Abstract
Caffeine is the active constituent in coffee. Continual consumption of caffeine can lead to an attenuated response also known as tolerance. Results from rat studies have shown that caffeine is an inducer of CYP1A2, the enzyme responsible for caffeine’s metabolism. This suggests that CYP1A2 induction by caffeine may be in part responsible for caffeine tolerance. However, whether caffeine induces CYP1A2 expression in humans remains unknown. Our results from luciferase assays performed in HepG2 cells showed that caffeine is not an activator of the aromatic hydrocarbon receptor (AhR), a major transcription factor involved in upregulation of CYP1A2. Furthermore, caffeine did not induce CYP1A2 expression in primary human hepatocytes at a concentration attained by ordinary coffee drinking. On the other hand, caffeine enhanced CYP1A2 expression by 9-fold in rat hepatocytes. Our results suggestthat caffeine from ordinary coffee drinking does not induce CYP1A2 expression in humans and that factors other than CYP1A2 induction by caffeine likely contribute to development of caffeine tolerance in humans.
Keywords: caffeine, tolerance, pharmacokinetics, AhR, CYP1A2, regulation
Introduction
Caffeine is a psychoactive compound found in many daily-consumed drinks and food such as tea, coffee, energy drinks, and chocolate [1]. Caffeine exhibits various pharmacological effects, including increased heart rate, diuresis, motor activity, and mental alertness. Repeated administration of caffeine (e.g., daily intake of coffee) can produce a tolerance to the pharmacological effects of caffeine [2]. Tolerance to the motor-activating effect of caffeine is in part explained by decreased sensitivity to adenosine receptors [3]. However, whether altered caffeine elimination potentially plays a role in development of the tolerance remains unknown.
Caffeine is eliminated mainly through hepatic metabolism by the CYP1A2 enzyme. CYP1A2 expression is regulated by a ligand-activated transcription factor, aromatic hydrocarbon receptor (AhR) (reviewed in [4, 5]). Upon binding of a ligand such as 3-methylchoanthrane (3-MC), AhR translocates into the nucleus and forms a heterodimer complex with a nuclear protein, aromatic hydrocarbon receptor nuclear translator protein. The complex binds to a DNA sequence, called dioxin-responsive element (DRE), in the upstream regulatory region of target genes (including CYP1A1 and CYP1A2) and enhances transcription.
Previous studies in rats have shown that caffeine induces CYP1A2 expression. In Fischer 344 rats, oral administration of caffeinated green tea and black tea led to an approximately 5-fold induction in hepatic CYP1A2 enzyme activity as compared to the vehicle-treated animals [6], and administration of caffeine alone led to an 11-fold increase in CYP1A2 activity in Wistar rats [7]. These results suggest that in rats, caffeine induces CYP1A2 expression. This CYP1A2 induction by caffeine may be in part responsible for development of caffeine tolerance. In humans, it remains unknown whether caffeine is capable of activating AhR and/or enhancing CYP1A2 expression.
In this exploratory study, we hypothesized that caffeine induces CYP1A2 via AhR activation in humans and this could be in part responsible for development of caffeine tolerance. We investigated whether caffeine is capable of activating human AhR and inducing human CYP1A2 expression.
Materials and Methods
Chemicals
DMSO, caffeine, omeprazole, and 3-MC were purchased from Sigma (St. Louis, MO).
Plasmids
pGudLuc1.1 is a gift from Dr. Michael Denison (UC Davis) [8]. β-Galactosidase expression vector has been previously described [9].
Hepatocytes
Primary human and rat hepatocytes were obtained from Liver Tissue Cell Distribution System (Pittsburgh, PA) and Invitrogen (Durham, NC), respectively. Upon receipt, media were replaced with serum-free Williams’ E media as previously described [10]. On the next day, the cells were used for drug treatment.
Luciferase Reporter Assay
HepG2 cells were seeded in 12-well plates at a density of 6.0 × 105 cells/ml, and on the next day transfected with 0.6 μg of luciferase constructand 0.1 μg of β-galactosidase expression plasmid using Fugene 6 transfection reagent (Roche Applied Sciences) following the manufacturer’s protocol. The transfected cells were grown for 24 hr and treated with vehicle or drugs. After 24 hr incubation, cells were harvested, and activities of luciferase and β-galactosidase were determined using assay kits from Promega (Madison, WI). The luciferase activity was normalized to β-galactosidase activity to control for differences in transfection efficiency. Statistical analysis was performed by using Student’s t-test.
RNA Isolation and Quantitative Real time-PCR (qRT-PCR)
Total RNAs were isolated from human hepatocytes using Trizol (Invitrogen, Carlsbad, CA) and used as a template for cDNA synthesis using High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). Using the cDNA as template, qRT-PCR was performed using StepOnePlus Real-Time PCR System and TaqMan® Gene expression assays (Applied Biosystems). The following TaqMan probes (Applied Biosystems and Integrated DNA Technologies) were used: human CYP1A2 (Hs01070369_m1), human GAPDH (Hs99999905_m1), rat CYP1A1 (Rn.PT.49.7201670), rat CYP1A2 (Rn00561082_m1), and rat β-actin (Rn0066789_m1). The fold change in mRNA levels of CYP upon drug treatment was determined after normalizing the gene expression levels by those of β-actin or GAPDH (2−ΔΔCt method) [11]. Statistical analysis was performed by using Student’s t-test.
Results
To determine whether caffeine activates human AhR, we performed luciferase reporter assays in human HepG2 cells using the pGudLuc1.1 vector, which harbors a luciferase gene driven by a DRE [8]. HepG2 cells were transfected with pGudLuc1.1 and β-galactosidase (for normalization of transfection efficiency). The transfected cells were treated with vehicle (DMSO), caffeine (50–400 μM), or 3-MC (a known AhR ligand) for 24 hours, and luciferase activities were measured. The 50 μM concentration of caffeine corresponds to the maximum plasma concentration obtained after administration of 12-oz. coffee [12]. The results showed that caffeine, at a concentration as high as 400 μM, showed minimal effects on the DRE-driven luciferase activity, whereas 3-MC caused a significant (41-fold) induction when compared to vehicle-treatment as expected (Fig. 1). These results indicate that caffeine, at concentrations attained from ordinary coffee drinking, is not an AhR activator in HepG2 cells.
Fig. 1.
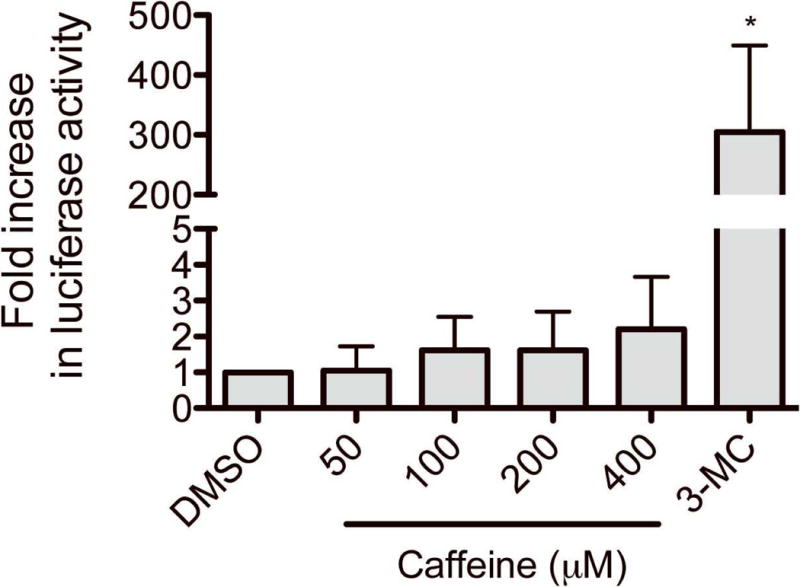
Effects of caffeine on AhR transactivation. HepG2 cells were transfected with a luciferase construct (pGudLuc1.1) and β-galactosidase expression plasmid. The transfected HepG2 cells were treated with vehicle (DMSO), caffeine, or 3-MC (0.5 μM) for 24 hr, and luciferase assay was performed. Results represent fold changes in luciferase activity by drug treatment relative to vehicle treatment (mean ± S.D.; n = 3). *, p < 0.05 compared with vehicle-treated group.
A regulatory mechanism distinct from AhR action is known to mediate induction ofCYP1A2 expression by chemicals, such as omeprazole [13, 14]. To determine whether caffeine is capable of inducing human CYP1A2 expression (despite not being an AhR activator), we examined the effects of caffeine on CYP1A2 expression in primary human hepatocytes. Primary hepatocytes were treated with DMSO (vehicle), caffeine (50–400μM), omperazole, or 3-MC for 72 hours, and mRNA levels of CYP1A2 were measured by using qRT-PCR. The results showed that 3-MC and omeprazole significantly induced CYP1A2 expression (by 48- and 130-fold, respectively) although caffeine up to 200 μM had a minimal effect on CYP1A2 expression (Fig. 2A). Interestingly, caffeine at 400 μM enhanced CYP1A2 expression by 2.3-fold in human hepatocytes (p < 0.05) although the magnitude of this change was small as compared to that by 3-MC or omeprazole. The lack of CYP1A2 induction at 50 μM caffeine concentration was shown in 3 additional different batches of human hepatocytes (data not shown). On the other hand, in rat hepatocytes, 3-MC and caffeine (50 μM) both led to significant induction in CYP1A2 expression (281-fold and 9-fold, respectively) (Fig. 2B), consistent with the previously reported induction of CYP1A2 expression upon caffeine administration in rats [6, 7, 15]. Similar result was obtained in additional batch of rat hepatocytes. Taken together, our results suggest that caffeine, at concentrations attained from ordinary coffee drinking, enhances CYP1A2 expression in rat hepatocytes but not in human hepatocytes.
Fig. 2.
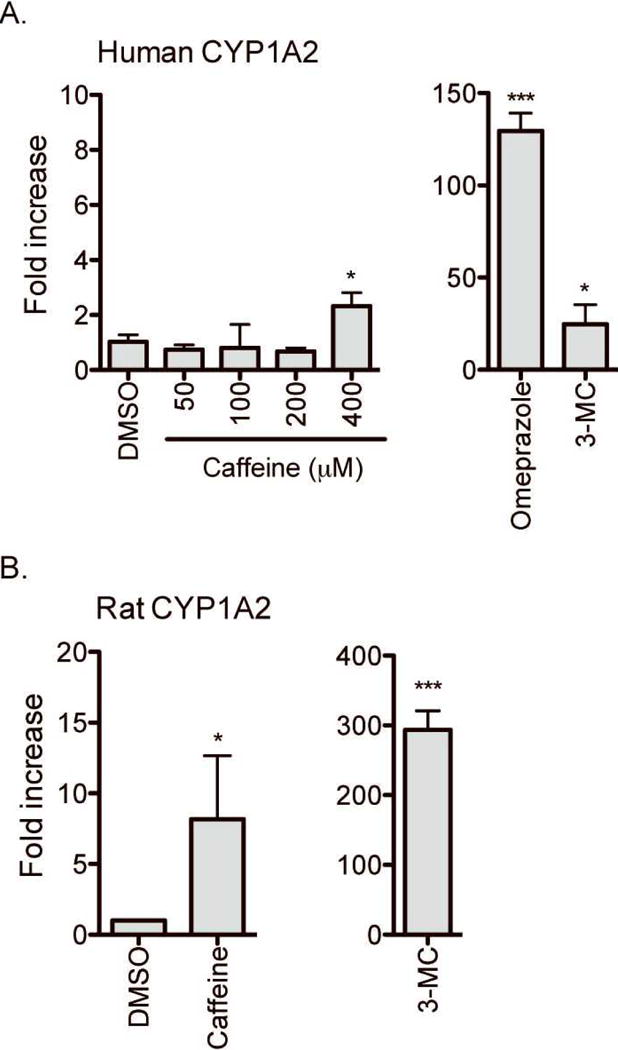
Effects of caffeine on mRNA expression of CYP1A2. Primary human hepatocytes (A) or rat hepatocytes (B) were treated with vehicle (DMSO), caffeine (50–400 μM for human hepatocytes; 50 μM for rat hepatocytes), omeprazole (100 μM), or 3-MC (0.5 μM) for 72 hr. mRNA expression levels of CYP1A2 were determined by qRT-PCR. The data shown are representative of results obtained from 4 and 2 different batches of human and rat hepatocytes, respectively (mean ± S.D.; n = 3 wells/drug treatment). *, p < 0.05 ; ***, p < 0.001 compared with vehicle-treated group.
Discussion
Our results from luciferase reporter assays and human hepatocytes revealed that caffeine, at concentrations attained from average coffee drinking (50 μM), is neither an activator of AhR nor an inducer of CYP1A2 expression in human cells. Interestingly however, at higher concentrations (≥ 400 μM), caffeine significantly induced CYP1A2 expression in human hepatocytes (Fig 2A) while not activating AhR in HepG2 cells (Fig 1). Possibly, mechanisms not involving direct AhR activation by caffeine may be responsible for the CYP1A2 induction in hepatocytes at 400 μM caffeine concentration. For example, metabolites of caffeine produced from human hepatocytes (that express drug-metabolizing enzymes at much higher levels than in HepG2 cells) may activate AhR and induce CYP1A2 expression. Regardless of the underlying mechanisms, the high concentration (400 μM) of caffeine is likely unattainable from average coffee drinking; thus its physiological significance appears minimal.
Studies have reported that consumption of caffeine-containing food or beverages modulates CYP1A2 activity in humans [16, 17]. Djordjevic et al reported that heavy coffee drinking (i.e., regular daily intake of ≥ 3 cups of coffee) is associated with increased CYP1A2 activity index (i.e., plasma paraxanthine/1,3,7-trimethylxanthine ratio) in Serbian and Swedish[16]. Also, another study in healthy Caucasians reported that caffeine increases CYP1A2 activity by 45% for every liter of coffee consumed per day [17]. Considering our data showing that caffeine at concentration attained by average coffee drinking does notactivate AhR, the induced CYP1A2 activity seen in previous clinical studies may be attributable to other substances present in roasted coffee beans (e.g., polycyclic aromatic hydrocarbons).
Unlike in human hepatocytes, caffeine is a weak inducer of CYP1A2 expression in rat hepatocytesat 50 μM (Fig. 2B and [6, 7, 15]). Whether this is due to AhR activation by caffeine (or its metabolites) is unknown. It was previously shown that caffeine was unable to displace TCDD from its binding to AhR [7]. As the experiment was performed using high concentrations of TCDD, which limits detection of low-affinity ligands to AhR [7], the possibility of AhR activation by caffeine (or its metabolites) still remains. In our study, caffeine treatment enhanced expression of CYP1A1, another AhR target gene, by 6-fold in rat hepatocytes (as compared to 53-fold induction by 3-MC; data not shown), suggesting potential AhR activation by caffeine. Furthermore, the chemical structure of caffeine resembles atypical AhR ligands that have been recently discovered [19]. The investigation on whether caffeine or its metabolites bind to and activate AhR is beyond the cope of this exploratory study. Together, the involvement of AhR in the CYP1A2 induction by caffeine remains to be further verified.
In conclusion, our results show that caffeine has minimal effects on AhR activity or CYP1A2 expression in humanhepatocytes. This suggests that in humans mechanisms other than CYP1A2 induction by caffeine mediate development of caffeine tolerance. Whether other components of coffee or tea affect CYP1A2 expression in humans remain to be determined.
References
- 1.Frary CD, Johnson RK, Wang MQ. Food sources and intakes of caffeine in the diets of persons in the United States. J Am Diet Assoc. 2005;105(1):110–113. doi: 10.1016/j.jada.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 2.Smith A. Effects of caffeine on human behavior. Food Chem Toxicol. 2002;40(9):1243–1255. doi: 10.1016/s0278-6915(02)00096-0. [DOI] [PubMed] [Google Scholar]
- 3.Ferre S, Ciruela F, Borycz J, Solinas M, Quarta D, Antoniou K, Quiroz C, Justinova Z, Lluis C, Franco R, Goldberg SR. Adenosine A1-A2A receptor heteromers: new targets for caffeine in the brain. Front Biosci. 2008;13:2391–9. doi: 10.2741/2852. [DOI] [PubMed] [Google Scholar]
- 4.Ma Q. Induction of CYP1A1. The AhR/DRE paradigm: Transcription, receptor regulation, and expanding biological roles. Curr Drug Metab. 2001;2(2):149–164. doi: 10.2174/1389200013338603. [DOI] [PubMed] [Google Scholar]
- 5.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 6.Sohn OS, Surace A, Fiala ES, Richie JP, Jr, Colosimo S, Zang E, Weisburger JH. Effects of green and black tea on hepatic xenobiotic metabolizing systems in the male F344 rat. Xenobiotica. 1994;24(2):119–27. doi: 10.3109/00498259409043226. [DOI] [PubMed] [Google Scholar]
- 7.Ayalogu EO, Snelling J, Lewis DFV, Talwar S, Clifford MN, Ioannides C. Induction of hepatic CYP1A2 by the oral administration of caffeine to rats: lack of association with the Ah locus. Biochimica et Biophysica Acta (BBA) – Molecular Basis of Disease. 1995;1272(2):89–94. doi: 10.1016/0925-4439(95)00071-b. [DOI] [PubMed] [Google Scholar]
- 8.Garrison PM, Tullis K, Aarts J, Brouwer A, Giesy JP, Denison MS. Species-specific recombinant cell lines as bioassay systems for the detection of 2,3,7,8-tetrachlorodibenzo-p-dioxin-like chemicals. Fundam Appl Toxicol. 1996;30(2):194–203. doi: 10.1006/faat.1996.0056. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Yang K, Choi S, Fischer JH, Jeong H. Up-regulation of UDP-glucuronosyltransferase (UGT) 1A4 by 17beta-estradiol: a potential mechanism of increased lamotrigine elimination in pregnancy. Drug Metab Dispos. 2009;37(9):1841–7. doi: 10.1124/dmd.109.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang K, Koh KH, Jeong H. Induction of CYP2B6 and CYP3A4 Expression by 1-Aminobenzotriazole (ABT) in Human Hepatocytes. Drug Metab Lett. 2010 doi: 10.2174/187231210791698410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 12.Robertson D, Wade D, Workman R, Woosley RL, Oates JA. Tolerance to the humoral and hemodynamic effects of caffeine in man. Journal of Clinical Investigation. 1981;67(4):1111–1117. doi: 10.1172/JCI110124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakuma T, Ohtake M, Katsurayama Y, Jarukamjorn K, Nemoto N. Induction of CYP1A2 by phenobarbital in the livers of aryl hydrocarbon-responsive and -nonresponsive mice. Drug Metab Dispos. 1999;27(3):379–84. [PubMed] [Google Scholar]
- 14.Yoshinari K, Ueda R, Kusano K, Yoshimura T, Nagata K, Yamazoe Y. Omeprazole transactivates human CYP1A1 and CYP1A2 expression through the common regulatory region containing multiple xenobiotic-responsive elements. Biochem Pharmacol. 2008;76(1):139–45. doi: 10.1016/j.bcp.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Chen LS, Bondoc FY, Lee MJ, Hussin AH, Thomas PE, Yang CS. Caffeine induces cytochrome P4501A2: Induction of CYP1A2 by tea in rats. Drug Metab Dispos. 1996;24(5):529–533. [PubMed] [Google Scholar]
- 16.Djordjevic N, Ghotbi R, Bertilsson L, Jankovic S, Aklillu E. Induction of CYP1A2 by heavy coffee consumption in Serbs and Swedes. Eur J Clin Pharmacol. 2008;64(4):381–5. doi: 10.1007/s00228-007-0438-6. [DOI] [PubMed] [Google Scholar]
- 17.Tantcheva-Poor I, Zaigler M, Rietbrock S, Fuhr U. Estimation of cytochrome P-450 CYP1A2 activity in 863 healthy Caucasians using a saliva-based caffeine test. Pharmacogenetics. 1999;9(2):131–44. [PubMed] [Google Scholar]
- 18.Kalow W, Tang BK. Use of caffeine metabolite ratios to explore CYP1A2 and xanthine oxidase activities. Clin Pharmacol Ther. 1991;50(5 Pt 1):508–19. doi: 10.1038/clpt.1991.176. [DOI] [PubMed] [Google Scholar]
- 19.Denison MS, Pandini A, Nagy SR, Baldwin EP, Bonati L. Ligand binding and activation of the Ah receptor. Chemico-Biological Interactions. 2002;141(1–2):3–24. doi: 10.1016/s0009-2797(02)00063-7. [DOI] [PubMed] [Google Scholar]


