SUMMARY
The intracellular signaling molecule TRAF6 is critical for Toll-like receptor (TLR)-mediated activation of dendritic cells (DCs). We now report that DC-specific deletion of TRAF6 (TRAF6ΔDC) resulted, unexpectedly, in loss of mucosal tolerance, characterized by spontaneous development of T helper 2 (Th2) cells in the lamina propria, and eosinophilic enteritis and fibrosis in the small intestine. Loss of tolerance required the presence of gut commensal microbiota, but was independent of DC-expressed MyD88. Further, TRAF6ΔDC mice exhibited decreased regulatory T (Treg) cell numbers in the small intestine, and diminished induction of iTreg cells in response to model antigen. Evidence suggested this defect was associated with diminished DC expression of interleukin-2 (IL-2). Finally, we demonstrate that aberrant Th2 cell-associated responses in TRAF6ΔDC mice could be mitigated via restoration of Treg cell activity. Collectively, our findings reveal a role for TRAF6 in directing DC maintenance of intestinal immune tolerance through balanced induction of Treg versus Th2 cell immunity.
INTRODUCTION
Microbial pathogens have long been understood to produce inflammatory stimuli essential to induction of adaptive immune responses, and there is now increasing interest in understanding the relationship between microbiota and maintenance of immune cell tolerance (Maloy and Powrie, 2011; Swiatczak and Rescigno, 2012). Pathogen-associated molecular patterns (PAMPs) derived from pathogenic as well as commensal microbiota are recognized by Toll-like receptors (TLRs) that are highly expressed by dendritic cells (DCs) along the mucosal lining (Maloy and Powrie, 2011; Pulendran et al., 2010). DCs are specialized antigen-presenting cells (APCs) that are capable under inflammatory conditions of providing costimulatory signals and efficient antigen presentation through MHC to T cells (Steinman, 2012). These functions are critical for an effective adaptive immune response, but DCs are now also recognized as key cellular keepers of immune tolerance (Manicassamy and Pulendran, 2011; Rescigno, 2010). What remains unclear is the degree to which this dual role is balanced by dispatchment of specialized subsets of DCs versus specific tuning of DC-intrinsic signaling mechanisms to affect certain immunologic outcomes. Past attempts to address these issues via ex vivo analyses of DC phenotypes have often been hampered by the keen sensitivity of DCs to external manipulation. The recent development of a transgenic mouse model enabling specific genetic targeting to the DC compartment (e.g., CD11c-Cre) represents a major step forward in conducting physiologic in vivo analysis of DC function (Caton et al., 2007).
The molecular adaptor protein TRAF6 integrates upstream signals from both MyD88-dependent IL-1R-TLR superfamily pathways, as well as some TNFR superfamily members, including CD40 and RANK (Inoue et al., 2007; Walsh et al., 2006). Activation of TRAF6, a non-conventional RING finger E3 ligase, which catalyzes formation of K63-linked ubiquitin chains, results in downstream activation of the NFκB, MAPK, and PI3K pathways (Deng et al., 2000; Inoue et al., 2007). Activation of TRAF6-dependent signaling is associated with induction of inflammatory gene expression products, including IL-6 and IL-12. We have previously shown that DCs derived from TRAF6-deficient (Traf6−/−) mice or Traf6−/− fetal liver cell chimeras exhibit substantial defects in maturation and inflammatory cytokine elaboration in response to IL-1R-TLR superfamily ligands and TNF superfamily members, and that Traf6−/− DCs adoptively transferred into third party hosts possess decreased capability to induce antigen-dependent T cell expansion and IFN-γ induction (Kobayashi et al., 2003). However, because Traf6−/− mice die perinatally (surviving no longer than about 15 days), and Traf6−/−fetal liver chimeras develop progressive inflammatory disease characterized by massive organ infiltration of Th2-like cells (Chiffoleau et al., 2003), it has not been possible to specifically examine the in situ role of DC-expressed TRAF6 in the context of physiologic immune responses or immune homeostasis.
Therefore, we have generated mice lacking TRAF6 specifically in the DC compartment (TRAF6ΔDC.) We report here that despite defective activation of TRAF6-deficient DCs in peripheral lymphoid organs like the spleen, we have made the striking observation that TRAF6ΔDC mice develop spontaneous loss of immune tolerance in the gut that is associated with both decreased Treg cell numbers and the presence of microbiota. These findings highlight a unique role for TRAF6 in DC function, and serve to elucidate the relationship between microbiota and DCs in the maintenance of immune tolerance.
RESULTS
Generation of Mice Lacking TRAF6 Specifically in the DC Compartment
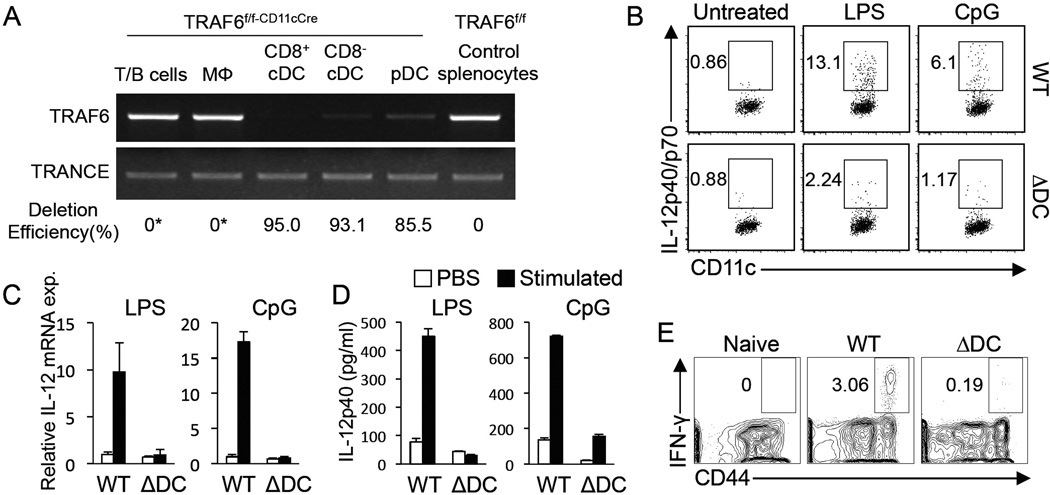
To generate TRAF6ΔDC mice lacking TRAF6 specifically in the DC compartment, we have crossed mice carrying a floxed TRAF6 exon 1 allele (which we have previously described (King et al., 2006)) with CD11c-Cre (ltgax-Cre) transgenic mice (Caton et al., 2007). DCs sorted from spleens of TRAF6ΔDC mice exhibit specific deletion of TRAF6 compared to other sorted cell types as determined by qPCR of genomic DNA samples (Figure 1A). Specific deletion was further observed in DCs isolated from other tissues as well (data not shown.) To determine whether splenic DCs from TRAF6ΔDC mice exhibit predicted defective responses to inflammatory stimuli, IL-12 expression was measured in response to both in vivo (Figure 1B) and in vitro (Figures 1C and 1D) treatment with the TLR ligands LPS and CpG DNA, and revealed that cytokine production is reduced in the absence of TRAF6. To demonstrate the effect of DC-specific TRAF6 deficiency on T cell activation under inflammatory conditions in vivo, TRAF6ΔDC mice were infected with LCMV and splenic CD4+ T cells assayed for CD44 upregulation and IFN-γ production 7 days later, revealing a severely reduced IFN-γ+ cell population in TRAF6ΔDC mice (Figure 1E). Together, these results demonstrate a requirement of DC-expressed TRAF6 for induction of DC activation in response to pathogenic stimuli.
Figure 1. Generation of Mice Lacking TRAF6 Specifically in the DC Compartment.
(A) Quantitative PCR of the floxed region of the Traf6 gene was performed with genomic DNA from TRAF6ΔDC (TRAF6f/f-CD11cCre) and control (TRAF6f/f) sorted FACS-sorted cells to measure degree of deletion in various cell types. A sequence from the TRANCE (Tnfsf11) gene was used as a control for sample standardization. (*, deletion not detected). (B) IL-12 expression in wild type (WT) or TRAF6ΔDC (ΔDC) mice after i.v. injection with PBS, LPS, or CpG. IL-12p40/p70 was detected by intracellular staining for splenocytes isolated at 2 hours post injection. The representative FACS plots were gated on CD11chiMHC class IIhi DCs. (C and D) IL-12p40 mRNA production after in vitro stimulation of MACS-sorted splenic DCs with LPS or CpG. (C) IL-12p40 expression was determined by Q-PCR from mRNA 1 hour after stimulation. (D) IL-12p40 from culture medium was measured by ELISA 12 hours after stimulation. (E) Intracellular IFN-γ level was determined from splenocytes on 7 days postinfection with LCMV. Shown are representative FACS plots gated on CD4+ T cells. The histograms in (C) and (D) represent mean ± SD (standard deviation). FACS plots in (B) and (E) are representative of at least 3 separate experiments.
TRAF6ΔDC Mice Exhibit Spontaneous Small Intestine Enteritis
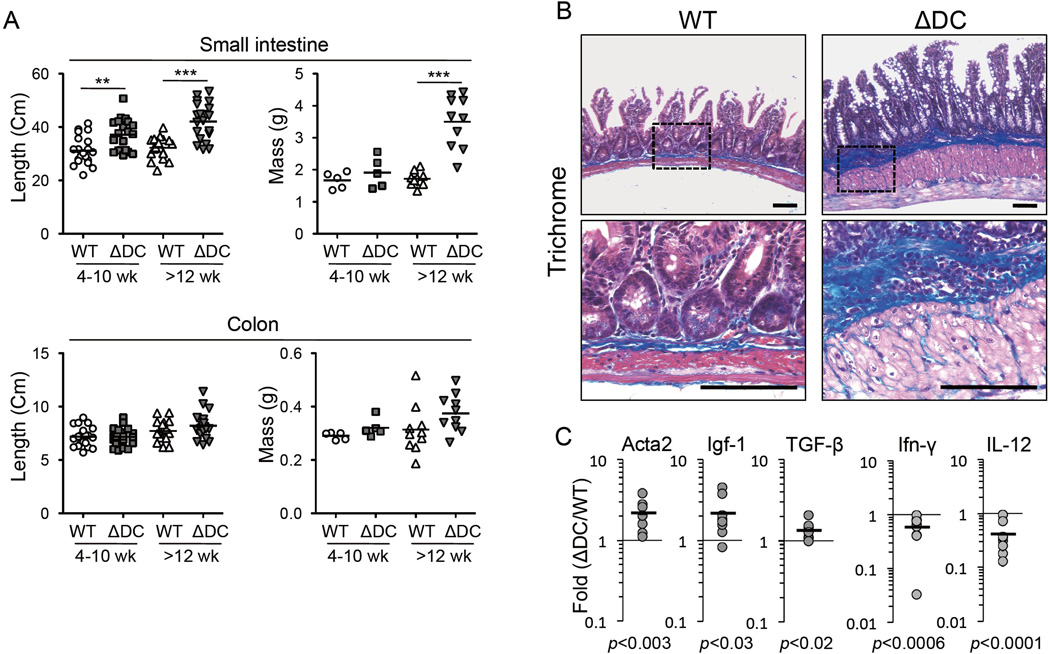
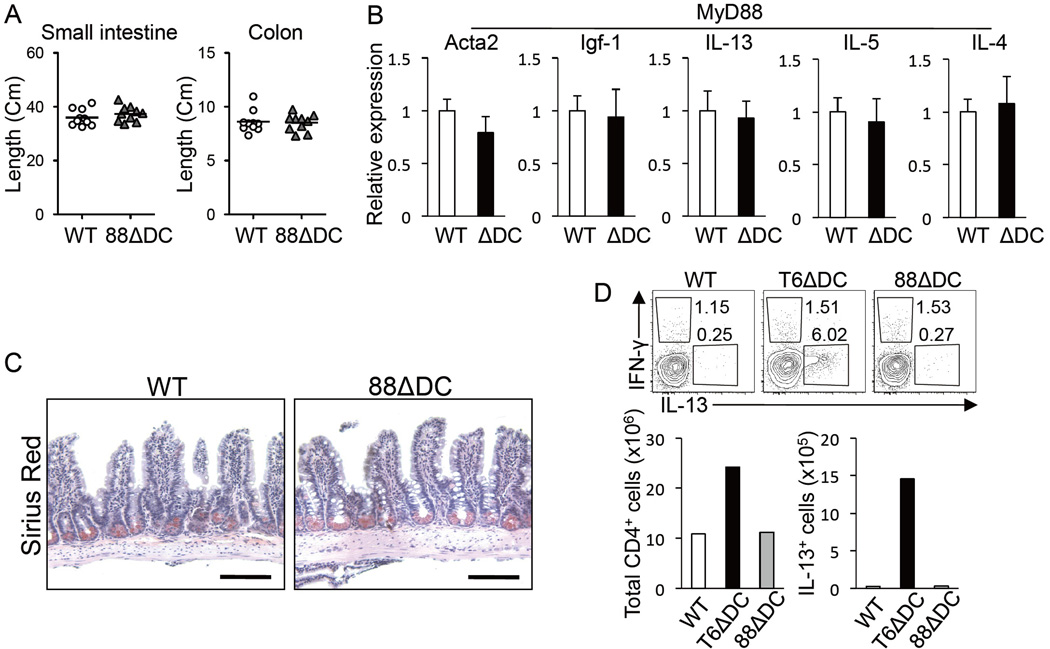
DCs are important mediators of mucosal immune tolerance (Maloy and Powrie, 2011; Pulendran et al., 2010). While gut TRAF6ΔDC DC subsets (characterized by CD103 and/or CD11b expression) were found in normal proportions and numbers, and with normal levels of membrane activation markers (Supplemental figures S1C and S1D), examination of the gut tissue of TRAF6ΔDC mice revealed that, by 12 weeks of age, TRAF6ΔDC mice begin to exhibit substantially increased tissue length and mass specifically in the small intestine (Figures 2A and Supplemental figure S1A). Histological analysis showed signs of spontaneous enteritis, characterized by thickening of the smooth muscle layer, hypertrophy of the intestinal crypts, blunted villi, and increased numbers of both goblet and Paneth cells (Supplemental figure S2). There was also evidence of developing fibrosis, with muscular hypertrophy and increased collagen staining (Figure 2B). Consistent with this observation, tissue mRNA levels of profibrogenic factors Acta2, Igf-1, and TGF-β were increased, while anti-fibrogenic (and Th1-related) factors IL-12 and IFN-γ were slightly reduced (Figure 2C). At the same time, similar analyses revealed TRAF6ΔDC colons to be unremarkable (Supplemental figure S3.) These results reveal a role for DC TRAF6 in maintaining gut homeostasis.
Figure 2. TRAF6ΔDC Mice Exhibit Spontaneous Small Intestine Enteritis.
(A) Lengths (n=20) and masses (n≥5) of small intestines and colons from littermate control (WT) and TRAF6ΔDC (ΔDC) mice organized according to age at sacrifice. Data were analyzed by one-way ANOVA with Tukey’s post-test of multiple comparisons. (B) Trichrome staining was performed on the muscularis propria of the ileum at 27 weeks of age. Collagen (blue, arrows) from Trichrome staining is a marker for gut fibrosis. Smooth muscle layer (arrowheads) corresponds with red staining in sections. (C) Fold increases in pro- (Acta2, Igf-1, and TGF-β) and anti- (IFN-γ and IL-12) fibrotic markers in mRNA isolated from ileum of TRAF6ΔDC (ΔDC) small intestines were compared to wild type (n≥9; ≥8 week-old littermate groups). Data were analyzed with two-tailed, paired student’s t-tests. **, p< 0.01; ***, p< 0.001. Scale bars, 100 µm. N.S., not significant. See also Figures S1,S2, and S3.
Spontaneous Th2 Cell Responses in the Small Intestine of TRAF6ΔDC Mice
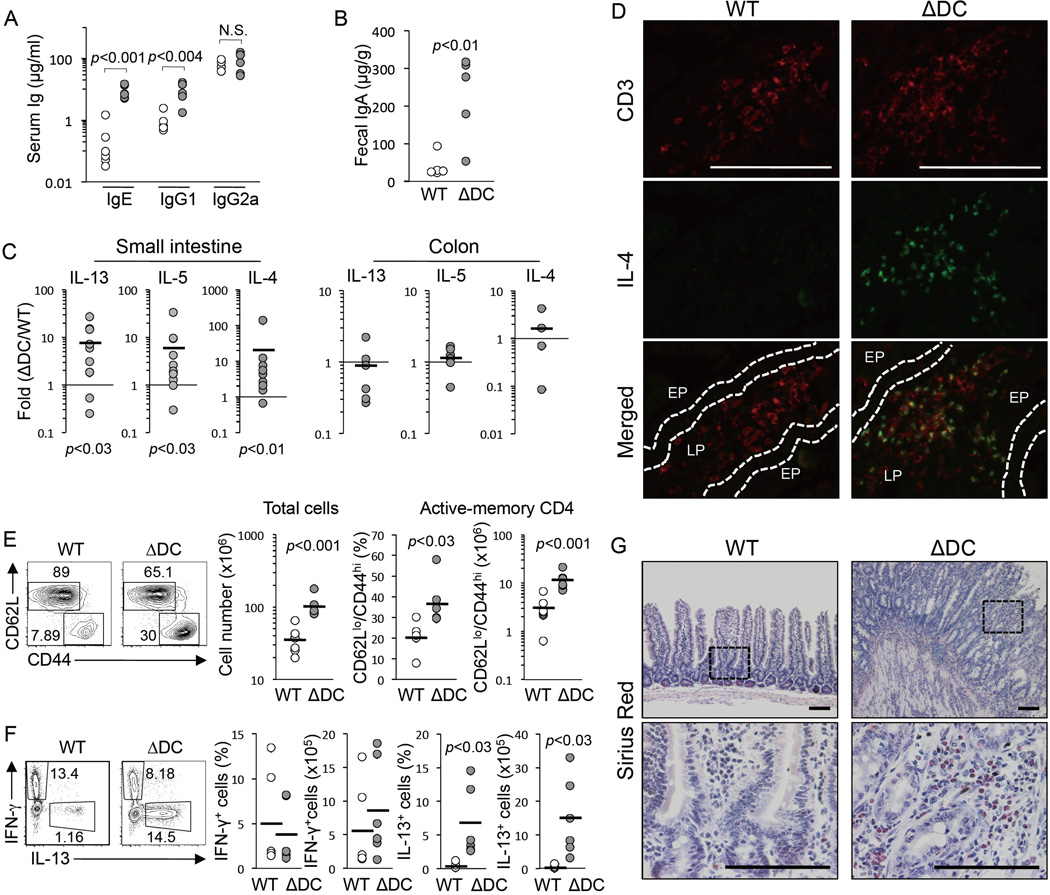
To elucidate potential cellular interactions driving small intestine enteritis in TRAF6ΔDC mice, we crossed TRAF6ΔDC mice to a SCID background. The small intestines of TRAF6ΔDC.SCID mice appear similar to control SCID mice, even past 20 weeks of age, and exhibit substantially reduced levels of fibrotic markers Acta2 and Igf-1 (Supplemental figure S4), suggesting that lymphocytes are critical to the development of the TRAF6ΔDC small intestine phenotype. Consistent with these findings, we observed development of lymphadenopathy of TRAF6ΔDC mesenteric lymph nodes with age (Supplemental figure S1B). Analysis of serum and fecal IgA titers, which are associated with B cell activity in the mucosal lining, showed substantial increases in TRAF6ΔDC mice (Figures 3A and 3B). Analysis of small intestine lamina propria (LP) tissue gene expression from TRAF6ΔDC and littermate control mice older than 8 weeks of age revealed substantial increases in Th2 cell-associated factors IL-13, IL-5, and IL-4 (Figure 3C), while colons from the same mice showed no significant increases in expression and the tissue was histologically normal in appearance (data not shown.) To investigate whether these cytokines were being produced by T cells in situ, we performed immunofluorescent staining, which revealed costaining of IL-4 and the T cell marker CD3 in TRAF6ΔDC small intestinal LP (Figure 3D). Further, in TRAF6ΔDC mesenteric lymph nodes we found substantially increased numbers and frequencies of activated and/or memory CD4+ T cells (Figure 3E), as well as more CD4+ T cells capable of producing IL-13 -- but not IFN-γ -- upon in vitro stimulation (Figure 3F). Th2 cell-mediated intestinal phenotypes are sometimes associated with eosinophil infiltration (Blanchard and Rothenberg, 2009). By staining small intestine sections with Sirius Red, we found that TRAF6ΔDC mice exhibit substantially increased eosinophil numbers (Figure 3G,) potentially revealing a parallel between the TRAF6ΔDC phenotype and human EGID (eosinophilic gastrointestinal diseases (Lucendo, 2010; Masterson et al., 2011).)
Figure 3. Spontaneous Th2 Cell Responses in the Small Intestine of TRAF6ΔDCMice.
Serum (A) and fecal (B) immunoglobulin isotype levels in mice (n≥5; ≥12 week-old littermate groups) were measured by ELISA. (C) Fold increases (between TRAF6ΔDC mice and paired littermate control mice) in IL-13, IL-5, and IL-4 mRNA expression in small intestine (n≥8) or colon (n≥4) tissue as measure by qPCR. (D) Double immunofluorescent staining for CD3 and IL-4. EP, epithelial cell lining. LP, lamina propria. (E and F) Increases of active-memory CD4+ cells and IL-13+ cells in mesenteric lymph nodes of TRAF6ΔDC mice compared to littermate control mice (WT). FACS plots gated on CD4+ T cells, and show population counts and frequencies of (E) naïve (CD44lowCD62Lhi) versus active-memory (CD44hiCD62Llow), and (F) IFN-γ+ and IL-13+ cells (n=6; ≥12 week-old littermate groups). (G) Image of eosinophils (in red) stained with Sirius Red. Eosinophil infiltration is observed in small intestinal sections of TRAF6ΔDC and littermate control (duodenum, 27 weeks old). FACS plots and histogram data are representative of at least 3 separated experiments. Data in (A) and (B) were analyzed with two-tailed, unpaired Student’s t-tests. Data in (C), (E), and (F) were analyzed with two-tailed, paired Student’s t-tests. Scale bars, 100 µm. (See also Supplemental Figure S4). See also Figure S1.
Disruption of TRAF6ΔDC Immune Homeostasis is Gut Microbiota-Dependent
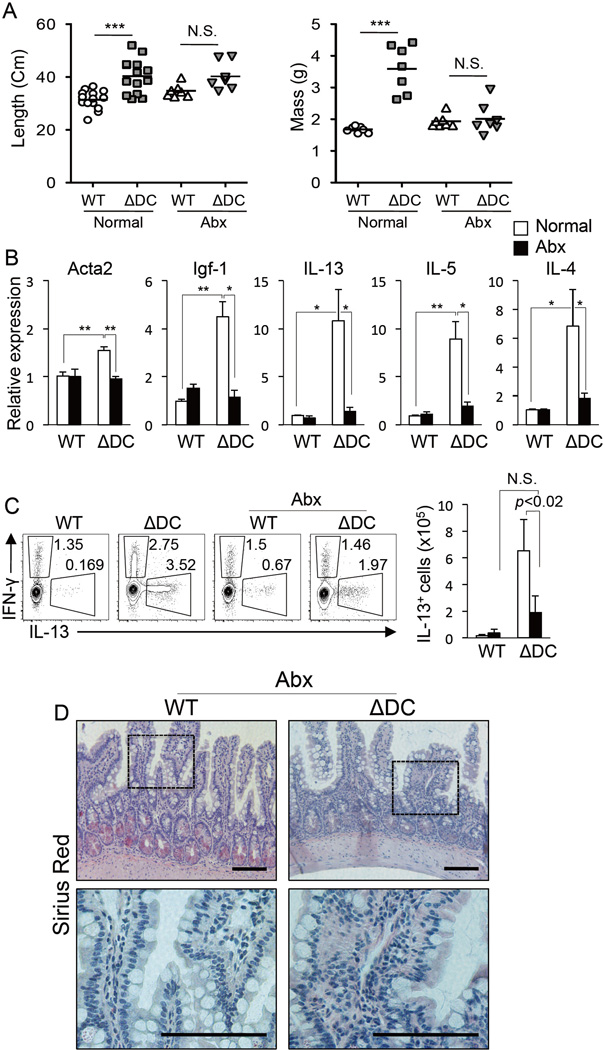
TRAF6 is a key transducer of TLR signaling pathways that are tasked in part with mediating inflammatory and homeostatic interactions between DCs and gut microbiota (Maloy and Powrie, 2011; Swiatczak and Rescigno, 2012). To determine whether gut microbiota interaction with small intestine DCs is relevant to the observed TRAF6ΔDC phenotype, TRAF6ΔDC and littermate control mice were treated with full-spectrum antibiotics for up to six weeks starting at 14 weeks of age. Intriguingly, we observed that antibiotics-treated TRAF6ΔDC intestines now (as compared to untreated intestines shown in Supplemental figure S1A) exhibited grossly normal appearance (Supplemental figure S4B), and normal mean length and mass (Figure 4A). Small intestine mRNA levels of both pro-fibrotic and Th2 cell-associated genes were substantially reduced under antibiotic treatment (as compared to untreated) conditions (Figure 4B.) Further, antibiotics-treated mice exhibited substantial reductions in both the frequency and number of IL-13-producing CD4+ T cells isolated from the small intestinal LP (Figure 4C.) Finally, histological sections of TRAF6ΔDC small intestine tissue from antibiotics-treated mice showed Sirius Red staining similar to littermate control mice, suggesting an absence of eosinophil infiltration (Figure 4D.) Therefore, aberrant immune homeostasis relating to TRAF6-deficient gut DCs is triggered only when commensal microbiota are present.
Figure 4. Disruption of TRAF6ΔDC Immune Homeostasis is Gut Microbiota-Dependent.
(A) Lengths and masses (n≥7 per group) of small intestines from 8–20 week-old littermate-matched control (WT) and TRAF6ΔDC (ΔDC) mice provided untreated or antibiotics-treated (Abx) water for the final 6 weeks. ***, p<0.001. (B) Fibrosis markers (Acta2 and Igf-1) and Th2 cell cytokines (IL-13, IL-5, and IL-4) mRNA expression level in the ileum region of small intestines from mice treated with full-spectrum antibiotics (Abx). Histograms (mean ± SD) are representative of 3 independent experiments. (C) Intracellular staining for IFN-γ, IL-13, and Foxp3 in mesenteric lymph nodes of antibiotics-treated (Abx) or untreated mice. FACS plots were gated on CD4+ T cells and cell counts of IL-13 producers cell are shown in the histogram. FACS plots are representative of 3 independent experiments. The histograms represent means ± SD. (D) Sirius Red staining of the duodenum region of small intestine from antibiotics-treated (Abx) mice. Antibiotic water containing 1 g/L Ampicillin, 1 g/L Neomycin, 0.5 g/L Vancomycin, 1 g/L Metronidazole was provided ad libitum in water to 14-week-old mice for 6 weeks. Data in (A), (B), and (C) were analyzed by one-way ANOVA with Tukey’s post-test of multiple comparisons. *, p<0.05; **, p<0.01. Scale bars, 100 µm. N.S., not significant. See also Figure S4 and S8.
MyD88ΔDC Mice Do Not Phenocopy Aberrant Immune Homeostasis of TRAF6ΔDC Mice
TRAF6 and MyD88 carry out critical non-redundant roles as mediators of TLR signaling (Inoue et al., 2007; Takeda and Akira, 2004), with MyD88-IRAK complexes leading to activation of TRAF6 and subsequently NFκB and MAPKs. DC-specific MyD88 conditional knockout mice (MyD88ΔDC) utilizing the same CD11c-Cre system that we have employed for TRAF6ΔDC have previously been studied, and while MyD88ΔDC exhibit defects in TLR-mediated activation and induction of Th1-associated immunity (consistent with our observations of TRAF6ΔDC mice), no aberrant immune homeostasis in the gut is reported (Hou et al., 2008). Given the major significance of the MyD88-TRAF6 signaling juncture to DC biology (especially in the context of TLR signaling), together with our observations that TRAF6ΔDC phenotypes are substantially influenced by TLR ligand-producing gut microbiota, we decided to re-examine MyD88ΔDC mice side-by-side with TRAF6ΔDC mice to determine whether previous analyses may have yielded different results based on housing-specific factors (e.g., quantitatively and/or qualitatively distinct intestinal microbiota.) Interestingly, we found that aged MyD88ΔDC mice exhibited no significant differences in small intestine length versus littermate controls (Figure 5A), no increases in pro-fibrotic or Th2 cell-associated mRNA levels in small intestine tissue samples (Figure 5B), and no apparent eosinophil infiltration of the small intestine (Figure 5C.) Further, CD4+ T cells isolated from small intestinal LP exhibited frequencies and numbers of IL-13-producing cells no different from littermate controls, and much lower than similar cells isolated at the same time from TRAF6ΔDC mice (Figure 5D), suggesting the existence of a MyD88-independent component to the TRAF6ΔDC small intestine phenotype. We further considered the possibility that genetic manipulation of DC signaling components may exert indirect external population effects on the surrounding commensal microbiota. However, profiling of fecal bacterial communities of control, TRAF6ΔDC, and MyD88ΔDC mice by deep sequencing showed that the most abundant types of bacterial taxa were common among communities of all three genotypes, and their proportions were not significantly different (Supplemental figure S5), implying that differences between TRAF6ΔDC and MyD88ΔDC phenotypes are not likely the result of the presence of altered bacteria-derived stimuli in TRAF6ΔDC intestines.
Figure 5. MyD88ΔDC Mice Do Not Phenocopy Aberrant Immune Homeostasis of TRAF6ΔDC Mice.
(A) Length of gut was measured in MyD88ΔDC mice (88ΔDC) and littermate controls (WT). (B) Relative mRNA levels in 20 week-old ileum of MyD88ΔDC mice and littermate controls (WT). The histograms (mean ± SD) are representative of 3 independent experiments. (C) Sirius Red staining was performed in the ileum section of small intestine from 20-week-old mice. (D) FACS plots gated on CD4+ T cells showing Intracellular staining for Th1 and Th2 cell cytokines (IFN-γ and IL-13, respectively) in mesenteric lymph nodes from 20 week-old mice and cytokine-producing cell counts. FACS plots and histogram data are representative of 3 independent experiments. Scale bars, 100 µm.
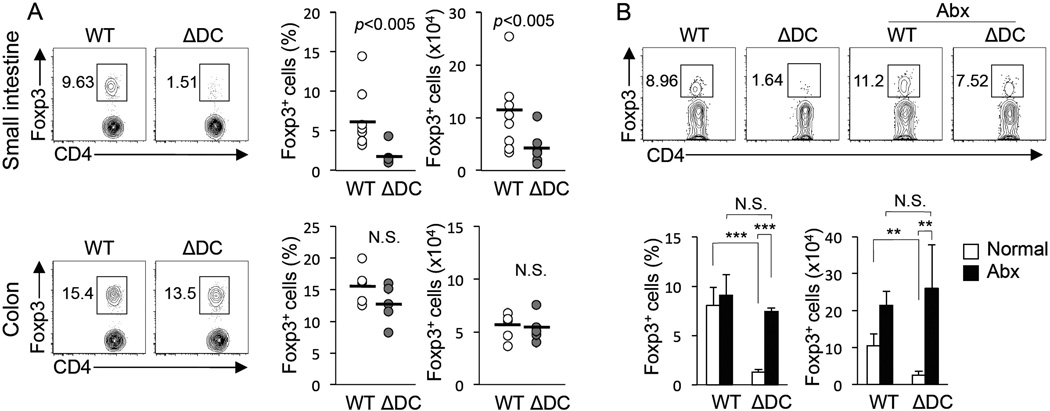
Microbiota-Dependent Reduction in TRAF6ΔDC Small Intestine Treg Cells
One mechanism by which mucosal DCs maintain tolerance is through the induction and maintenance of iTreg cells (Pulendran et al., 2010; Rescigno, 2010), regulatory T cells that acquire their suppressive phenotype in peripheral tissues rather than in the thymus. We analyzed TRAF6ΔDC intestinal LP and found decreased numbers and frequencies of Treg cells (by Foxp3 expression) in mice as young as 6 weeks of age, and importantly, these defects were found in the small intestine, but not the colon (Figure 6A.) Consistent with their lack of spontaneous enteritis, MyD88ΔDC mice of similar ages and genders to analyzed TRAF6ΔDC mice were found to have normal Treg cell populations (Supplemental figure S6.) To determine whether the TRAF6ΔDC Treg cell defect is also dependent on gut microbiota, we again treated mice with full-spectrum antibiotics, but this time started at 4 weeks of age to account for the early presentation of the Treg cell defect. Interestingly, we found that two weeks of treatment was sufficient to partially restore the frequency (within the total CD4+ T cell population), and completely restore the number, of Treg cells located in the TRAF6ΔDC small intestine (Figure 6B). Therefore, defective TRAF6ΔDC Treg cell induction appears to depend on the presence of microbiota.
Figure 6. Microbiota-Dependent Reduction in TRAF6ΔDC Small Intestine Treg Cells.
(A) FACS plots gated on CD4+ T cells show intracellular staining for small intestine or colon lamina propria lymphocytes from 6–8 week-old TRAF6ΔDC and littermate control mice and Foxp3+ as a percentage of CD4+ and cell counts. Data were analyzed with two-tailed, paired Student’s t-test. (B) Intracellular staining for Foxp3 in small intestinal lamina propria of antibiotics-treated (Abx) or untreated mice. FACS plots were gated on CD4+ T cells. Water containing 1 g/L Ampicillin, 1 g/L Neomycin, 0.5 g/L Vancomycin, 1 g/L Metronidazole was provided to 4 week-old mice for 2 weeks. The histograms (mean ± SD) were analyzed by one-way ANOVA with Tukey’s post-test of multiple comparisons. FACS plots in (A) and (B) are representative of at least 3 separated experiments. **, p< 0.01; ***, p< 0.001. N.S., not significant. See also Figure S5.
Defective DC-Expressed IL-2 and Rescue of Treg Cells via Exogenous IL-2 in TRAF6ΔDC Mice
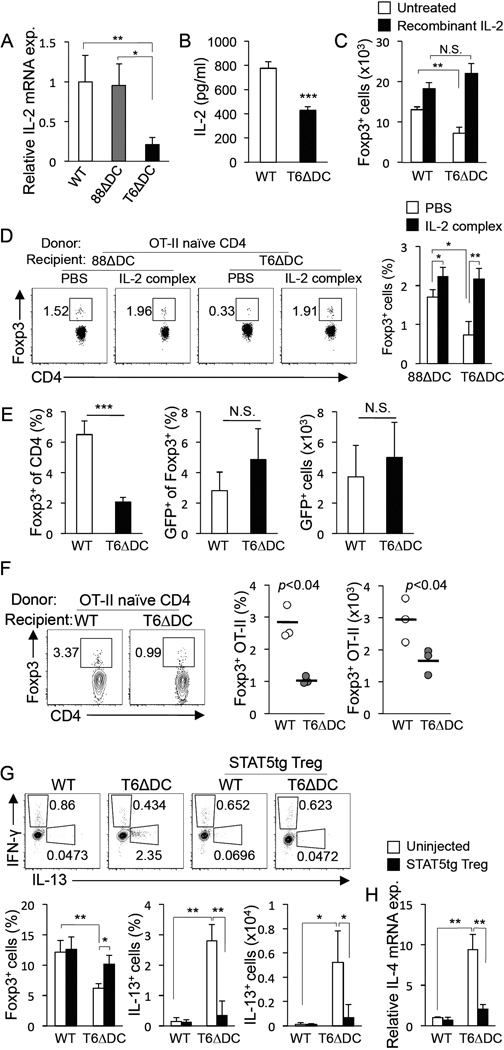
To uncover potential mechanistic bases for diminished Treg cell numbers in TRAF6ΔDC LP, we investigated the potential roles of cell surface markers and other proteins reportedly involved in the induction of intestinal iTreg cells by DCs. Numerous factors, including Aldh1a2, TGF-β, integrin-αv, and integrin-β8 showed no apparent difference between control and TRAF6ΔDC (Supplemental figure S7). This process led us to focus on IL-2, which is not only critical to Treg cell development and maintenance (Malek and Castro, 2010), but which has also been shown to be expressed by DCs (Granucci et al., 2001), and its expression by DCs may play a role in Treg cell maintenance (Kulhankova et al., 2012; Sgouroudis et al., 2011). We have also found that blocking IL-2 or use of IL-2-deficient DCs results in diminished Treg cell conversion (Supplemental figure S8). Interestingly, we examined IL-2 expression in control, MyD88ΔDC, and TRAF6ΔDC LP DCs, and found that while IL-2 mRNA levels in both control and MyD88ΔDC samples were similar, levels in TRAF6ΔDC LP DCs were dramatically reduced (Figure 7A). TRAF6ΔDC LP DCs similarly exhibited decreased IL-2 protein elaboration during brief ex vivo culture (Figure 7B). Next, we showed that while DCs from TRAF6ΔDC mice are defective inducers of Foxp3 in in vitro Treg cell conversion assays, provision of IL-2 to these cultures had a corrective effect on this defect (Figure 7C). Finally, we similarly showed that defective in vivo model antigen-induced Treg cell conversion of adoptively-transferred TCR transgenic T cells into TRAF6ΔDC recipients could be restored to the higher levels observed in MyD88ΔDC recipients (which are similar to those observed for control recipients) through provision of bioactive IL-2–αIL-2 complexes (Figure 7D). Together, these findings suggest that the decreased capacity of DCs from TRAF6ΔDC mice to induce the Treg cell phenotype can be overcome by provision of the pro-Treg cell cytokine IL-2, and raises the possibility that DC-expressed IL-2 is required for normal Treg cell homeostasis in the gut.
Figure 7. Treg homeostasis and gut immune tolerance in TRAF6ΔDC mice is linked to regulation of DC-expressed IL-2.
(A) IL-2 mRNA expression levels in MACS-purified lamina propria DCs from wild type (WT), MyD88ΔDC (88 Δ DC), and TRAF6 Δ DC (T6 Δ DC) mice. (B) ELISA of IL-2 proteins levels elaborated by wild type (WT) and TRAF6ΔDC (Δ DC) lamina propria DCs cultured for 12 hours with 50 ng/mL PMA and 500 ng/mL ionomycin. ***, p<0.001. (C) Counts of Foxp3+ cells converted from naïve-effector OT-II CD4+ T cells after 4 days culture with purified lamina propria DCs (5 × 104 T cells and 5 × 103 DCs) from wild type (WT) or TRAF6ΔDC (T6ΔDC) (1 µ M OVA 323–332 and 1 ng/ml TGF-β in all cultures) in the presence (IL-2(+)) or absence (IL-2(−)) of 10U/mL recombinant IL-2. (D) FACS plots and histograms show the frequencies of Foxp3+ OT-II donor cells in mesenteric lymph nodes after 5 days of OVA feeding. MyD88ΔDC (88 Δ DC) and TRAF6 Δ DC (T6 Δ DC) mice were used as recipients. IL-2-mAb complex (IL-2 complex) was injected as indicated for the first 3 days of OVA-feeding. (E) FACS sorted Foxp3-GFP+ cells (0.5 × 106) were transferred to WT or TRAF6ΔDC recipients. Cells were isolated and analyzed from small intestinal lamina propria 7 days post transfer. The histograms show the Foxp3-GFP+ (donor) cells as a ratio of total Foxp3+ cells and counts of Foxp3-GFP+ cells in the gut (n=3). (F) Intracellular staining and cell counts of converted donor OT-II cells recovered from mesenteric lymph nodes of mice fed OVA protein for 5 days. FACS plots depict gated CD45.1+ (donor marker) and CD4+ T cells. (G and H) STAT5bCA transgenic Foxp3-GFP+ regulatory T cells (STAT5tg Treg cell) were transferred to TRAF6ΔDC and littermate control recipients. (G) FACS plots showing Intracellular staining for Th1 and Th2 cell cytokines (IFN-γ and IL-13, respectively) 4 weeks post-transfer. The percentages and counts for Foxp3+ or IL-13+ from CD4+ T cells in mesenteric lymph nodes, and (H) Th2 cell cytokine (IL-4) mRNA expression level from ileum of both adoptively-transferred and no transfer control groups. FACS plots and graphs are representative of at least 3 separated experiments. The histograms are represented as mean ± SD. Data in (C), (D), and (G) were analyzed by oneway ANOVA with Tukey’s post-test of multiple comparisons. *, p< 0.05; **, p< 0.01; ***, p< 0.001. N.S., not significant. See also Figure S6 and S7.
Defective TRAF6ΔDC iTreg Cell Induction is Linked to Aberrant Th2 Cell Development
Decreased Treg cell numbers in the small intestine raised the possibility that Treg cell maintenance and/or iTreg cell induction are defective in TRAF6ΔDC mice. To address the first possibility, we sorted GFP-expressing peripheral Treg cells from transgenic mice expressing GFP under the control of the Foxp3 promoter (Fontenot et al., 2005) and transferred these cells into control or TRAF6ΔDC recipient mice. After one week the populations of transferred cells were identified by GFP expression and enumerated. While endogenous levels of TRAF6ΔDC Treg populations were decreased (as expected: Figure 7E left), the transferred populations appeared at normal levels (Figure 7E middle and right.) Therefore, to address the alternative possibility of defective iTreg cell induction, we adoptively transferred sorted naïve (CD44loCD62LhiCD25−) Thy1.1 congenic OVA-specific OT-II CD4+ T cells into TRAF6ΔDC or littermate control mice followed by 5 days of oral administration of OVA peptide. Using congenic markers, transferred cells were identified after one week and Foxp3 staining revealed that TRAF6ΔDC recipient mice exhibit substantially reduced capability to induce Treg cells in response to model oral antigen (Figure 7F).
These findings raised the possibility that reduced Treg cell function in TRAF6ΔDC mice may be linked to spontaneous induction of intestinal Th2 cell immunity. To restore robust Treg cell function experimentally, we crossed Foxp3-GFP mice with transgenic mice expressing a constitutively-active form of the pro-Treg cell transcription factor STAT5b (Burchill et al., 2003) (Foxp3-GFP.STAT5bCA), and then adoptively transferred sorted Foxp3-GFP+ Treg cells from those mice into either TRAF6ΔDC or littermate control recipients. Foxp3-GFP.STAT5bCA Treg cells were used because they were obtainable in large numbers, and, we reasoned, would be more stable as a population (capable of reliably providing Treg cell activity) once transferred. This was important because the TRAF6ΔDC gut phenotype developed over an extended time period. Four weeks post-transfer we observed that TRAF6ΔDC mice which had received STAT5bCA Treg cells had partially restored Treg cell numbers, and more importantly, exhibited reduced frequencies and numbers of small intestinal LP Th2 cell cells and cytokine expression (Figures 7G and 7H.) These findings suggested a direct link between Treg cell function and spontaneous development of aberrant gut immune responses in TRAF6ΔDC mice.
DISCUSSION
DCs are important for both activation of inflammatory T cell immune responses and maintenance of T cell tolerance. By generating mice that specifically lack DC-expressed TRAF6, we have been able to characterize a new role for TRAF6 in directing DC maintenance of intestinal immune tolerance. While TRAF6ΔDC mice were found to exhibit some predicted (given our previous work with Traf6−/−DCs) phenotypes, such as defective induction of TLR-mediated peripheral Th1 cell-associated responses, we were surprised to find that these mice also exhibit defective intestinal iTreg cell induction, and subsequent spontaneous Th2 cell-associated disruption of immune homeostasis. Intriguingly, these TRAF6ΔDC phenotypes were found to be dependent on the presence of gut microbiota, while mice lacking MyD88, the signaling protein that links TLRs to TRAF6, in DCs (MyD88ΔDC) did not exhibit any signs of disease onset or decreased Treg cell numbers. Furthermore, we have shown that LP DCs harvested from TRAF6ΔDC mice exhibit dramatically reduced levels of IL-2 mRNA, and that IL-2 provision can rescue defective iTreg cell induction by TRAF6ΔDC DCs in multiple contexts. These findings represent a potentially significant link between TRAF6 signaling pathways and the production of cytokines functionally relevant to the maintenance of immune tolerance. Further, in light of recent work by Kulhankova et al. regarding juxtacrine relationships of IL-2 with DCs and Treg cells (Kulhankova et al., 2012), it will be especially interesting to further investigate the potential role of TRAF6-dependent signaling in this relationship. It is also important to point out that identification of a potential role for DC-expressed IL-2 in TRAF6ΔDC Treg cell homeostasis does not exclude the possibility that other Treg cell-associated cytokines, such as IL-10, are also relevant. However, at this time, we have not identified any irregular expression patterns of such factors in DCs isolated from TRAF6ΔDC mice.
Decoding the complex signaling networks that govern the relationships between DC-mediated tolerance and immunity, and being able to do so in a physiologic setting (and avoiding the difficulties associated with studying DCs ex vivo), represented significant motivations for generating and studying TRAF6ΔDC mice. It is instructive to consider our observations of the in vivo relevance of DC-expressed TRAF6 in the context of some TRAF6-associated signaling factors that have been similarly analyzed. To begin with, because of the divergent phenotypes described above in TRAF6ΔDC and MyD88ΔDC mice in the presence of TLR ligand-bearing microbiota, we infer either: 1) that TLRs activate a MyD88-independent, TRAF6-dependent pathway that serves to promote a tolerogenic phenotype in DCs (this pathway could involve the TLR adapter TRIF (Takeda and Akira, 2004), and/or the NOD2-RIP2 signaling complex (Brand, 2009; Fritz et al., 2011)); 2) that microbiota act directly or indirectly on DCs through pattern recognition receptor (PRR)-independent pathways (such as extracellular ATP, which has been shown to act through P2X and P2Y receptors to modulate regulation of immune tolerance by DCs (Atarashi et al., 2008; Idzko et al., 2007)); or 3) that TLRs stimulate production of factors by non-DCs (e.g., epithelial, Paneth, goblet, other lymphoid or myeloid cells) that then act on DCs through known TRAF6-dependent, MyD88-independent pathways (e.g., TGF-β, CD40L, RANKL, IL-25 (Chang and Dong, 2011; Landstrom, 2010; Walsh et al., 2006)).
TAK1 is the primary kinase activated by TRAF6, but TAK1ΔDC mice that lack TAK1 in the DC compartment, are not reported to develop a similar gut phenotype (Wang et al., 2007). However, TAK1ΔDC mice do exhibit a Treg cell defect. The reason TRAF6ΔDC mice exhibit loss of gut immune tolerance in addition to the similar Treg cell phenotype is likely because DCs in TRAF6ΔDC mice retain sufficient TAK1 activity to avoid the massive survival defect of TAK1ΔDC DCs that would preclude the development of Th2 cells. Unlike TAK1, the ubiquitin-editing molecule A20 is a negative regulator of TRAF6-mediated signaling (Shembade et al., 2010). Recent reports describe mice lacking DC-expressed A20 (A20ΔDC) as developing spontaneous Th1-associated peripheral autoimmunity (Hammer et al., 2011; Kool et al., 2011), and one study identifies the DC-intrinsic pathway responsible for T cell expansion and inflammatory cytokine elaboration as being MyD88-dependent (and therefore, possibly TRAF6-dependent.) Consistent with an inverted phenotype (in relation to TRAF6ΔDC), Treg cell numbers in A20ΔDC mice are reported to be normal (Hammer et al., 2011) or even increased (Kool et al., 2011).
TRAF6 is also involved in TGFβR signaling (Landstrom, 2010), primarily through activation of TAK1 (Sorrentino et al., 2008). Recent reports indicate that complete ablation of TGFβR1-ALK-5 signaling in DCs results in loss of Langerhans cells (Kel et al., 2010), while DC-specific deletion of αvβ8 integrin, which activates the precursor form of TGF-β, reveals a phenotype bearing some similarity to TRAF6ΔDC, including age-related development of inflammatory bowel disease associated with decreased gut Treg cells (though in the colon rather than the small intestine) (Travis et al., 2007). These findings may imply a role for the TRAF6-specific component of TGFβR signaling in DCs for maintaining mucosal homeostasis.
One of the most curious features of the TRAF6ΔDC gut phenotype is the exacerbating role that gut microbiota play. The fact that depletion of commensal bacteria increased small intestinal LP Treg cells seems to contrast recent findings that commensal bacteria boost gut Treg cell numbers (Atarashi et al., 2011), though these differences may be explained by the fact that these studies were performed for Treg cells in the colon, where commensal-related stimuli are substantially higher than and qualitatively different from the small intestine (Santaolalla et al., 2011; Swiatczak and Rescigno, 2012). Additionally, it is possible that TRAF6 expression in DCs differentially impacts the distribution between the small and large intestines of DC subsets. Specifically, the major subset of mucosal DCs responsible for inducing iTreg cells, the vitamin Ametabolizing CD103+ subset, is present at higher numbers in the small intestine (Agace and Persson, 2012), and might be more dependent on TRAF6-dependent signals. The primary defect in TRAF6ΔDC mice might derive first from failure to sufficiently induce small intestine iTreg cells, though thus far we have found TRAF6ΔDC CD103+ DCs present in normal numbers expressing normal levels of Retinaldehyde dehydrogenases RALDH1 and RALDH2 (data not shown).
It should also be noted that while we have not identified any phenotypic differences in small intestinal DCs in TRAF6ΔDC mice that might explain why these mice exhibit an iTreg cell defect and Th2 cell-associated autoimmunity, there are certain caveats attached to DC analysis in the gut. First, regarding enumeration, preps are relatively complex and can lead to significant variability between labs and even within labs (Rescigno, 2011). This difficulty must be given special consideration in the case of TRAF6ΔDC because the small intestine tissues become enlarged and fibrotic with disease onset, thereby altering, with respect to control mice, expectations about DC prep efficiency. Second, beyond CD103 and CD11b, there are numerous other markers, including many chemokine receptors (e.g., CX3CR1 and CCR7, as well as CD70), which identify specialized DC subsets. It is difficult to determine, therefore, whether relevant defects in TRAF6ΔDC DCs exist in specific subsets that constitute a minority population. Alternatively, it is possible that disease in TRAF6ΔDC mice is mediated by a DC subset not yet characterized. Clearly additional effort will be required to determine the specific nature of the relevant cellular and signaling defect(s) in TRAF6ΔDC mice.
Overall, the TRAF6ΔDC mouse dramatically identifies a novel example of a DC signaling mediator that is critical for activation of the immune response in one anatomic and stimulatory context, and critical for maintaining immune homeostasis in another. We believe this unusual status makes TRAF6ΔDC a valuable model for further elucidating, in a physiologic setting, the mechanisms employed by DCs to balance active immunity and tolerance.
EXPERIMENTAL PROCEDURES
Mice
TRAF6ΔDC and MyD88ΔDC mice were generated by crossing mice carrying floxed alleles of TRAF6 (King et al., 2006) or MyD88 (Hou et al., 2008) with CD11c Cre transgenic mice (Caton et al., 2007). All mice were backcrossed to B6 background more than 10 generations. OT-II transgenic (B6.Cg-Tg(TcraTcrb)425Cbn/J), SCID (B6.CB17-Prkdcscid/SzJ), and congenic (CD45.1, B6.SJL-Ptprca Pepcb/BoyJ) mice were purchased from Jackson laboratories. STAT5b-CA transgenic mice (Burchill et al., 2003), Foxp3-GFP mice (Fontenot et al., 2005), and IL-2-GFP knock-in mice (McKarns and Schwartz, 2008; Naramura et al., 1998) were kind gifts from M. A. Farrar (University of Minnesota), A. Y. Rudensky (Sloan Kettering), and Ronald Schwartz (NIH), respectively. All experimental pairs of mice were co-housed. Mouse care and experimental procedures were performed in accordance with protocols from Institutional Animal Care and Use Committees of the University of Pennsylvania.
Buffers and Media
Sort buffer containing 2% FBS and 0.7 mM EDTA was used to prepare, stain, and wash isolated cells. Complete RPMI-1640 medium containing 10% FBS, 10 U/ml Penicillin, 10 µg/ml Streptomycin, 2 mM L-glutamin (Invitrogen), and 50 µM β-mercapotethanol (Sigma) was used for cell culture. Gey’s solution composed of 155 mM NH4Cl (Sigma) and 10 mM KHCO3 (Sigma) was used for red blood cell lysis.
Quantitative PCR
Tissues or purified cells were homogenized in TRIzol (Invitrogen) after freezing in liquid nitrogen. cDNA were established by using Superscript III (invitrogen) and random hexamer (Qiagen). TaqMan gene probes were used with TaqMan Universal PCR Master Mix (Applied Biosystems) and run on 7300 Realtime PCR System (Applied Biosystems): 2 min at 50 °C, 10 min at 95 °C, 50 cycles of 15 sec 95 °C, 1 min at 60 °C, and signals were detected during annealing step (60 °C). Relative mRNA expression levels of all samples were normalized to 18S mRNA. The TaqMan gene probes (Applied Biosystems) used: Acta2 (Mm01546113_m1), Igf-1 (Mm00439560_m1), Tgf-β (Mm00441724_m1), IFN-γ (Mm01168134_m1), Il-2 (Mm00434256_m1), Il-12p40 (Mm99999067_m1), Il-13 (Mm99999190_m1), Il-5 (Mm00439646_m1), Il-4 (Mm00445259_m1), and 18S (Hs99999901_s1).
ELISA
For serum immunoglobulin levels, serum was diluted to 1:10,000-100,000 and immunoglobulin isotype levels measured by ebioscience ELISA kit. For fecal IgA, feces were weighed and homogenized in 10 fold (v/w) PBS containing 0.01% NaN3. Supernatants were diluted 1:1000 after centrifuging at 4 °C and 10,000 rpm for 5 min. IgA level was determined by ebioscience ELISA kit. For IL-2 detection, 3 × 104 DCs (purified from small intestine LP with anti-CD11c MACS beads (Miltenyi Biotech)) per triplicate well were stimulated with 500 ng/ml ionomycin and 50 ng/ml PMA (Sigma) for 12 hours. Supernatant concentration of IL-2 was determined according by ELISA kit according to manufacturer instructions (eBioscience). Purified α-IL-2 (JES6-aA12) was used for capture and biotin-conjugated α-IL-2 (JES6-5H4) for detection.
Cell Isolation
Single cells were isolated from spleen and mesenteric lymph nodes by mechanical disruption on 70µm cell strainers (BD). For small intestinal LP lymphocyte (LPL) isolation, epithelial cells were removed by incubation in stripping RPMI-1640 medium containing 10% FBS, 5mM EDTA, 1mM DTT, and 20mM HEPES at 37 °C for 15 min in shaker. The remaining pieces were minced and incubated in serum-free RPMI-1640 medium containing 52 U/ml Liverase TM (Roche), 50 µg/ml DNaseI (Sigma), and 20 mM HEPES at 37 °C for 15 min in while shaking. For T cell enrichment, LPLs were separated with Percoll gradient (GE Healthcare). Cells were collected at the interface between 40% and 70% after centrifuging at 2500 rpm at room temperature for 20 min with no brake. For DC enrichment, 145 µg/ml Histodenz (Sigma) dissolved in sort buffer was used at 2500 rpm, 4 °C for 15 min with no brake. For gene expression assay, LP dendritic cells (LpDCs) were isolated by MACS purification (Miltenyi Biotec) using magnetic bead-conjugated anti-CD11c antibody.
Flow Cytometry
1 × 106 cells were used for surface staining in FACS buffer containing α-CD16/32 (2.4G2) and LIVE/DEAD dye (Invitrogen) with combinations of fluorochrome-conjugated antibodies including CD44 (IM7), CD62L (MEL-14), CD25 (PC61.5), CD8 (53-6.7), CD4 (RM4–5), I-Ab (AF6–120.1), CD45.1 (A20), CD45.2 (104), CD11b (M1/70), CD11c (HL3), B220 (RA3-6B2), CD90.2 (53-2.1), and CD103 (2E7). For intracellular staining, cells were stimulated in round-bottom 96-well plates with complete RPMI1640 medium containing 500 ng/ml Ionomycin and 5 ng/ml PMA (Sigma) in the presence of Brefeldin A (BD) for the last 3 hours. After surface staining, the cells were fixed and permeabilized with Fixation/Permeabilization reagent (eBioscience) and stained with intracellular protein-specific antibodies reconizing IL-12p40/p70 (C15.6), IL-13 (eBio13A), IFN-γ (XMG1.2), and Foxp3 (FJK-16s). All stained samples were analyzed on an LSRII flow cytometer (BD) and the raw data were calculated and visualized with FlowJo software (Tree Star Inc.). All of antibodies were purchased from BD or eBioscience.
LCMV infection
2 × 105 PFU of LCMV-Armstrong were administrated to mice by intraperioneal injection. 1 × 106 isolated cells from spleen of the mice 7 day post infection were stimulated by PMA and ionomycin with Brefeldin A treatment for the last 3 hours. After surface marker staining with CD4 and CD44 antibodies, the cells were fixed and permeabilzed by BD Cytofix/Cytoperm Fixation and Permeabilization Solution kit for intracellular staining with IFN-γ antibody.
Histology and Immunofluorescence
Intestinal specimens were cut longitudinally and formed into inside-out Swiss rolls. Paraffin-embedded sections were performed after fixation in 10% Formalin solution (Fisher diagnostics). Trichrome staining and Sirius Red staining were performed by the Penn Center for Molecular Studies in Digestive and Liver Diseases. Briefly, for Sirius Red staining, hematocylin was used for nucleus staining and Sirius red solution was used for eosinophil staining. Trichrome staining was used for staining muscle, cytoplasm, and collagen. For immunofluorescence staining, antigen retrieval of paraffin slides was performed in boiled 0.01 M Sodium citrate buffer (pH 6.0) followed by deparaffinization and rehydration. Primary staining with α-CD3 (ab5690, Abcam) and α-IL-4 (BVD4-1D11, Abcam) was performed overnight at 4 °C after blocking specimens with 5% host serum of secondary antibodies. Specimens were examined using a fluorescence microscope (Olympus) after staining with fluorochrome-conjugated secondary antibodies including Alexa Fluor 488 α-rat IgG (A21208, Invitrogen) and Alexa Fluor 568 α-rabbit IgG (A10042, Invitrogen), and then mounting.
Antibiotic Treatment
Full-spectrum antibiotic treatment was supplied for 2–6 weeks in drinking water containing ampicillin (1 g/L, Cellgro), vancomycin (0.5 g/L, Calbiochem), neomycin sulfate (1 g/L, Calbiochem), and metronidazole (1 g/L, Santa Cruz Biotechnology). The antibiotic solution was refreshed every 4 days.
In Vitro Treg cell Conversion
5×104 Naive CD4 OT-II cells isolated by FACS Aria cell sorter (BD) and 5×103 LpDCs were mixed in a 96-well round bottom plate with 1 µM OVA 323–332 and 1 ng/ml TGF-β. After 4 days, cells were counted, fixed and permeabilized with Fixation/Permeabilization reagent (eBioscience), and stained for surface markers and Foxp3. Stained cells were analyzed on an LSRII flow cytometer (BD).
IL-2-mAb Complex Treatment
Naïve congenic OT-II (CD45.1+CD4+CD62LhighCD44lowCD25−) cells isolated with a FACS Aria cell sorter (BD) were transferred intravenously into TRAF6ΔDC or gender and age-matched control recipients (1×106 donor cells per mouse). 5 mg OVA protein (Sigma) dissolved in PBS was provided orally to recipients for 5 consecutive days starting 24 hours post-transfer. IL-2-mAb complex was prepared in PBS mixed with 1 µg IL-2 (R&D) and 5 µg anti-IL-2 mAb (clone JES6-1; gift from Charles D. Surh (Webster et. al., 2009)). The complex was injected intraperitoneally on a daily basis during the first 4 days of OVA treatment. The donor cells were harvested and analyzed from mesenteric lymph nodes on day 6.
In Vivo Treg cell Conversion
Congenic OT-II naïve (CD45.1+CD4+CD62LhighCD44lowCD25−) cells isolated with a FACS Aria cell sorter (BD) were transferred intravenously into TRAF6ΔDC or gender-matched littermate control recipients (1×106 donor cells per mouse). 5 mg OVA protein (Sigma) dissolved in PBS was orally provided to recipients for 5 days in a row from the following day of transfer. Cells were harvested from mesenteric lymph nodes and analyzed on day 6.
Adoptive Transfer of Treg Cells
Single cells were isolated from spleen and lymph nodes of Foxp3-GFP mice or STAT5b transgenic Foxp3-GFP mice. CD4, GFP double-positive cells were collected with a FACS Aria cell sorter (BD), and 0.5–1×106 cells injected intravenously to each recipient. Mice were sacrificed and analyzed after 4 weeks.
Statistics
Data were analyzed using Prism software (GraphPad) with an unpaired or paired Student’s t-test. P values less than 0.05 were considered significant.
Supplementary Material
HIGHLIGHTS.
Dendritic cell (DC)-expressed TRAF6 is critical for small intestine immune tolerance.
Spontaneous gut Th2 cell responses develop in the absence of DC-expressed TRAF6.
Gut microbiota trigger small intestine autoimmunity absent DC-expressed TRAF6.
DC-expressed TRAF6 controls IL-2-associated iTreg cell induction in small intestine.
ACKNOWLEDGEMENTS
The authors wish to thank Eddy Chen and Jang Eun Lee for helpful discussion. This work was supported in part by the World Class University (WCU) program, NRF, MEST, Korea (R31-10105 to D.H. and Y.C), by the Korea Institute of Oriental Medicine (KIOM), MEST, Korea (No. K13050 to Y.C.), and by the National Institutes of Health (AI064909 to Y.C., AI43620 to Y.C. and L.A.T., AI039368 to G.D.W., AI079724, AI083022, and AI095740 to H.S., and AI037691 to L.A.T.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agace WW, Persson EK. How vitamin A metabolizing dendritic cells are generated in the gut mucosa. Trends Immunol. 2012;33:42–48. doi: 10.1016/j.it.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard C, Rothenberg ME. Biology of the eosinophil. Adv Immunol. 2009;101:81–121. doi: 10.1016/S0065-2776(08)01003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand S. Crohn’s disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn’s disease. Gut. 2009;58:1152–1167. doi: 10.1136/gut.2008.163667. [DOI] [PubMed] [Google Scholar]
- Burchill MA, Goetz CA, Prlic M, O’Neil JJ, Harmon IR, Bensinger SJ, Turka LA, Brennan P, Jameson SC, Farrar MA. Distinct effects of STAT5 activation on CD4+ and CD8+ T cell homeostasis: development of CD4+CD25+ regulatory T cells versus CD8+ memory T cells. J Immunol. 2003;171:5853–5864. doi: 10.4049/jimmunol.171.11.5853. [DOI] [PubMed] [Google Scholar]
- Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J Exp Med. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SH, Dong C. Signaling of interleukin-17 family cytokines in immunity and inflammation. Cell Signal. 2011;23:1069–1075. doi: 10.1016/j.cellsig.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiffoleau E, Kobayashi T, Walsh MC, King CG, Walsh PT, Hancock WW, Choi Y, Turka LA. TNF receptor-associated factor 6 deficiency during hemopoiesis induces Th2-polarized inflammatory disease. J Immunol. 2003;171:5751–5759. doi: 10.4049/jimmunol.171.11.5751. [DOI] [PubMed] [Google Scholar]
- Deng L, Wang C, Spencer E, Yang L, Braun A, You J, Slaughter C, Pickart C, Chen ZJ. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103:351–361. doi: 10.1016/s0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- Fritz T, Niederreiter L, Adolph T, Blumberg RS, Kaser A. Crohn’s disease: NOD2, autophagy and ER stress converge. Gut. 2011;60:1580–1588. doi: 10.1136/gut.2009.206466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granucci F, Vizzardelli C, Pavelka N, Feau S, Persico M, Virzi E, Rescigno M, Moro G, Ricciardi-Castagnoli P. Inducible IL-2 production by dendritic cells revealed by global gene expression analysis. Nat Immunol. 2001;2:882–888. doi: 10.1038/ni0901-882. [DOI] [PubMed] [Google Scholar]
- Hammer GE, Turer EE, Taylor KE, Fang CJ, Advincula R, Oshima S, Barrera J, Huang EJ, Hou B, Malynn BA, et al. Expression of A20 by dendritic cells preserves immune homeostasis and prevents colitis and spondyloarthritis. Nat Immunol. 2011;12:1184–1193. doi: 10.1038/ni.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou B, Reizis B, DeFranco AL. Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity. 2008;29:272–282. doi: 10.1016/j.immuni.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MA, Muskens F, Hoogsteden HC, Luttmann W, Ferrari D, Di Virgilio F, et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med. 2007;13:913–919. doi: 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- Inoue J, Gohda J, Akiyama T. Characteristics and biological functions of TRAF6. Adv Exp Med Biol. 2007;597:72–79. doi: 10.1007/978-0-387-70630-6_6. [DOI] [PubMed] [Google Scholar]
- Kel JM, Girard-Madoux MJ, Reizis B, Clausen BE. TGF-beta is required to maintain the pool of immature Langerhans cells in the epidermis. J Immunol. 2010;185:3248–3255. doi: 10.4049/jimmunol.1000981. [DOI] [PubMed] [Google Scholar]
- King CG, Kobayashi T, Cejas PJ, Kim T, Yoon K, Kim GK, Chiffoleau E, Hickman SP, Walsh PT, Turka LA, Choi Y. TRAF6 is a T cell-intrinsic negative regulator required for the maintenance of immune homeostasis. Nat Med. 2006;12:1088–1092. doi: 10.1038/nm1449. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Walsh PT, Walsh MC, Speirs KM, Chiffoleau E, King CG, Hancock WW, Caamano JH, Hunter CA, Scott P, et al. TRAF6 is a critical factor for dendritic cell maturation and development. Immunity. 2003;19:353–363. doi: 10.1016/s1074-7613(03)00230-9. [DOI] [PubMed] [Google Scholar]
- Kool M, van Loo G, Waelput W, De Prijck S, Muskens F, Sze M, van Praet J, Branco-Madeira F, Janssens S, Reizis B, et al. The ubiquitin-editing protein A20 prevents dendritic cell activation, recognition of apoptotic cells, and systemic autoimmunity. Immunity. 2011;35:82–96. doi: 10.1016/j.immuni.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Kulhankova K, Rouse T, Nasr ME, Field EH. Dendritic Cells Control CD4(+)CD25(+) Treg Cell Suppressor Function In Vitro through Juxtacrine Delivery of IL-2. PLoS One. 2012;7:43609. doi: 10.1371/journal.pone.0043609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landstrom M. The TAK1-TRAF6 signalling pathway. Int J Biochem Cell Biol. 2010;42:585–589. doi: 10.1016/j.biocel.2009.12.023. [DOI] [PubMed] [Google Scholar]
- Lucendo AJ. Eosinophilic diseases of the gastrointestinal tract. Scand J Gastroenterol. 2010;45:1013–1021. doi: 10.3109/00365521003690251. [DOI] [PubMed] [Google Scholar]
- Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity. 2010;33:153–165. doi: 10.1016/j.immuni.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- Manicassamy S, Pulendran B. Dendritic cell control of tolerogenic responses. Immunol Rev. 2011;241:206–227. doi: 10.1111/j.1600-065X.2011.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masterson JC, Furuta GT, Lee JJ. Update on clinical and immunological features of eosinophilic gastrointestinal diseases. Curr Opin Gastroenterol. 2011 doi: 10.1097/MOG.0b013e32834b314c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKarns SC, Schwartz RH. Biphasic regulation of Il2 transcription in CD4+ T cells: roles for TNF-alpha receptor signaling and chromatin structure. J Immunol. 2008;181:1272–1281. doi: 10.4049/jimmunol.181.2.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naramura M, Hu RJ, Gu H. Mice with a fluorescent marker for interleukin 2 gene activation. Immunity. 1998;9:209–216. doi: 10.1016/s1074-7613(00)80603-2. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Tang H, Manicassamy S. Programming dendritic cells to induce T(H)2 and tolerogenic responses. Nat Immunol. 2010;11:647–655. doi: 10.1038/ni.1894. [DOI] [PubMed] [Google Scholar]
- Rescigno M. Intestinal dendritic cells. Adv Immunol. 2010;107:109–138. doi: 10.1016/B978-0-12-381300-8.00004-6. [DOI] [PubMed] [Google Scholar]
- Rescigno M. Dendritic cells in oral tolerance in the gut. Cell Microbiol. 2011;13:1312–1318. doi: 10.1111/j.1462-5822.2011.01626.x. [DOI] [PubMed] [Google Scholar]
- Santaolalla R, Fukata M, Abreu MT. Innate immunity in the small intestine. Curr Opin Gastroenterol. 2011;27:125–131. doi: 10.1097/MOG.0b013e3283438dea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgouroudis E, Kornete M, Piccirillo CA. IL-2 production by dendritic cells promotes Foxp3(+) regulatory T-cell expansion in autoimmuneresistant NOD congenic mice. Autoimmunity. 2011;44:406–414. doi: 10.3109/08916934.2010.536795. [DOI] [PubMed] [Google Scholar]
- Shembade N, Ma A, Harhaj EW. Inhibition of NF-kappaB signaling by A20 through disruption of ubiquitin enzyme complexes. Science. 2010;327:1135–1139. doi: 10.1126/science.1182364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino A, Thakur N, Grimsby S, Marcusson A, von Bulow V, Schuster N, Zhang S, Heldin CH, Landstrom M. The type I TGF-beta receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat Cell Biol. 2008;10:1199–1207. doi: 10.1038/ncb1780. [DOI] [PubMed] [Google Scholar]
- Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- Swiatczak B, Rescigno M. How the interplay between antigen presenting cells and microbiota tunes host immune responses in the gut. Semin Immunol. 2012;24:43–49. doi: 10.1016/j.smim.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, Wang Y, Bernstein X, Huang X, Reichardt LF, et al. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MC, Kim N, Kadono Y, Rho J, Lee SY, Lorenzo J, Choi Y. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol. 2006;24:33–63. doi: 10.1146/annurev.immunol.24.021605.090646. [DOI] [PubMed] [Google Scholar]
- Wang FL, Qin WJ, Wen WH, Tian F, Song B, Zhang Q, Lee C, Zhong WD, Guo YL, Wang H. TGF-beta insensitive dendritic cells: an efficient vaccine for murine prostate cancer. Cancer Immunol Immunother. 2007;56:1785–1793. doi: 10.1007/s00262-007-0322-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.