Abstract
The APOE ε4 allele correlates with increased risk of Alzheimer disease (AD) and increased parenchymal amyloid plaques. We tested how the APOE genotype correlated with cerebral amyloid angiopathy (CAA) by analyzing 371 brains for parenchymal and meningeal CAA in 4 brain regions (frontal, parietal, temporal, and occipital neocortex). The overall severity of CAA was highest in the occipital lobe. APOE-ε4/4 brains (n = 22) had the highest levels of CAA across regions. In the occipital lobe, nearly all APOE-ε4/4 cases were scored with the highest level of CAA (meninges, 95% of cases; parenchyma, 81%). In this brain region as in others, APOE-ε3/4 brains (n = 115) showed consistently less CAA that APOE-ε4/4 brains (meninges, 43%; parenchyma, 43%). APOE-ε3/3 brains (n = 182) showed even less CAA (meninges, 19%; parenchyma, 19%). Interestingly, APOE-ε2/3 cases (n = 42) had more CAA than APOE-ε3/3 (meninges, 44%; parenchyma, 32%), despite a reduced risk for AD in the APOE-ε2/3 individuals. APOE-ε4/4 brains also had the fewest regions without CAA, whereas APOE-ε3/3 brains had the most. Ordinal regression analyses demonstrated significant impacts of APOE-ε2 and APOE-ε4 on CAA in at least some brain region. These data demonstrate that APOE genotype correlations with Ab deposition in CAA only incompletely correspond to other AD-linked brain pathologies.
Keywords: Alzheimer disease, Apolipoprotein, CAA, Dementia, Hemorrhagic stroke, Risk factor
INTRODUCTION
Alzheimer disease (AD) is the most prevalent form of dementia in aged populations. AD brains demonstrate neurofibrillary tangles and amyloid plaques (1), and in most cognitively impaired individuals there are comorbid neuropathological changes such as microinfarcts that could also contribute to cognitive impairment (2). Identifying risk factors and pinpointing the molecular mechanisms behind cognitive decline is critical to develop approaches to diagnose the dementia early and develop treatments. Cerebral amyloid angiopathy (CAA) is a common pathological feature in the brains of the elderly and patients with AD (3, 4) resulting from deposition of the Ab protein in vessel walls of small arteries and capillaries of the leptomeninges and brain parenchyma. CAA is also an important cause of intracerebral hemorrhage in the elderly (4).
The risk of late-onset AD is affected by many factors, with APOE alleles the strongest genetic factor (5). The APOE-ε4 allele is associated with increased risk and the APOE-ε2 allele is associated with decreased risk, compared to the APOE-ε3 allele (5, 6). The correlations of APOE genotypes with clinical AD mirror their effects on levels of Ab deposition in plaques (7, 8). CAA is observed in a large proportion of AD brains (9), and APOE-ε4 affects the incidence of CAA in AD brains independent of its effects on AD risk (10).
Here, we analyzed the associations between APOE genotype and CAA pathology in a large neuropathological data set including semiquantitative assessment of CAA in both the parenchyma and the meninges of various brain areas. We found that either inheritance of APOE-ε4 or APOE-ε2 increased the incidence of amyloid in the parenchymal or meningeal cerebrovasculature.
MATERIALS AND METHODS
Patients
371 autopsied brain samples were collected as part of ongoing studies of normal aging and AD at the University of Kentucky, Sanders Brown Center on Aging, Lexington, KY (Table 1). These brains span the spectrum of cases with minimal pathological changes to those with AD, with many cases of mixed pathological changes, including dementia with Lewy bodies, cerebrovascular disease, and hippocampal sclerosis, as is usual for any cohort of aged brains. APOE genotypes were determined by polymerase chain reaction, as previously described (11). The distribution of APOE genotypes was as follows: APOE-ε2/2 (n = 2), APOE-ε2/3 (n = 42), APOE-ε3/3 (182), APOE-ε2/4 (n = 8), APOE-ε3/4 (n = 115), and APOE-ε4/4 (n = 22). Distribution of sexes and average ages at death for each group are listed in Table 1.
Table 1. Characteristics of Brain Samples Analyzed, Separated by APOE Genotype.
| APOE | n | % Males | Average Age (y ± sd) |
Average Braak stage |
Average CERAD score |
|---|---|---|---|---|---|
| ε2/2 | 2 | 50 | 91.0 ± 8.9 | 2.5 | 0.50 |
| ε2/3 | 42 | 57 | 86.5 ± 8.3 | 2.6 | 1.65 |
| ε2/4 | 8 | 62 | 85.5 ± 5.0 | 3.9 | 1.73 |
| ε3/3 | 182 | 64 | 84.4 ± 8.5 | 3.1 | 1.74 |
| ε3/4 | 115 | 53 | 82.4 ± 8.3 | 4.1 | 2.20 |
| ε4/4 | 22 | 59 | 84.9 ± 8.4 | 5.4 | 1.76 |
CERAD, Consortium to Establish a Registry for Alzheimer Disease.
Neuropathological Evaluation
Specimens for histologic evaluation included samples from both cortical parenchyma and meninges of 4 neocortical regions (i.e. frontal, parietal, occipital, and superior/mid-temporal cortex, corresponding Brodmann areas 9, 39/40, 17/18, and 21/22), as described (12). Briefly, tissue blocks were cut from the left cerebral hemisphere at the time of autopsy and fixed in 4% formaldehyde. Eight-μm-thick sections were stained with hematoxylin and eosin and the modified Bielschowsky method. Gallyas stain was used for sections of the entorhinal cortex, hippocampus, and amygdala. Sections of neocortex were stained with 10D5 amyloid-β (Aβ) antibody (Novocastra, Newcastle, UK), which was raised against the N-terminal portion of Ab and recognizes amino acids 3-7 (13). Braak staging (14) and CERAD plaque scores (15) were used to determine NIA-Reagan diagnosis of pathological AD (Table 1). All pathological diagnoses were made blinded to clinical information.
CAA Scale
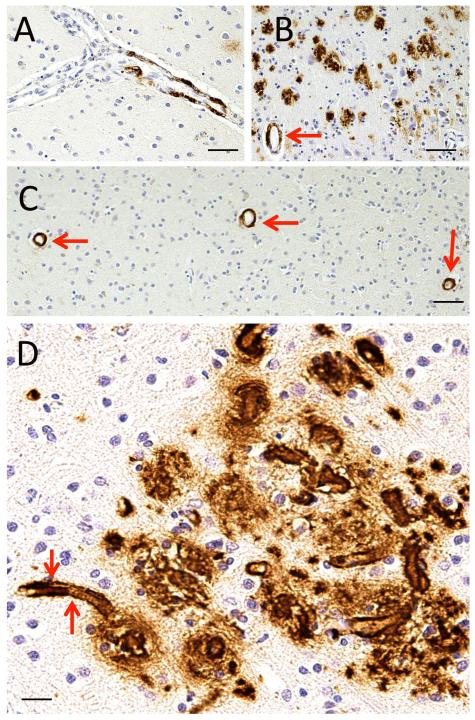
We defined 4 stages of cerebrovascular amyloid deposition based on Ab immunostaining in the leptomeninges and in the brain parenchyma, (Fig. 1, 2). This staging system was based on an overall impression of a given Ab-immunostained slide rather than a count-based method. “Stage 0” CAA is the absence of any Ab immunostaining. “Stage 1” CAA is defined as a focal aggregation of Ab immunopositivity in an isolated blood vessel (Figs. 1A, 2A). “Stage 2” CAA is defined as scattered Ab immunopositivity of intermediate severity (Figs. 1B, 2B). “Stage 3” CAA is defined as relatively strong uniform Ab immunostaining of vessels (Figs. 1C, 2C). Many cases with “Stage 3” CAA show strong Ab immunopositivity that outlines small blood vessels, including capillaries (Fig. 2D). The assessment of CAA indicates an estimate of overall severity rather than an individual vessel (16, 17).
Figure 1.
Amyloid-β (Aβ) immunohistochemistry demonstrating cerebral amyloid angiopathy (CAA) severity scale in meningeal blood vessels. (A) “Stage 1” CAA in a meningeal vein with a focal aggregation of Ab. (B) “Stage 2” CAA in meningeal veins. In this photomicrograph the subarachnoid space is a “V”-shaped wedge in the middle; some leptomeningeal blood vessels show patchy CAA (red arrow) but others are unstained (green arrow). (C) “Stage 3” CAA in meningeal vessels. In the area between the red arrows the Ab immunohistochemistry in the blood vessel walls is consistently dark and nearly uniformly distributed. Scale bars: A, C, 200 mm; B, 50 mm.
Figure 2.
Amyloid-β (Aβ) immunohistochemistry demonstrating cerebral amyloid angiopathy (CAA) severity scale in parenchymal blood vessels. (A) CAA in a narrow artery with a focal aggregation of Ab immunopositivity. A section in which this pattern predominates was designated “Stage 1” CAA. (B) A case in which there are many parenchymal Ab plaques but there is only scattered CAA of intermediate severity (arrow), which would be designated “Stage 2” CAA. (C) A section with minimal parenchymal Ab plaques but relatively strong uniform Ab immunostaining of vessels (arrows); this would still be “Stage 3” CAA. (D) Many cases with “Stage 3” CAA showed strong Ab immunopositivity outlining small blood vessels, including capillaries (arrows). Scale bars: A, C, = 200 mm; B, 50 mm; D, 40 mm.
Statistical Analyses
Ordinal Logistic Regression was conducted on the data set. Ordinal outcomes rated as 0, 1, 2, 3 (least to most severe) from each of parenchymal and meningeal CAA in the 4 brain subregions were regressed on gender, age at death, Braak stage (low–I/II [reference category], medium–III/IV and high–V/VI) and APOE genotype (3/3 [reference category], 2/3, 3/4, 4/4). Subjects with APOE genotypes of 2/2 and 2/4 were not considered in this analysis. Briefly, a proportional odds logistic regression, or cumulative link, model (18) was employed to relate the cumulative outcome probabilities to the linear predictors. For example, parenchymal CAA in the frontal (Y) is modeled using:
where j = 0,1,2, logit(x) = log(x/(1-x)) and the covariates are gender, age at death, Braak stage and APOE genotype.
RESULTS
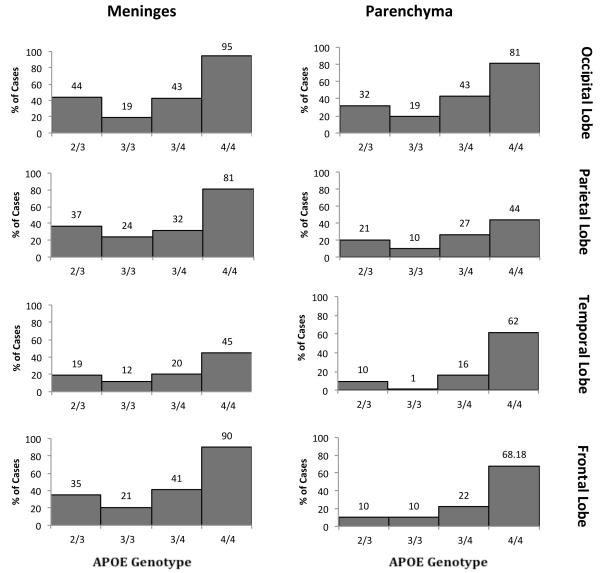
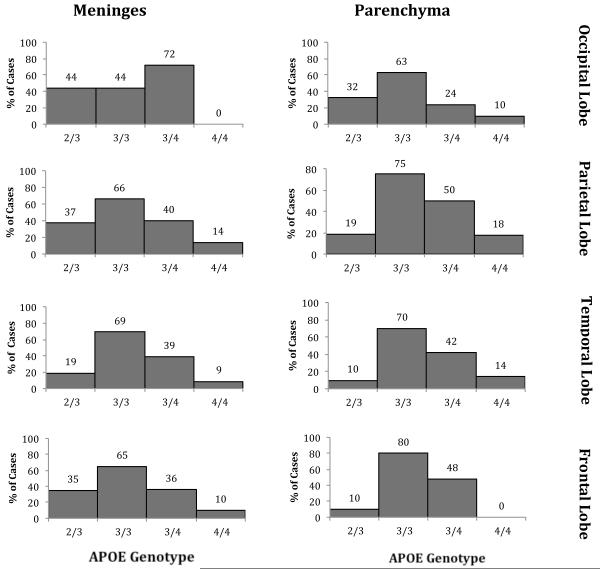
We first compared the frequency of the extremes of CAA burden as a function of APOE genotype (Figs. 3, 4). Among the various brain regions in the 182 (most common) APOE-ε3/3 cases, 62% to 79% of brains showed no parenchymal CAA in the various brain regions; 43% to 68% of brains showed no meningeal CAA. At the other extreme, 9% to 19% of brains showed the highest level of parenchymal CAA, and 21% to 41% of brains showed the highest level of meningeal CAA (Figs. 3, 4). Across brain regions there was consistently higher burden of CAA in the meninges compared to the parenchyma. There were also consistently greater levels of CAA in the occipital lobe (19% and 41% scores of CAA 3 in the parenchyma and meninges, respectively) compared to the other brain regions examined (frontal: 9% and 21%; parietal: 12% and 23%; temporal: 11% and 23%). Thus, CAA was common in autopsy samples, with higher levels in the meninges compared to the parenchyma and in the occipital lobe compared to other brain regions.
Figure 3.
Percentage of cases with highest levels of cerebral amyloid angiopathy (CAA) by APOE genotype. The frequency of the highest level of CAA pathology (CAA score of 3) in the meninges and parenchyma of the frontal, occipital, parietal, and temporal lobes in individuals with each of the more common APOE genotypes (ε2/3, ε3/3, ε3/4, and ε4/4).
Figure 4.
Percentage of cases without cerebral amyloid angiopathy (CAA) by APOE genotype. The frequency of cases showing a lack of observed CAA pathology (CAA score of 0) in the meninges and parenchyma of the frontal, occipital, parietal, and temporal lobes for individuals with each of the more common APOE genotypes (ε2/3, ε3/3, ε3/4, and ε4/4).
We compared these results to those of other APOE genotypes, focusing on the more common genotypes observed i.e. APOE ε4/4, ε3/4 and ε2/3. Among APOE genotypes, APOE-ε4/4 brains showed the highest CAA scores in every brain region in both the meninges and parenchyma. Interestingly, both APOE-ε3/4 and APOE-ε2/3 cases showed CAA levels intermediate between APOE-ε3/3 and APOE-ε4/4 cases. For example, the percentages of cases with the highest levels of CAA in the occipital lobe parenchyma were APOE-ε3/3 19%, APOE-ε2/3 31%, APOE-ε3/4 42%, and APOE-ε4/4 77% (Fig. 4). Similarly, the percentages of cases with the highest levels of CAA in the occipital lobe meninges were APOE-ε3/3, 41%; APOE-ε2/3, 43%; APOE-ε3/4, 66% and APOE-ε4/4, 91% (Fig. 4).
Analysis of the frequency of cases with no apparent CAA pathology showed the same relationship of CAA to APOE genotype (Fig. 4). In nearly all areas, the highest frequency of the cases without CAA was in the APOE ε3/3 group; the lowest frequency of cases without CAA was the APOE ε4/4 genotype. Individuals of the APOE-ε3/4 and APOE-ε2/3 genotypes were again intermediate between APOE-ε3/3 and APOE-ε4/4 (Fig. 3). For example, the percentages of cases without CAA in the occipital lobe parenchyma were APOE-ε3/3, 62%; APOE-ε2/3, 48%; APOE-ε3/4, 23% and APOE-ε4/4, 9% (Fig. 3). Similarly, the percentages of cases without CAA in the occipital lobe meninges were APOE-ε3/3, 43%; APOE-ε2/3. 33%; APOE-ε3/4, 15%; and APOE-ε4/4, 0% (Fig. 3).
To test whether these apparent correlations between APOE genotype and CAA were significant, and to determine whether other measured variables affected the incidence and severity of CAA, we conducted ordinal regression analysis (Table 2). We used CAA data from each cerebral cortical lobe with measures from both the parenchyma and the meninges. In addition to APOE genotype, we tested whether CAA score was associated with age at death, Braak stages of neurofibrillary tangle distribution (0-II, III-IV, and V-VI), and sex. This analysis allowed us to determine whether each of the variables we studied had independent effects on CAA, controlling for the effects of the other variables.
Table 2. p Values of Ordinal Logistic Regression Analyses.
| Parenchyma | Meninges | |||||||
|---|---|---|---|---|---|---|---|---|
| Frontal | Temporal | Parietal | Occipital | Frontal | Temporal | Parietal | Occipital | |
| APOE ε2/3* | 0.05 | 0.45 | 0.10 | 0.05 | 0.05 | 0.18 | 0.18 | 0.40 |
| APOE 3/4* | 0.001 | 0.05 | 0.003 | 0.001 | 0.001 | 0.001 | 0.05 | 0.001 |
| APOE 4/4* | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.02 |
| Braak Stage | ||||||||
| V/VI# | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| Braak Stage | ||||||||
| III/IV# | 0.005 | 0.004 | 0.10 | 0.03 | 0.02 | 0.38 | 0.11 | 0.10 |
| Age | 0.15 | 0.38 | 0.08 | 0.27 | 0.06 | 0.03 | 0.07 | 0.01 |
| Sex | 0.06 | 0.002 | 0.02 | 0.07 | 0.31 | 0.03 | 0.14 | 0.37 |
Actual p values are below the numbers listed in the table.
Compared to samples of the APOE ε3/3 genotype.
Compared to samples of the Braak 0/I/II designations.
CAA scores across brain regions were consistently affected by Braak staging and APOE genotypes. Brains with the highest Braak levels (V or VI) had significantly more CAA than the brains with lowest Braak levels (0-II) in all brain regions. Brains with intermediate Braak scores (III-IV) also generally had significantly more CAA, although not in all brain regions (e.g. the meninges in the temporal and parietal lobes). APOE-ε4 was significantly correlated with CAA score in all 8 regions, in both APOE-ε3/4 and APOE-ε4/4 individuals compared to APOE-ε3/3 cases (Table 2). APOE-ε2/3 brains had significantly higher CAA scores compared to APOE-ε3/3 brains in 3 regions (frontal parenchyma, frontal meninges, and occipital parenchyma).
The correlations between CAA score and age at death or sex were more complex (Table 2). Age was significantly correlated with increased odds of higher CAA score in the meninges in 2 brain regions and showed trends (p < 0.07) towards significant correlations in the other 2 regions; however, age was not significantly associated with CAA score in any of the parenchymal regions. In contrast, sex had a stronger correlation with parenchymal CAA. Males showed significantly higher odds of having higher CAA scores in the temporal and parenchymal lobes, with trends (p < 0.07) to significance in frontal and occipital lobes.
DISCUSSION
In this study, analysis of CAA in a large number of brains with varying pathological involvement confirmed that inheritance of APOE-ε4 strongly increased the frequency of CAA. For example, 81% and 95% of individuals with the APOE-ε4/4 genotype had severe CAA in the parenchyma and meninges of the occipital lobe, respectively; between 44% and 81% of these individuals also had severe CAA in other brain regions. In comparison, 43% of the individuals with the APOE-ε3/4 genotype had severe CAA in the occipital parenchyma and 43% in the occipital meninges. Even fewer of the individuals with the APOE-ε3/3 genotype had severe CAA in these regions (19% for each). These findings are consistent with pathological and clinical observations that APOE-ε4 is associated with increased risk of CAA and CAA-related hemorrhages (7, 10, 19, 20).
The effect of APOE-ε2 on CAA pathology was surprising. Overall, the incidence of CAA in APOE-ε2/ε3 individuals was similar to that seen in APOE-ε3/4 individuals. There were fewer APOE-ε2/ε3 cases (n = 42) than APOE-ε3/4 cases (n = 115), which limited our statistical power. The significant effect of APOE-ε2 on CAA persisted when we statistically controlled for the slight association of APOE-ε2 with older age (Tables 1, 2). APOE-ε2 is associated with decreased risk of AD (5), and decreased brain amyloid levels in both humans (21, 22) and in animal models of AD (23). We observed that APOE-ε2 brains showed lower average Braak scores although the average CERAD plaque scores were similar across APOE genotypes (Table 1). We also observed that brains exhibiting lower Braak scores showed statistically lower levels of CAA overall (Table 2), again making the association of APOE-ε2 with higher levels of CAA surprising.
Inheritance of APOE-ε2 has previously been linked clinically to a greater risk of CAA-related hemorrhage (24-26), and our findings support those data. The earlier pathological and clinical analyses suggested that the effect of APOE-ε2 was on increased risk of the breakage of amyloid-affected vessels due to fibrinoid necrosis (24, 27). However, the present study measured only amyloid associated vessels and not whether amyloid-laden vessels showed signs of vessel breakage. Our data suggest that the APOE-ε2 promotes the accumulation of Ab in the cerebral vasculature; this higher level of amyloid could account for the increased risk of hemorrhages observed clinically in APOE-ε2 positive individuals.
The presence of CAA is a major consideration in potential approaches for the removal of amyloid as a treatment for AD. For example, the clinical trial of active Ab immunotherapy was halted due to cerebral inflammation, likely caused by activated T cells around cerebral vessels with CAA (28, 29). Removal of amyloid in blood vessels could also sensitize a brain to hemorrhage (30), as was observed in passive immunotherapy in mice (31). Analysis of APOE genotype is now routinely used in clinical trials to evaluate whether individuals expressing APOE-ε4 are more responsive to treatment or are more susceptible to adverse side effects (32). We would suggest that APOE-ε2 individuals might also show differences related to clearance of Ab from the brain and risks of CAA-related hemorrhages.
Strategies for clearance of Ab from the brain to the periphery could lead to amyloid accumulation in the vasculature if Ab is not cleared efficiently from the parenchyma (33). APOE4 knock-in mice have an increased incidence of CAA (34, 35) and amyloid-associated intracerebral hemorrhages (36), suggesting that apoE4 does not efficiently promote clearance of Ab into the vasculature. Other support for this clearance hypothesis is found in studies of mutant Ab species (E22Q “Dutch” and D23N “Iowa” Ab mutations) linked to strong CAA with less parenchymal Ab (37, 38). In vitro, these versions of Ab show increased aggregation on the surface of smooth muscle cells that line some of the cerebrovasculature (39), suggesting that Ab may be blocked in clearance at that level. The higher levels of CAA in APOE-ε2 and APOE-ε4 brains suggest that both apoE4 and apoE2 may cause deficits in the clearance of Ab. In mice expressing Dutch and Iowa mutant Ab, the expression of any of the 3 human apoE isoforms had similar effects: reduced Ab in the microvasculature and increased parenchymal Ab (40, 41). Human apoE seems to have less ability to clear Ab from the brain compared to mouse Ab (42), and in these models, Ab clearance may be so impaired that it is not even moved from the parenchyma to the vasculature.
Overall, our analysis of 371 brains demonstrates that APOE-ε2 and APOE-ε4 both potentiate the accumulation of Ab in parenchymal and meningeal blood vessels. These genetic associations suggest that individuals with either of these APOE alleles may show complications in clinical trials based on clearance of Ab.
Acknowledgments
G. William Rebeck and Steven Estus were supported by NIH P01 AG030128. Peter T. Nelson was supported by NIH P30 AG028383.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Schellenberg GD, Montine TJ. The genetics and neuropathology of Alzheimer’s disease. Acta Neuropathol. 2012;124:305–23. doi: 10.1007/s00401-012-0996-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arvanitakis Z, Leurgans SE, Barnes LL, et al. Microinfarct pathology, dementia, and cognitive systems. Stroke. 2011;42:722–7. doi: 10.1161/STROKEAHA.110.595082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arvanitakis Z, Leurgans SE, Wang Z, et al. Cerebral amyloid angiopathy pathology and cognitive domains in older persons. Ann Neurol. 2011;69:320–7. doi: 10.1002/ana.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viswanathan A, Greenberg SM. Cerebral amyloid angiopathy in the elderly. Ann Neurol. 2011;70:871–80. doi: 10.1002/ana.22516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertram L, McQueen MB, Mullin K, et al. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 6.Corder EH, Saunders AM, Strittmatter WJ, et al. Science. 1993;261:921–3. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 7.Schmechel DE, Saunders AM, Strittmatter WJ, et al. Proc Natl Acad Sci U S A. 1993;90:9649–53. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rebeck GW, Reiter JS, Strickland DK, et al. Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions. Neuron. 1993;11:575–80. doi: 10.1016/0896-6273(93)90070-8. [DOI] [PubMed] [Google Scholar]
- 9.Vonsattel JP, Myers RH, Whyte ET, et al. Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann Neurol. 1991;30:637–49. doi: 10.1002/ana.410300503. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg SM, Rebeck GW, Vonsattel JP, et al. Apolipoprotein E epsilon 4 and cerebral hemorrhage associated with amyloid angiopathy. Ann Neurol. 1995;38:254–9. doi: 10.1002/ana.410380219. [DOI] [PubMed] [Google Scholar]
- 11.Pulliam JF, Jennings CD, Kryscio RJ, et al. Association of HFE mutations with neurodegeneration and oxidative stress in Alzheimer’s disease and correlation with APOE. Am J Med Genet B Neuropsychiatr Genet. 2003;119B:48–53. doi: 10.1002/ajmg.b.10069. [DOI] [PubMed] [Google Scholar]
- 12.Nelson PT, Jicha GA, Schmitt FA, et al. Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J Neuropathol Exp Neurol. 2007;66:1136–46. doi: 10.1097/nen.0b013e31815c5efb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bard F, Barbour R, Cannon C, et al. Epitope and isotype specificities of antibodies to beta -amyloid peptide for protection against Alzheimer’s disease-like neuropathology. Proc Natl Acad Sci U S A. 2003;100:2023, 8. doi: 10.1073/pnas.0436286100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braak H, Braak E. Diagnostic criteria for neuropathologic assessment of Alzheimer’s disease. Neurobiol Aging. 1997;18:S85–8. doi: 10.1016/s0197-4580(97)00062-6. [DOI] [PubMed] [Google Scholar]
- 15.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 16.Nelson PT, Abner EL, Schmitt FA, et al. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol. 2010;20:66–79. doi: 10.1111/j.1750-3639.2008.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sudduth TL, Schmitt FA, Nelson, et al. Neurobiol Aging. 2013;34:1051–9. doi: 10.1016/j.neurobiolaging.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agresti A. An introduction to categorical data analysis. John Wiley & Sons; New Jersey: 2007. [Google Scholar]
- 19.Premkumar DR, Cohen DL, Hedera P, et al. Apolipoprotein E-epsilon4 alleles in cerebral amyloid angiopathy and cerebrovascular pathology associated with Alzheimer’s disease. Am J Pathol. 1996;148:2083–95. [PMC free article] [PubMed] [Google Scholar]
- 20.Olichney JM, Hansen LA, Galasko D, et al. The apolipoprotein E epsilon 4 allele is associated with increased neuritic plaques and cerebral amyloid angiopathy in Alzheimer’s disease and Lewy body variant. Neurology. 1996;47:190–6. doi: 10.1212/wnl.47.1.190. [DOI] [PubMed] [Google Scholar]
- 21.Nagy Z, Esiri MM, Jobst KA, et al. Influence of the apolipoprotein E genotype on amyloid deposition and neurofibrillary tangle formation in Alzheimer’s disease. Neuroscience. 1995;69:757–61. doi: 10.1016/0306-4522(95)00331-c. [DOI] [PubMed] [Google Scholar]
- 22.Pirttila T, Soininen H, Mehta PD, et al. Apolipoprotein E genotype and amyloid load in Alzheimer disease and control brains. Neurobiol Aging. 1997;18:121–7. doi: 10.1016/s0197-4580(96)00204-7. [DOI] [PubMed] [Google Scholar]
- 23.Bales KR, Liu F, Wu S, et al. Human APOE isoform-dependent effects on brain beta-amyloid levels in PDAPP transgenic mice. J Neurosci. 29(67):71–9. doi: 10.1523/JNEUROSCI.0887-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenberg SM, Vonsattel JP, Segal AZ, et al. Association of apolipoprotein E epsilon2 and vasculopathy in cerebral amyloid angiopathy. Neurology. 1998;50:961–5. doi: 10.1212/wnl.50.4.961. [DOI] [PubMed] [Google Scholar]
- 25.Biffi A, Sonni A, Anderson CD, et al. Variants at APOE influence risk of deep and lobar intracerebral hemorrhage. Ann Neurol. 2010;68:934–43. doi: 10.1002/ana.22134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicoll JA, Burnett C, Love S, et al. High frequency of apolipoprotein E epsilon 2 in patients with cerebral hemorrhage due to cerebral amyloid angiopathy. Ann Neurol. 1996;39:682–3. doi: 10.1002/ana.410390521. [DOI] [PubMed] [Google Scholar]
- 27.McCarron MO, Nicoll JA, Stewart J, et al. The apolipoprotein E epsilon2 allele and the pathological features in cerebral amyloid angiopathy-related hemorrhage. J Neuropathol Exp Neurol. 1999;58:711–8. doi: 10.1097/00005072-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Nicoll JA, Wilkinson D, Holmes C, et al. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med. 2003;9:448–52. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 29.Boche D, Denham N, Holmes C. Neuropathology after active Abeta42 immunotherapy: implications for Alzheimer’s disease pathogenesis. Acta Neuropathol. 2010;120:369–84. doi: 10.1007/s00401-010-0719-5. [DOI] [PubMed] [Google Scholar]
- 30.Atwood CS, Bishop GM, Perry G, et al. Amyloid-beta: a vascular sealant that protects against hemorrhage? J Neurosci Res. 2002;70:356. doi: 10.1002/jnr.10388. [DOI] [PubMed] [Google Scholar]
- 31.Pfeifer M, Boncristiano S, Bondolfi L, et al. Cerebral hemorrhage after passive anti-Abeta immunotherapy. Science. 2002;298:1379. doi: 10.1126/science.1078259. [DOI] [PubMed] [Google Scholar]
- 32.Aisen PS. Clinical trial methodologies for disease-modifying therapeutic approaches. Neurobiol Aging. 2011;32(Suppl 1):S64–6. doi: 10.1016/j.neurobiolaging.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herzig MC, Van Nostrand WE, Jucker M. Mecchanism of cerebral beta-amyloid angiopathy: murine and cellular models. Brain Pathol. 2006;16:40–54. doi: 10.1111/j.1750-3639.2006.tb00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holtzman DM, Fagan AM, Mackey B, et al. Apolipoprotein E facilitates neuritic and cerebrovascular plaque formation in an Alzheimer’s disease model. Ann Neurol. 2000;47:739–47. [PubMed] [Google Scholar]
- 35.Fryer JD, Taylor JW, DeMattos RB. Apolipoprotein E markedly facilitates age-dependent cerebral amyloid angiopathy and spontaneous hemorrhage in amyloid precursor protein transgenic mice. J Neurosci. 2003;23:7889–96. doi: 10.1523/JNEUROSCI.23-21-07889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sullivan PM, Mace BE, Estrada JC, et al. Human apolipoprotein E4 targeted replacement mice show increased prevalence of intracerebral hemorrhage associated with vascular amyloid deposition. J Stroke Cerebrovasc Dis. 2008;17:303–11. doi: 10.1016/j.jstrokecerebrovasdis.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 37.Grabowski TJ, Cho HS, Vonsattel JP, et al. Novel amyloid precursor protein mutation in an Iowa family with dementia and severe cerebral amyloid angiopathy. Ann Neurol. 2001;49:697–705. doi: 10.1002/ana.1009. [DOI] [PubMed] [Google Scholar]
- 38.Coria F, Castano EM, Frangione B. Brain amyloid in normal aging and cerebral amyloid angiopathy is antigenically related to Alzheimer’s disease beta-protein. Am J Pathol. 1987;129:422–8. [PMC free article] [PubMed] [Google Scholar]
- 39.Van Nostrand WE, Melchor JP, Cho HS, et al. Pathogenic effects of D23N Iowa mutant amyloid beta-protein. J Biol Chem. 2001;276:32860–6. doi: 10.1074/jbc.M104135200. [DOI] [PubMed] [Google Scholar]
- 40.Xu F, Vitek MP, Colton CA, et al. Human apolipoprotein E2 promotes parenchymal amyloid deposition and neuronal loss in vasculotropic mutant amyloid-β protein Tg-SwDI mice. J Alzheimers Dis. 2012;31:359–69. doi: 10.3233/JAD-2012-120421. [DOI] [PubMed] [Google Scholar]
- 41.Xu F, Vitek MP, Colton CA, et al. Human apolipoprotein E redistributes fibrillar amyloid deposition in Tg-SwDI mice. J Neurosci. 2008;28:5312–20. doi: 10.1523/JNEUROSCI.1042-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]