Abstract
Previous studies have reported cognitive deficits among HIV-positive individuals infected with clade C virus. However, no study has examined whether individuals predominately infected with clade C virus exhibit brain atrophy relative to healthy controls. This study examined volumetric differences between 28 HIV+ individuals and 23 HIV− controls from South Africa. Volumetric measures were obtained from six regions of interest--caudate, thalamus, corpus callosum, total cortex, total gray matter, and total white matter. HIV+ participants had significantly lower volumes in the total white matter (p<.01), thalamus (p<.01) and total gray matter (inclusive of cortical and subcortical regions, p<.01). The current study is the first to provide evidence of brain atrophy among HIV+ individuals in South Africa, where HIV Clade C predominates. Additional research that integrates neuroimaging, comprehensive neuropsychological testing, genetic variance in clade-specific proteins, and the impact of treatment with ARVs are necessary to understand the development of HIV-related neurocognitive disorders in South Africa.
Introduction
HIV-1 has a number of subtypes, or clades, that are regionally distributed. HIV clade B is predominant in the US and Europe and has been extensively studied in terms of associated neurovirulence (for review see Shapshak 2011). In contrast, relatively little is understood regarding the neurovirulence of clade C, the most prevalent genetic variation of HIV worldwide. Early reports of clade C HIV-associated neurocognitive disorders (HAND) have suggested a lower prevalence of cognitive impairment (~2%;Satishchandra et al 2000) compared to clade B (~30%; Lindl et al 2010).
These observed differences prompted subsequent studies that possible differences in mechanisms exist between clades B and C within the central nervous system (CNS). In 2004, Ranga et al. (2004) reported a defect in the Tat envelope protein that resulted in reduced migrations of infected monocytes into the brain with clade C. A subsequent study using a mouse model (Rao et al 2008) demonstrated that clade B mice exhibited greater cognitive compromise, elevated frontal astrogliosis, and increased monocyte attraction compared to the clade C mouse. Recent studies have demonstrated that compared to clade B, clade C cultures induce fewer proinflammatory cytokines (Gandhi et al 2009), have reduced neurotoxicity in hippocampal neurons (Samikkannu et al 2011), and replicate more slowly in monocytes (Constantino et al 2011). Both mouse and in vitro models provide insight into the differences that might exist in the neuropathology of clade B and clade C respectively. Conversely, studies of neuropsychological function have provided evidence that the prevalence of neurocognitive disorders in clade C is higher than the earliest studies suggest.
A number of studies have assessed cognitive function in clade C using both brief screening tools as well as longer test batteries, but the number of studies are limited and few have been replicated in the same geographical regions. The prevalence of neurocognitive disorders as suggested by brief screening tools such as the International HIV Dementia Scale (IHDS; Sacktor et al 2005) has been reported to be 35% in India (2006), 24% in South Africa (Joska et al., 2010). In an Ethiopian study comparing HIV+ individuals and controls (Clifford et al 2007) no differences in test performance were detected. A more comprehensive battery administered in India (2006) reported that the HIV+ group scored below healthy controls on most neuropsychological measures with approximately 50% of the HIV+ group meeting criteria for impairment in two cognitive domains. Another study in India (Gupta et al 2007) showed that approximately 60% of the HIV+ group demonstrated deficits on a number of the measures, but none of the deficits were severe and there was no notable functional impairment in the cohort. However, in a South African study using a comprehensive neuropsychological battery to assess HAND, 25% of HIV+ individuals indicated severe impairment (Joska et al 2009) and an additional 50% were classified as having milder forms of neurocognitive impairment. Overall, the studies described above suggest that cognitive impairment is present among HIV+ patients residing in regions dominated by clade C virus, however, the underlying pathophysiological mechanisms responsible for the varying degree of impairment remains unclear.
Neuroimaging provides a non-invasive means for studying the effects of HIV in the brain. An extensive number of studies have used a variety of neuroimaging methods to describe neuropathology associated with clade B HIV (Paul et al 2002, Tucker et al 2004). Compared to other methods, neuroimaging is free of cultural biases, can be used in conjunction with neuropsychological testing, and can be performed at many institutions throughout the world. To date, no studies have reported structural volumetric neuroimaging outcomes among individuals presumed to be infected with clade C HIV; we addressed this issue in the present study. In addition, the IHDS battery was administered to assess neuropsychological functioning in a group of untreated HIV+ individuals and HIV− controls from Cape Town, South Africa. We hypothesized that the HIV patients would exhibit smaller volumetric measures in select subcortical structures (caudate, thalamus, corpus callosum) in HIV+ individuals compared to HIV− controls. In addition, we expected to find evidence of global atrophy in HIV+ individuals.
Methods
Participants
Twenty-eight HIV+ individuals (5 males, 23 females) and 23 HIV− controls (13 males, 10 females) were recruited as part of an ongoing study. All participants provided consent to participate in the study as prescribed by the Human Research Ethics Committee at the University of Cape Town. HIV serostatus was documented by ELISA and confirmed with Western Blot for the HIV+ individuals. All participants were naïve to ARV treatment and had a CD4 count less than 500 cells/μL Controls and participants were selected from two primary care clinics in the Cape Town area. Controls were recruited based on the same criteria as the HIV+ group, though not infected with HIV. Seronegative family and friends who met criteria for the study were also selected for participation. Individuals were excluded from participation if they had a history of schizophrenia or bipolar disorder, substance abuse or alcoholism as defined by the Mini-International Neuropsychiatric Interview Plus (MINI-Plus), other confounding neurological conditions, head injury with loss of consciousness greater than 30 minutes, active substance abuse, clinical evidence of opportunistic CNS infections, or contraindications for MRI. The primary language spoken by all the participants is Xhosa. Items on all of the measures were reviewed to determine cultural relevancy in the study population. Instructions were translated from English to Xhosa and back-translated to English to verify accuracy.
Measures
The IHDS was administered as a screening tool to determine HIV-associated dementia (HAD) in the HIV+ cohort (Sacktor et al, 2005). The IHDS consists of three basic measures of motor speed (Finger Tapping), psychomotor speed (fist-palm-side), and memory (five words with brief recall). Individuals can score up to four points on each task for a total score of 12 points. Scores that are less than 10 are indicative of HAD.
Blood was drawn from each of the participants to determine CD4 nadir and viral load. All patients completed a universal drug screen to determine current use of opiates, amphetamines, marijuana, and benzodiazepines at the time of the neuropsychological testing. Persons who tested positive on the screen were excluded.
Neuroimaging acquisition and processing
Imaging was performed on a Siemens 3T Magnetom Allegra head-only scanner equipped with a four-channel phased array head coil (Siemens AG, Erlangen, Germany). Structural images were acquired using a T1-weighted three dimensional magnetization-prepared rapid acquisition gradient echo (MPRAGE) sequence (Time of repetition (TR)/inversion time (TI)/echo time (TE) = 2400/1000/2.38 milliseconds, flip angle = 8°, and voxel size = 1 × 1 × 1 mm3). Images were subsequently converted to a format appropriate for use in the Freesurfer image analysis suite (.mgz, v 4.5 Martinos Center, Harvard University, Boston, MA). Specific methods have been previously described (Fischl et al 2002). Preliminary MRI processing was done using the most recent N4ITK intensity correction software to decrease MR bias (Tustison et al 2010). In short, Freesurfer processing included extraction of non-brain tissue, normalization of voxel intensity as a result of MR bias, transformation of each brain to Talairach space, and segmentation of the subcortical white matter and deep gray matter volumetric structures (Fischl et al 2002, Fischl et al 2004) using voxel identity probabilities. Proper segmentation was reviewed independently by two trained reviewers in order to ensure proper classification of structures. Six regions of interest (ROIs) were chosen for statistical analyses and included the Total Cortex, Total Gray Matter, Total White matter, Corpus Callosum, Thalamus, and Caudate (see Figure 1). These regions were chosen as HIV has been shown to affect both subcortical and cortical areas (Thompson et al 2005). In all cases the sum of the structures from the R and L hemispheres were used for analyses. Volumes for each of these regions of interest were normalized using intracranial volume to correct for possible differences in head size (Zatz & Jernigan 1983).
Figure 1.
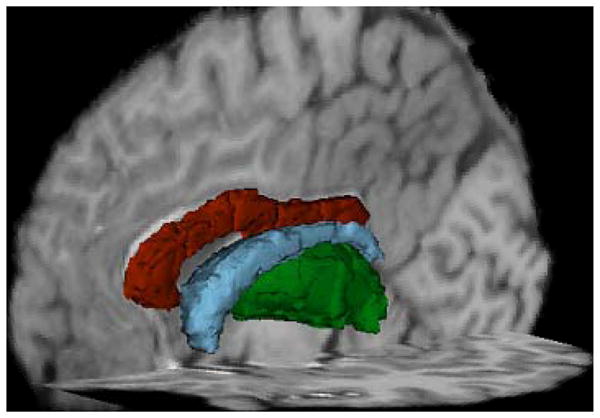
Is a 3D representation of the subcortical segmentations obtained from freesurefer. Red is the segmentation of the corpus callosum, Blue represents the caudate, and green represents the thalamus
Statistical Analysis
All variables were plotted to ensure a normal distribution. Independent means t-tests (two-tailed) were completed to assess for group differences in demographic variables such as age, education, IHDS scores. Separate ANOVAs were used to assess group volumetric differences for each of the six brain regions chosen for analysis. Bonferroni corrections were applied to correct for multiple comparisons. Pearson’s correlation coefficients were used to determine correlations between the volumetric measures, age, education, IHDS scores, gender, and clinical markers of HIV such as nadir CD4 cell count, and plasma HIV viral load (VL).
Results
Preliminary analyses revealed that the two groups differed significantly on age, education, gender, and IHDS scores (see Table I). While both groups were on average within the same young adult age band (<40) the HIV+ group was significantly older than the HIV− control subjects (p <.01). Correlational analyses were conducted to determine whether age correlated with any of the primary neuroimaging variables. Results from these analyses revealed significant small correlations between age and cortex volume (r =.33 p <.05) as well as total gray matter volume (r =.31, p <.05). Education was significantly correlated to callosal volumes (r =.37, p <.01). Both age and education were entered as covariate in subsequent analyses.
Table 1.
The demographic characteristics of the HIV+ and Control samples. Values shown are the mean values (SD) for the groups. Significance *(p) was determined using independent samples t-tests and chi-square analyses (group x gender). For IHDS, the significance was determined using ANCOVA, with education as a covariate.
| Demographic Table | ||||
|---|---|---|---|---|
| HIV+ Clade C (n=28) | HIV-Control (n=23) | Sig. (p=) | ||
| Age (years old) | 33 (4.52) | 22 (3.36) | <0.0001* | |
| Education | 10 (1.98) | 11 (.72) | 0.046* | |
| IHDS scores | 9.67 (1.57) | 10.93 (.96) | 0.07 | |
| Nadir CD4(cells/μL) | 190.30 (140) | n/a | ||
| Log Plasma Viral Load | 4.58 (.77) | n/a | ||
| Gender | Male | 18% (5) | 57% (13) | 0.014* |
HIV+ participants had a reduction in volumetric measures within select brain areas
Compared to controls, the HIV+ group had significantly lower volumes within both cortical and subcortical ROIs. In particular, significantly smaller volumes were observed in total gray matter volume (F =13.835 p < 0.01), total white matter volume (F = 7.629, p < 0.01) and the thalamus (F = 15.968, p <0.01) for HIV+ participants (see Fig. 2). However, no significant differences were observed between the groups in the caudate, corpus callosum, or total cortex.
Figure 2.
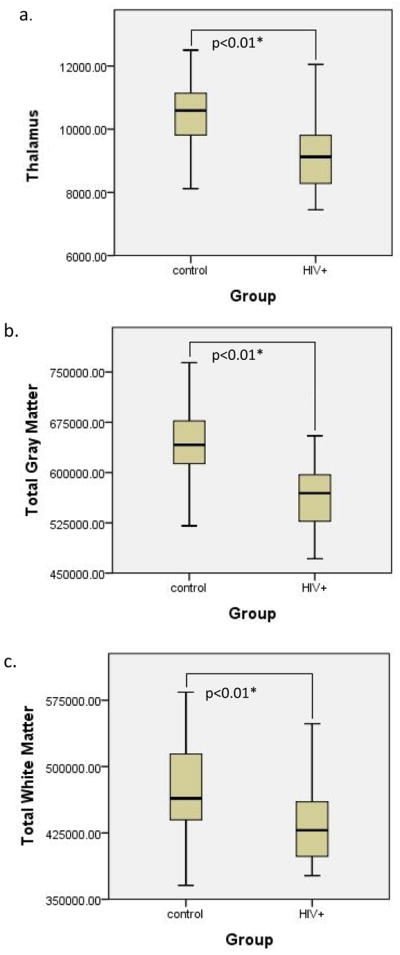
Boxplots of the volumes for each group with significant group differences noted. The dark lines in the boxes represent the mean values for the volumes of the groups (HIV+ and controls) in each region a) Total Thalamus (p<0.01) b) Total Gray Matter (p<0.01) c) Total White Matter (p<.01)
Because the HIV+ group was predominantly female and the control group had a number of males we completed additional analysis comparing the HIV+ females to the HIV− control females to confirm that observed results shown were not attributable to gender differences associated with brain volume. Using ANCOVA in the three significantly different ROIs from the previous analysis and using age as a co-variate two of the three regions remained significantly different between the HIV+ and HIV− female groups. Total gray matter volume was significantly lower in the HIV+ female only group (F= 10.856, p<.01) as were the thalamus volumes (F=13.2, p<.01). A trend was seen in total white matter volume (F=3.746, p=.06), the HIV+ group having lower volumes in this area compared to the HIV− control group
Changes in brain volume occurred regardless of degree of cognitive impairment or clinical laboratory values
An ANCOVA was performed to determine the effects of HIV status on IHDS performance using education as a covariate. The effect of HIV status, after controlling for education, was not significant (F(1, 35) = 3.584, p=0.07). Performance on the IHDS did not correlate significantly with any of the volumetric measures. In addition, reductions with select regions did not correlate with typical clinical laboratory markers (i.e. current CD4 cell count, nadir CD cell count, and VL). Nadir CD4 was moderately correlated with total cortex volume (r = .43 p <.05) and VL was inversely correlated with total caudate volume (r= −.46 p<.05).
Conclusions
In summary, this is the first study to provide evidence that there is a significant amount of cortical and subcortical atrophy in South Africa, where clade C HIV predominates. Lower volumes were observed in the total gray matter, thalamus, and total white matter. There were no significant differences detected in the caudate, corpus callosum, or total cortex as anticipated. Previous volumetric studies in clade B HIV+ individuals (Elovaara et al 1990, Kieburtz et al 1996, Patel et al 2002, Ances et al 2012) also reported cortical atrophy, general gray matter atrophy, atrophy in the basal ganglia--most notably the caudate, and white matter changes. The lack of significant atrophy in the caudate or white matter in this study may indicate that clade C HIV affects different brain regions compared to clade B. It should be noted, however, that the volumes of all ROIs sampled were lower in the HIV+ group suggesting that these areas may also be vulnerable. While this study did not find evidence of atrophy in white matter, a recent study published by members of this group did see evidence of white matter abnormalities within specific tracts in HIV+ individuals compared to HIV− controls in South Africa using diffusion tensor imaging (Hoare et al 2011).
The HIV+ group scored significantly lower than controls on the IHDS, which is in keeping with previous studies demonstrating impaired cognitive function in this South African region (Joska et al 2010); however, the scores were not significantly different between the two groups. Not all of the control subjects complete the IHDS for this study, so the results are not representative of the complete sample Performance on the IHDS did not correlate with any of the volumes analyzed, which may be in part due to a limited range of scores on the measure. The IHDS was the only neuropsychological measure available for this study. As mentioned previously the measure may lack the sensitivity needed to identify more subtle forms of cognitive impairment or provide information about the relationship between cognitive status and neuroimaging biomarkers. The use of more comprehensive neuropsychological testing may provide additional insight into the relationship between structural changes and performance in the South African region.
The HIV+ cohort was naïve to ART and many of them had low CD4 counts and high viral loads. Since they were untreated at the time of testing the CD4 counts obtained were recorded as the nadir value. Early studies in clade B HIV infection demonstrated volume loss in patients with low CD4 values before treatment (for review see (Thurnher & Donovan Post 2008). Although the tissues are unlikely to increase or recover volume after treatment it is possible that access to treatment in South Africa will improve cognitive function as has been shown in studies implemented in various regions across the globe (see Joska et al 2010, Liner et al 2010, Ances et al 2012).
This study had limitations as we did not have confirmed clade sequencing for all of the participants. It is possible that some of the individuals in the HIV positive group were of a different clade (A, B, or recombinant); however, it has been estimated that the percentage of people who are infected with clade C HIV in this area is between 89 % and 95% (Jacobs et al 2008, Jacobs et al 2009). Individuals in previous studies from this area with non-clade C HIV infection were less likely to be native to the region. We did not have information about other infections often co-morbid with HIV, such as hepatits C; however, the prevalence of hepatitis C in the region is less than 2% (Amin et al., 2004). Additionally, our sample is relatively small, with fewer controls than HIV+ individuals. Despite the small sample, we were able to detect significant differences between the groups indicating a strong effect of HIV on the volumetric measures.
The use of volumetric measures, DTI, and other neuroimaging methods provide an in vivo depiction of the effects of the virus; however, they are not capable of providing detailed information about mechanisms of disease available through in vitro methods and mouse models. A number of the in vitro studies indicate Clade C is less neurovirulent primarily due to the Tat defect (Constantino et al, Gandhi et al 2009, Ranga et al 2004, Rao et al 2008, Samikkannu et al 2011). While the data from this study do not confer specific information about the magnitude of neurovirulence our results do provide evidence that clade C HIV has a deleterious influence on the CNS. Studies using a larger sample are necessary to confirm the results of this study. Furthermore, future research is needed to determine the association between the Tat defect, neuroimaging indices, and HAND. It is unlikely that Tat is independently responsible for neurocognitive impairment in HIV; moreover, it is possible that the Tat defect may not be completely conserved in the clade C population (Engelbrecht, unpublished data). Other factors that may contribute to clade C neurovirulence (e.g. gp120, CSF markers, inflammatory processes, genetic variance of HIV), will need to be investigated in order to develop a better understanding of the mechanisms leading to brain atrophy and cognitive impairment in clade C HIV. Until a more complete model is defined, results from neuropsychological studies, and the current neuroimaging study suggest that individuals likely infected with clade C exhibit significant impact on the brain.
References
- Amin J, Kaye M, Skidmore S, Pillay D, Cooper DA, Dore GJ. HIV and hepatitis C coinfection within the CAESAR study. HIV Medicine. 2004;5:174–179. doi: 10.1111/j.1468-1293.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- Ances BA, Ortega M, Vaida F, Heaps J, Paul R. Independent effects of HIV, aging, and HAART on brain volumetric measures. JAIDS. 2012 doi: 10.1097/QAI.0b013e318249db17. Post acceptance 20 Jan 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford DB, Mitike MT, Mekonnen Y, Zhang J, Zenebe G, Melaku Z, Zewde A, Gessesse N, Wolday D, Messele T, Teshome M, Evans S. Neurological evaluation of untreated human immunodeficiency virus infected adults in Ethiopia. J Neurovirol. 2007;13:67–72. doi: 10.1080/13550280601169837. [DOI] [PubMed] [Google Scholar]
- Constantino AA, Huang Y, Zhang H, Wood C, Zheng JC. HIV-1 clade B and C isolates exhibit differential replication: relevance to macrophage-mediated neurotoxicity. Neurotox Res. 2011;20:277–88. doi: 10.1007/s12640-011-9241-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovaara I, Poutiainen E, Raininko R, Valanne L, Virta A, Valle SL, Lahdevirta J, Iivanainen M. Mild brain atrophy in early HIV infection: the lack of association with cognitive deficits and HIV-specific intrathecal immune response. J Neurol Sci. 1990;99:121–36. doi: 10.1016/0022-510x(90)90149-h. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Gupta JD, Satishchandra P, Gopukumar K, Wilkie F, Waldrop-Valverde D, Ellis R, Ownby R, Subbakrishna DK, Desai A, Kamat A, Ravi V, Rao BS, Satish KS, Kumar M. Neuropsychological deficits in human immunodeficiency virus type 1 clade C-seropositive adults from South India. J Neurovirol. 2007;13:195–202. doi: 10.1080/13550280701258407. 779950376 [pii] [DOI] [PubMed] [Google Scholar]
- Hoare J, Fouche JP, Spottiswoode B, Sorsdahl K, Combrinck M, Stein DJ, Paul RH, Joska JA. White-Matter Damage in Clade C HIV-Positive Subjects: A Diffusion Tensor Imaging Study. J Neuropsychiatry Clin Neurosci. 2011;23:308–15. doi: 10.1176/appi.neuropsych.23.3.308. [DOI] [PubMed] [Google Scholar]
- Jacobs GB, Laten A, van Rensburg EJ, Bodem J, Weissbrich B, Rethwilm A, Preiser W, Englebrecht S. Phylogenetic diversity and low level antiretroviral resistance mutations in HIV type 1 treatment-naive patients from Cape Town, South Africa. AIDS Res Hum Retroviruses. 2008;24:1009–12. doi: 10.1089/aid.2008.0028. [DOI] [PubMed] [Google Scholar]
- Jacobs GB, Loxton AG, Laten A, Robson B, van Rensburg EJ, Engelbrecht S. Emergence and diversity of different HIV-1 subtypes in South Africa, 2000–2001. J Med Virol. 2009;81:1852–9. doi: 10.1002/jmv.21609. [DOI] [PubMed] [Google Scholar]
- Joska JA, Fincham DS, Stein DJ, Paul RH, Seedat S. Clinical correlates of HIV-associated neurocognitive disorders in South Africa. AIDS Behav. 14:371–8. doi: 10.1007/s10461-009-9538-x. [DOI] [PubMed] [Google Scholar]
- Joska JA, Gouse H, Paul RH, Stein DJ, Flisher AJ. Does highly active antiretroviral therapy improve neurocognitive function? A systematic review. J Neurovirol. 2010;16:101–14. doi: 10.3109/13550281003682513. [DOI] [PubMed] [Google Scholar]
- Joska JA, Westgarth-Taylor J, Myer L, Hoare J, Thomas KG, Combrinck M, Paul RH, Stein DJ, Flisher AJ. Characterization of HIV-Associated Neurocognitive Disorders among individuals starting antiretroviral therapy in South Africa. AIDS Behav. 2010;15:1197–203. doi: 10.1007/s10461-010-9744-6. [DOI] [PubMed] [Google Scholar]
- Kieburtz K, Ketonen L, Cox C, Grossman H, Holloway R, Booth H, Hickey C, Feigin A, Caine ED. Cognitive performance and regional brain volume in human immunodeficiency virus type 1 infection. Arch Neurol. 1996;53:155–8. doi: 10.1001/archneur.1996.00550020059016. [DOI] [PubMed] [Google Scholar]
- Lindl KA, Marks DR, Kolson DL, Jordan-Sciutto KL. HIV-associated neurocognitive disorder: pathogenesis and therapeutic opportunities. J neuroimm pharmaco. 2010;5:294–309. doi: 10.1007/s11481-010-9205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liner KJ, 2nd, Ro MJ, Robertson KR. HIV, antiretroviral therapies, and the brain. Curr HIV/AIDS rep. 2010;7:85–91. doi: 10.1007/s11904-010-0042-8. [DOI] [PubMed] [Google Scholar]
- Patel SH, Kolson DL, Glosser G, Matozzo I, Ge Y, Babb JS, Mannon LJ, Grossman RI. Correlation between percentage of brain parenchymal volume and neurocognitive performance in HIV-infected patients. AJNR Am J Neuroradiol. 2002;23:543–9. [PMC free article] [PubMed] [Google Scholar]
- Paul R, Cohen R, Navia B, Tashima K. Relationships between cognition and structural neuroimaging findings in adults with human immunodeficiency virus type-1. Neurosci Biobehav Rev. 2002;26:353–9. doi: 10.1016/s0149-7634(02)00006-4. S0149763402000064 [pii] [DOI] [PubMed] [Google Scholar]
- Ranga U, Shankarappa R, Siddappa NB, Ramakrishna L, Nagendran R, Mahalingam M, Mahadevan A, Jayasuryan N, Satishchandra P, Shankar SK, Prasad VR. Tat protein of human immunodeficiency virus type 1 subtype C strains is a defective chemokine. J Virol. 2004;78:2586–90. doi: 10.1128/JVI.78.5.2586-2590.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VR, Sas AR, Eugenin EA, Siddappa NB, Bimonte-Nelson H, Berman JW, Ranga U, Tyor WR, Prasad VR. HIV-1 clade-specific differences in the induction of neuropathogenesis. J Neurosci. 2008;28:10010–6. doi: 10.1523/JNEUROSCI.2955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel D, Ghate M, Nene M, Paranjape R, Mehendale S, Bollinger R, Sacktor N, McArthur J, Nath A. Screening for human immunodeficiency virus (HIV) dementia in an HIV clade C-infected population in India. J Neurovirol. 2006;12:34–8. doi: 10.1080/13550280500516500. [DOI] [PubMed] [Google Scholar]
- Sacktor NC, Wong M, Nakasujja N, Skolasky RL, Selnes OA, Musisi S, Robertson K, McArthur JC, Ronald A, Katabira E. The International HIV Dementia Scale: a new rapid screening test for HIV dementia. AIDS. 2005;19:1367–74. [PubMed] [Google Scholar]
- Samikkannu T, Agudelo M, Gandhi N, Reddy PV, Saiyed ZM, Nwankwo D, Nair MP. Human immunodeficiency virus type 1 clade B and C gp120 differentially induce neurotoxin arachidonic acid in human astrocytes: implications for neuroAIDS. J Neurovirol. 2011;17:230–8. doi: 10.1007/s13365-011-0026-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satishchandra P, Nalini A, Gourie-Devi M, Khanna N, Santosh V, Ravi V, Desai A, Chandramuki A, Jayakumar PN, Shankar SK. Profile of neurologic disorders associated with HIV/AIDS from Bangalore, south India (1989–96) Indian J Med Res. 2000;111:14–23. [PubMed] [Google Scholar]
- Shapshak P, Kangueane P, Fujimura RK, Commins D, Chiappelli F, Singer E, Levine AJ, Minagar A, Novembre FJ, Somboonwit C, Nath A, Sinnott Editorial neuroAIDS review. AIDS. 2011;25:123–41. doi: 10.1097/QAD.0b013e328340fd42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, Becker JT. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proc Natl Acad Sci U S A. 2005;102:15647–52. doi: 10.1073/pnas.0502548102. 0502548102 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurnher MM, Donovan Post MJ. Neuroimaging in the brain in HIV-1-infected patients. Neuroimaging Clin N Am. 2008;18:93–117. viii. doi: 10.1016/j.nic.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Tucker KA, Robertson KR, Lin W, Smith JK, An H, Chen Y, Aylward SR, Hall CD. Neuroimaging in human immunodeficiency virus infection. J Neuroimmunol. 2004;157:153–62. doi: 10.1016/j.jneuroim.2004.08.036. S0165-5728(04)00346-7 [pii] [DOI] [PubMed] [Google Scholar]
- Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, Gee JC. N4ITK: improved N3 bias correction. IEEE Transact Med Imag. 2010;29:1310–20. doi: 10.1109/TMI.2010.2046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepthomi T, Paul R, Vallabhaneni S, Kumarasamy N, Tate DF, Solomon S, Flanigan T. Neurocognitive consequences of HIV in southern India: a preliminary study of clade C virus. J Int Neuropsych Soc : JINS. 2006;12:424–30. doi: 10.1017/s1355617706060516. [DOI] [PubMed] [Google Scholar]
- Zatz LM, Jernigan TL. The ventricular-brain ratio on computed tomography scans: validity and proper use. Psychiatry Res. 1983;8:207–14. doi: 10.1016/0165-1781(83)90064-1. [DOI] [PubMed] [Google Scholar]


