that is affixed to a plastic bottle inside of which the entire glaucoma medication bottle is stored. When the patient opens the MEMS SmartCap to retrieve his or her glaucoma medication bottle for eye drop instillation, the SmartCap records the date and time of the event. The assumption was made that if patients were prescribed IOP-lowering medications for both eyes, the bottle would be opened only once for bilateral eye drop instillation. Electronic data regarding the events are stored in the cap continuously. The device also acts as a passive mnemonic aid by displaying the number of the last dose taken within a 24-hour period on the liquid crystal display screen on the top of the bottle. Patients received a separate MEMS SmartCap for each prescribed IOP-lowering medication (whether the medication was prescribed for 1 or both eyes). The information regarding medication events was downloaded from the MEMS SmartCaps to companion software (Powerview; Aardex) when the patient returned for his or her next regularly scheduled clinic visit. Because our primary outcome was medication adherence during the first 30 days, the calculations were performed for the first 30 days of use of the MEMS SmartCap. However, most patients used the MEMS SmartCaps for more than 30 days, so in post hoc analyses, adherence metrics were calculated for days 31 through 60 and 61 through 90 and compared with metrics from the first 30 days.
The medication event data allow for the computation of several measures for describing adherence to the prescribed medication regimen. First, adherence, defined as the proportion of prescribed doses taken, was calculated. These values were averaged for all IOP-lowering medications for a given individual. As such, the calculated adherence value represents all medications for a given individual. Second, the proportion of doses taken on schedule was assessed. The default setting of the Powerview software defines “on schedule” as doses taken within 25% of the prescribed dosing interval. For example, if the second dose of an IOP-lowering medication prescribed to be taken twice daily was taken between 9 and 15 hours after the first dose, it would be considered “on schedule.” Because a more stringent criterion—doses taken within a 2-hour window of the nominal dosing interval—has been reported in the literature of glaucoma medication adherence,6 the proportion of doses taken on schedule was also calculated for the 2-hour window. Similarly to the calculation for adherence, the proportion of doses taken on schedule was averaged for all medications taken by a given patient.
Additional calculations included the number of dosing errors, defined as the number of overdosing or underdosing events in a 24-hour period, and the interval between doses, defined as the number of hours between doses taken according to the MEMS SmartCaps. For dosing errors and interval between doses calculations, each medication (whether used in 1 or both eyes of a given patient) was considered separately and not averaged for a given patient.
Descriptive statistics were obtained, including mean (SD) and median. Groups were compared with paired or unpaired t tests where appropriate. Continuous explanatory variables and continuous outcomes were assessed with linear regression. P < .05 (2-sided) was considered significant. Adjustments were not made for multiple comparisons.
RESULTS
Of the 50 patients whose parent(s) provided informed consent for study participation, 46 completed the study, which included using the electronic medication monitor for at least 30 days. No patients declined to participate, but 3 patients did not return the MEMS SmartCaps and 1 returned the cap but reported not having used it during the study period. Characteristics of the 46 patients who completed the study are provided in Table 1. For all patients but 1, only 1 parent attended the appointment, and for the 1 patient with 2 parents present, the mother volunteered to take the health literacy assessment (REALM). Although the study was designed to capture 30 days of medication use, 32 patients used the monitors for at least 90 days; therefore, a comparison of adherence measures for extended monitoring was included.
Table 1.
Characteristics of Patients and Prescribed Glaucoma Medications
| Characteristic | Finding |
|---|---|
| Sex, No. | |
| Male | 24 |
| Female | 22 |
| Race, No. | |
| White | 30 |
| African American | 13 |
| Other | 3 |
| Age, y | |
| Mean (SD) | 10.8 (3.6) |
| Median (range) | 10 (5–17) |
| No. of prescribed topical glaucoma medications, | |
| Mean (SD) | 2.0 (0.9) |
| Median (range) | 2 (1–4) |
| Prescribed dosing intervals of medications, No. of medications (No. of patients)a | |
| Once daily | 30 (26) |
| Twice daily | 55 (36) |
| 3 times daily | 4 (3) |
The total number of medications in the study with the stated dosing frequency (the total number of patients in the study prescribed medications with the stated dosing frequency [patients counted more than once if prescribed more than 1 medication with disparate dosing frequency]).
ADHERENCE
Adherence ranged from 43% to 107% (median, 95%; mean, 93% [12%]). Adherence was not associated with age (slope, 0.09 [0.52]; P = .86) or whether the parent (n = 25) vs the child (n = 21) instilled the eye drops (92% [13%] vs 96% [4%], respectively; P = .20). Adherence was lower for African American patients (n = 13) than for white patients (n = 30) (85% [16%] vs 94% [9%], respectively; P = .02), and adherence decreased significantly as the health literacy level of the parent decreased (slope, 0.62 [0.24]; P = .01). In multivariable analyses considering race and parental health literacy together, only decreased parental health literacy was associated with decreased adherence (P = .01).
PROPORTION OF DOSES TAKEN ON SCHEDULE
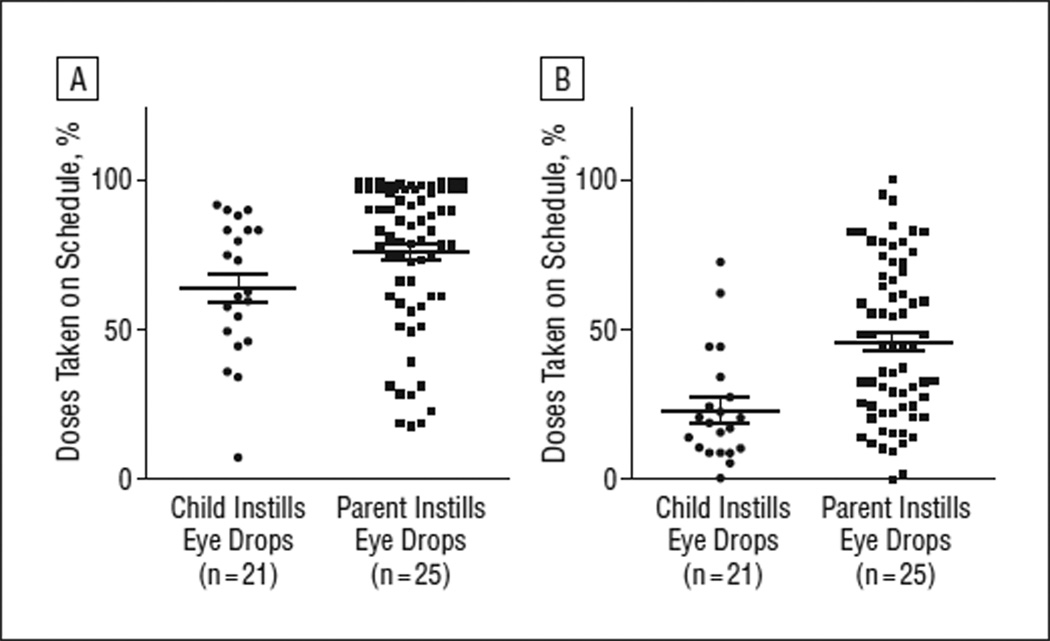
The proportion of doses taken on schedule, as defined by a window within 25% of the nominal dosing schedule, ranged from 17% to 99% (median, 78%; mean, 72% [22%]) and was higher when the parent vs the child was responsible for eye drop instillation (76% [24%] vs 64% [24%], respectively; P = .04; Figure 2A). For the more stringent definition of proportion of doses taken on schedule—within a 2-hour window of the nominal dosing interval—values ranged from 3% to 97% (median, 34%; mean, 41% [24%]). The proportion of doses taken on schedule according to the more stringent definition was also higher when the parent vs the child instilled the eye drops (46% [26%] vs 23% [19%], respectively; P < .001; Figure 2B).
Figure 2.
Proportion of glaucoma medication doses taken on schedule. For each patient, the proportion of doses taken on schedule was averaged for all medications prescribed. Means and SEMs are represented by bars and whiskers, respectively. A, Proportion of prescribed doses taken within 25% of the nominal dosing interval. B, Proportion of prescribed doses taken within 2 hours of the nominal dosing interval.
DOSING ERRORS
The number of dosing errors (during a 30-day period) for IOP-lowering medications prescribed to be taken daily vs twice daily was similar (mean, 3.3 [4.9] [n = 30 bottles] vs 2.9 [3.6] [n = 55 bottles]; P = .66). In only 4 instances were medications prescribed to be taken 3 times daily, and the mean number of dosing errors (9.0 [8.9]) was higher compared with dosing errors for those medications prescribed daily, but the difference was not statistically significant (P = .06).
INTERVALS BETWEEN DOSES
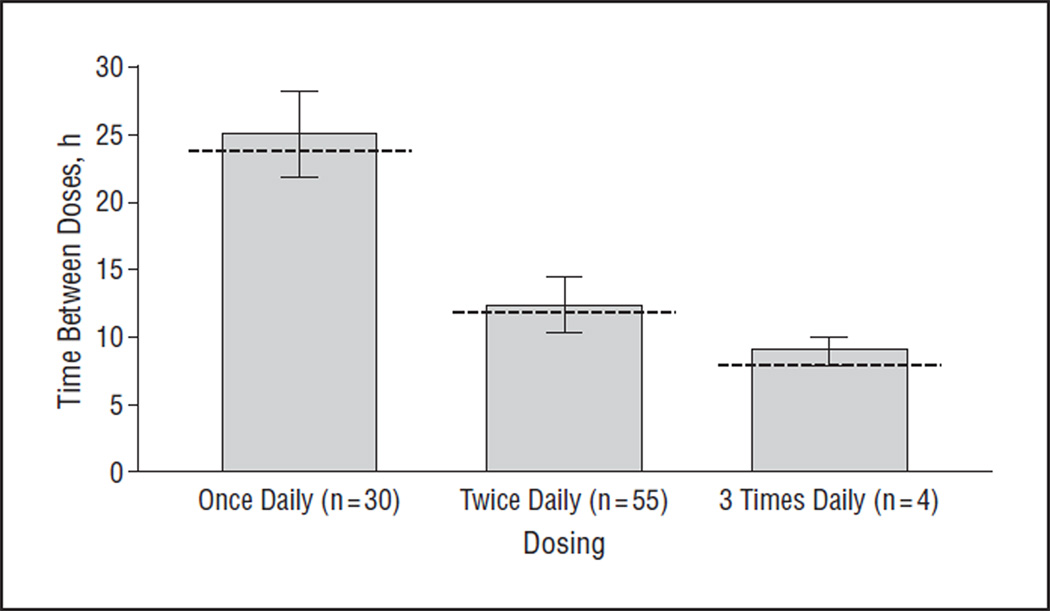
For IOP-lowering medications prescribed once daily, the intervals between doses ranged from 12 to 53 hours (median, 24 hours; mean, 25 [6] hours). For medications prescribed twice daily, the intervals between doses ranged from 11 to 22 hours (median, 12 hours; mean, 12 [1] hours). For medications prescribed 3 times daily, the intervals between doses ranged from 8 to 10 hours (median, 9 hours; mean, 9 [1] hours; Figure 3).
Figure 3.
Intervals between doses of glaucoma medications prescribed once, twice, and 3 times daily according to the data recorded from the Medication Event Monitoring System. Whiskers represent SDs and the dotted line represents the ideal dosing interval for each class of medications (ie, 24 hours for once-daily drugs, 12 hours for twice-daily drugs, and 8 hours for 3-times-daily drugs).
RESULTS OF EXTENDED MONITORING
Adherence and proportion of doses taken on schedule were compared for the first 30 days, second 30 days, and third 30 days for the 32 patients who used the MEMS SmartCaps for an extended period. The results of the extended monitoring are presented in Table 2. Pairwise comparisons for adherence and proportion of doses taken on schedule for the first, second, and third 30-day periods, respectively, revealed a statistically significant difference in adherence for the first month of monitoring vs the third (95% [6%] vs 88% [15%]; P = .02). The remaining pairwise comparisons did not reveal significant differences (P = .20 to .90).
Table 2.
Results of Extended Monitoring
| Mean (SD), % | ||||
|---|---|---|---|---|
| Time in Study, d |
No. of Patients |
Adherence | Doses Taken on Schedule, 25% Windowa |
Doses Taken on Schedule, 2-Hour Windowb |
| 0–30 | 32 | 95 (6) | 75 (20) | 44 (23) |
| 31–60 | 32 | 92 (9) | 72 (23) | 40 (23) |
| 61–90 | 32 | 88 (15) | 66 (28) | 36 (26) |
Percentage of prescribed doses taken within a window of 25% of the nominal dosing interval according to the electronic medication monitor.
Percentage of prescribed doses taken within a window of 2 hours of the nominal dosing interval according to the electronic medication monitor.
COMMENT
In this population of children with glaucoma requiring topical ocular hypotensive medications, patients took, on average, 93% of the prescribed doses. Despite the overall high levels of adherence, we identified factors associated with poorer adherence. As the health literacy level of the parent decreased, so did the child’s adherence to prescribed IOP-lowering medications. For example, children whose parents read at a high school level or better took, on average, 94% of the prescribed doses compared with 81% of children whose parents read at an eighth-grade level or below. Consistent with this finding, it has been reported that adults with poor health literacy are less adherent with their own glaucoma medications17 and more likely to experience progressive visual field loss.23 We cannot infer, however, from this observational study that poor health literacy is the cause of poor medication adherence. The links between health literacy and health outcomes are likely complex and multidirectional.
Comparison of glaucoma medication adherence among different populations is complicated by various definitions and metrics for measuring adherence. The mean adherence of 93% in this study is higher than reported in some studies in adults with glaucoma using electronic medication monitors24 and similar to others.6 On average, patients in this study took their prescribed IOP-lowering medication within 2 hours of the nominal dosing interval only 41% of the time, although time-appropriate dosing improved to 72% when a more generous definition of “appropriate” was used (ie, within a window defined as 25% of the nominal dosing interval). For comparison, in a study6 of adults with glaucoma, patients took on average 97% of the prescribed doses within a 2-hour window of the prescribed dosing interval.
For this group of children with glaucoma treated by a single physician prescriber, families were orally instructed to take their once-, twice-, and 3-times-daily medications specifically at intervals of 24, 12, and 8 hours, respectively. In other practices, however, these dosing instructions may vary. For 1 patient in this study who was prescribed a twice-daily medication, the MEMS SmartCap recorded regular medication events at 7 am and 11 pm every day, which resulted in low scores for the “proportion of doses taken on schedule” according to either definition but adherence of 100%. Data on the effect of consistent gaps of coverage of 4 hours on diurnal IOP lowering or on the long-term preservation of visual function are insufficient. With medication, such as prostaglandin analogs prescribed to be taken once daily,25 the effect may be negligible, but with medications prescribed to be taken multiple times daily, such as α-agonists, the effect may be greater. Likewise, we do not know the effects of taking a medication more often than suggested. Could there be increased adverse events associated with this or decreased efficacy such as seen when prostaglandins are taken too frequently?26 Indeed, we do not yet know which element of medication adherence, such as overall adherence or time-appropriate dosing, correlates best with clinical outcomes; it may vary among patients. In this study, time-appropriate dosing was better when the parent rather than the child was responsible for eye drop instillation. Further research on the clinical significance of time-appropriate dosing is needed.
Although this study was designed to measure 30 days of medication use, patients used the MEMS SmartCaps until their regularly scheduled return appointment, resulting in more than 30 days of data for most study participants. Given that others have reported worsening medication adherence with longer duration of monitoring,2 we compared adherence and proportion of doses taken on schedule from the first 30 days to the second and third 30-day periods of monitoring. The difference in adherence between the first and third months of monitoring for patients who completed at least 90 days was statistically significant. We must, however, qualify this because glaucoma is a lifelong disease and we do not know whether 90 days of follow-up in a limited number of children with a longer life expectancy can accurately reflect long-term patterns of adherence.
This study is limited by the sample size of 46 patients cared for in a tertiary care pediatric glaucoma clinic. In addition, all patients knew that they were being monitored. We would expect that knowledge of monitoring would increase overall levels of adherence but would not account for differences between groups. An additional limitation was that the electronic monitors could not capture all aspects of adherence. For example, a patient may open the MEMS SmartCap but never use the medication or attempt to use the medication and miss the eye.8,9 A patient could take the medication out of the MEMS SmartCap and never replace it but continue to use the medication. Electronic monitors, however, provide more comprehensive information about dosing than is available from self-report or pharmacy records. In addition, the relatively short duration of this study may not have captured a true decrease in adherence variables that might have been detectible during a longer monitoring period. Furthermore, the patients and parents in this study may not be representative of the complete population of pediatric glaucoma patients because of either specific geography or factors specific to the physician prescriber or the academic practice setting.
The results of this study are encouraging in that overall medication adherence is generally good, at least for this group of children with glaucoma. There are, however, opportunities for improvement. Even though the parents of patients in this study demonstrated health literacy skills that were, on average, higher than levels reported in the literature,27 significant associations between parental health literacy and the child’s medication adherence were found. The children of parents with worse health literacy skills were less likely to adhere to the prescribed medication regimen than the children of parents with better literacy skills. It is possible that initial identification of these susceptible individuals could facilitate different communication techniques (visual, oral, and written) that might improve adherence. Despite the known prevalence of low health literacy in the United States,18 few ophthalmic educational materials are appropriate for patients (or their parents) with poor literacy skills.28 Literacy level–appropriate education may help; in a study of individuals with poorly controlled diabetes, less literate individuals who received literacy level–appropriate education demonstrated improved glycemic control29 As investigators and clinicians work to improve glaucoma medication adherence, we have the opportunity to incorporate clear communication strategies with the patients and the entire family affected by this disease.
Acknowledgments
Funding/Support: This work was supported by grant 5K12 EY016333-05 and the Mentoring for the Advancement of Physician Scientists grant from the American Glaucoma Society. Dr Muir also receives salary support from the Veterans Affairs Health Services Research and Development Career Development Award 10-019-2.
Footnotes
Financial Disclosure: None reported.
REFERENCES
- 1.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 2.Krejci-Manwaring J, Tusa MG, Carroll C, et al. Stealth monitoring of adherence to topical medication: adherence is very poor in children with atopic dermatitis. J Am Acad Dermatol. 2007;56(2):211–216. doi: 10.1016/j.jaad.2006.05.073. [DOI] [PubMed] [Google Scholar]
- 3.Friedman DS, Quigley HA, Gelb L, et al. Using pharmacy claims data to study adherence to glaucoma medications: methodology and findings of the Glaucoma Adherence and Persistency Study (GAPS) Invest Ophthalmol Vis Sci. 2007;48(11):5052–5057. doi: 10.1167/iovs.07-0290. [DOI] [PubMed] [Google Scholar]
- 4.Gurwitz JH, Glynn RJ, Monane M, et al. Treatment for glaucoma: adherence by the elderly. Am J Public Health. 1993;83(5):711–716. doi: 10.2105/ajph.83.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kass MA, Meltzer DW, Gordon M, Cooper D, Goldberg J. Compliance with topical pilocarpine treatment. Am J Ophthalmol. 1986;101(5):515–523. doi: 10.1016/0002-9394(86)90939-6. [DOI] [PubMed] [Google Scholar]
- 6.Robin AL, Novack GD, Covert DW, Crockett RS, Marcic TS. Adherence in glaucoma: objective measurements of once-daily and adjunctive medication use. Am J Ophthalmol. 2007;144(4):533–540. doi: 10.1016/j.ajo.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Muir KW, Lee PP. Glaucoma medication adherence: room for improvement in both performance and measurement. Arch Ophthalmol. 2011;129(2):243–245. doi: 10.1001/archophthalmol.2010.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hennessy AL, Katz J, Covert D, Protzko C, Robin AL. Videotaped evaluation of eyedrop instillation in glaucoma patients with visual impairment or moderate to severe visual field loss. Ophthalmology. 2010;117(12):2345–2352. doi: 10.1016/j.ophtha.2010.03.040. [DOI] [PubMed] [Google Scholar]
- 9.Stone JL, Robin AL, Novack GD, Covert DW, Cagle GD. An objective evaluation of eyedrop instillation in patients with glaucoma. Arch Ophthalmol. 2009;127(6):732–736. doi: 10.1001/archophthalmol.2009.96. [DOI] [PubMed] [Google Scholar]
- 10.Siatkowski RM, Cotter S, Miller JM, Scher CA, Crockett RS, Novack GD US Pirenzepine Study Group. Safety and efficacy of 2% pirenzepine ophthalmic gel in children with myopia: a 1-year, multicenter, double-masked, placebo-controlled parallel study. Arch Ophthalmol. 2004;122(11):1667–1674. doi: 10.1001/archopht.122.11.1667. [DOI] [PubMed] [Google Scholar]
- 11.Tan DT, Lam DS, Chua WH, Shu-Ping DF, Crockett RS Asian Pirenzepine Study Group. One-year multicenter, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. Ophthalmology. 2005;112(1):84–91. doi: 10.1016/j.ophtha.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 12.Tsai JC, McClure CA, Ramos SE, Schlundt DG, Pichert JW. Compliance barriers in glaucoma: a systematic classification. J Glaucoma. 2003;12(5):393–398. doi: 10.1097/00061198-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Patel SC, Spaeth GL. Compliance in patients prescribed eyedrops for glaucoma. Ophthalmic Surg. 1995;26(3):233–236. [PubMed] [Google Scholar]
- 14.Hermann MM, Bron AM, Creuzot-Garcher CP, Diestelhorst M. Measurement of adherence to brimonidine therapy for glaucoma using electronic monitoring. J Glaucoma. 2011;20(8):502–508. doi: 10.1097/IJG.0b013e3181f3eb4a. [DOI] [PubMed] [Google Scholar]
- 15.Nordstrom BL, Friedman DS, Mozaffari E, Quigley HA, Walker AM. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol. 2005;140(4):598–606. doi: 10.1016/j.ajo.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 16.Friedman DS, Hahn SR, Gelb L, et al. Doctor-patient communication, health-related beliefs, and adherence in glaucoma results from the Glaucoma Adherence and Persistency Study. Ophthalmology. 2008;115(8):1320–1327. doi: 10.1016/j.ophtha.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 17.Muir KW, Santiago-Turla C, Stinnett SS, et al. Health literacy and adherence to glaucoma therapy. Am J Ophthalmol. 2006;142(2):223–226. doi: 10.1016/j.ajo.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen-Bohlman L. Health Literacy: A Prescription to End Confusion. Washington, DC: National Academies Press; 2004. Institute of Medicine, Committee on Health Literacy. [PubMed] [Google Scholar]
- 19.Schillinger D, Grumbach K, Piette J, et al. Association of health literacy with diabetes outcomes. JAMA. 2002;288(4):475–482. doi: 10.1001/jama.288.4.475. [DOI] [PubMed] [Google Scholar]
- 20.Williams MV, Baker DW, Honig EG, Lee TM, Nowlan A. Inadequate literacy is a barrier to asthma knowledge and self-care. Chest. 1998;114(4):1008–1015. doi: 10.1378/chest.114.4.1008. [DOI] [PubMed] [Google Scholar]
- 21.Loudon SE, Fronius M, Looman CW, et al. Predictors and a remedy for noncompliance with amblyopia therapy in children measured with the occlusion dose monitor. Invest Ophthalmol Vis Sci. 2006;47(10):4393–4400. doi: 10.1167/iovs.05-1428. [DOI] [PubMed] [Google Scholar]
- 22.Davis TC, Long SW, Jackson RH, et al. Rapid Estimate of Adult Literacy in Medicine: a shortened screening instrument. Fam Med. 1993;25(6):391–395. [PubMed] [Google Scholar]
- 23.Juzych MS, Randhawa S, Shukairy A, Kaushal P, Gupta A, Shalauta N. Functional health literacy in patients with glaucoma in urban settings. Arch Ophthalmol. 2008;126(5):718–724. doi: 10.1001/archopht.126.5.718. [DOI] [PubMed] [Google Scholar]
- 24.Friedman DS, Okeke CO, Jampel HD, et al. Risk factors for poor adherence to eyedrops in electronically monitored patients with glaucoma. Ophthalmology. 2009;116(6):1097–1105. doi: 10.1016/j.ophtha.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Gross RL, Peace JH, Smith SE, et al. Duration of IOP reduction with travoprost BAK-free solution. J Glaucoma. 2008;17(3):217–222. doi: 10.1097/IJG.0b013e31815a3472. [DOI] [PubMed] [Google Scholar]
- 26.Herndon LW, Asrani SG, Williams GH, Challa P, Lee PP. Paradoxical intraocular pressure elevation after combined therapy with latanoprost and bimatoprost. Arch Ophthalmol. 2002;120(6):847–849. [PubMed] [Google Scholar]
- 27.Davis TC, Mayeaux EJ, Fredrickson D, Bocchini JA, Jr, Jackson RH, Murphy PW. Reading ability of parents compared with reading level of pediatric patient education materials. Pediatrics. 1994;93(3):460–468. [PubMed] [Google Scholar]
- 28.Muir KW, Lee PP. Health literacy and ophthalmic patient education. Surv Ophthalmol. 2010;55(5):454–459. doi: 10.1016/j.survophthal.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothman RL, DeWalt DA, Malone R, et al. Influence of patient literacy on the effectiveness of a primary care–based diabetes disease management program. JAMA. 2004;292(14):1711–1716. doi: 10.1001/jama.292.14.1711. [DOI] [PubMed] [Google Scholar]