Abstract
OBJECTIVES
The aim of this study was to demonstrate the feasibility and efficacy of a novel simulation software called, virtual segmentectomy.
METHODS
We developed the segmentectomy simulation system, which was programmed to analyse the detailed 3D bronchovascular structure and to predict the appropriate segmental surface and surgical margin, based on lung modelling from CT images.
RESULTS
We have attempted this novel technique for 3 cases of pulmonary metastases and 1 case of multiple lung cancer. For validation, the predicted resection margin was compared with the actual resected specimen. The surgical surface, as estimated by the simulation, was compared with the surface of the specimen and a surgical video. To test its feasibility, the operation time, blood loss, durations of chest tube placement and hospitalization as well as pathological findings were assessed.
CONCLUSIONS
Preoperative simulation and intraoperative guidance by virtual segmentectomy could contribute significantly to determining the most appropriate anatomical segmentectomy and curative resection.
Keywords: 3D computed tomography, Surgical techniques, Lung segmentectomy, Simulation, Computer applications
INTRODUCTION
New technologies can considerably improve preoperative planning, enhance the surgeon's skill, and simplify the approach to complex surgical procedures. Recently, surgical simulation based on preoperative 3D computed tomography (CT) scan has been developed in the fields of head and neck surgery, neurosurgery, orthopaedic surgery and general surgery.
Because of the increasing detection of early small lung cancer lesions due to the expansion of low-dose CT screening, sub-lobar resections of the lung, including segmentectomy and wedge resection, are becoming common procedures [1]. However, segmentectomy is a complicated operative procedure because of its anatomical complexity, including the high variability of vascular and bronchial structures and the technical difficulty in obtaining an adequate surgical margin due to tumour location and the number of tumours. There have been reports of a recently developed hepatectomy simulation software, regarding its feasibility and efficacy in the fields of both general surgery and liver transplantation [2–5]. This software was designed to analyse detailed 3D vascular structure and to predict liver resection volume and margins. We have developed a segmentectomy simulation system for thoracic called virtual segmentectomy, based on high-quality 3D lung modelling from CT images using the Fujifilm Synapse Vincent system (Fujifilm Corporation, Tokyo, Japan). In this article, we describe 2 representative cases and report the preliminary results of the preoperative and intraoperative assessment via this simulation software in 4 consecutive cases.
TECHNOLOGY
Computed tomography, 3D angiography and bronchial tree
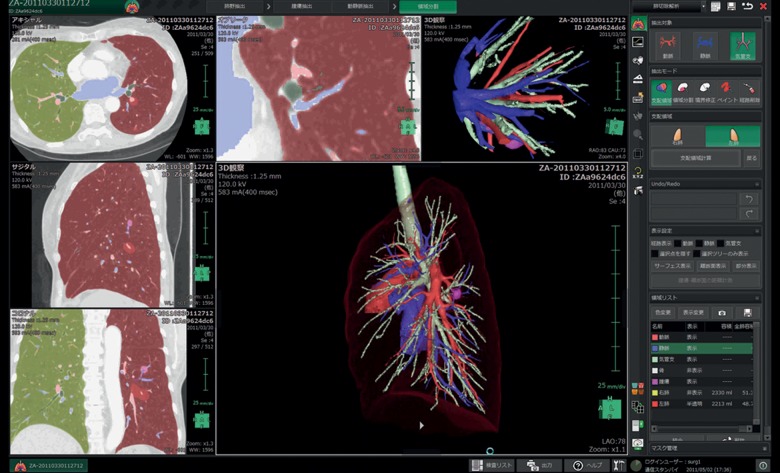
Virtual segmentectomy, surgical simulation of a segmentectomy using 3D lung modelling, was performed with a 64-channel multidetector CT (MDCT) (Light Speed VCT, General Electric Company, CT, USA). A total of 100 ml of contrast agent was injected by a mechanical injector at a rate of 1.5–2.0 ml/s. Scanning parameters used for the contrast examination were as follows: a slice thickness of 1.25 mm, a table displacement of 39.37 mm/rot, and a reconstruction interval of 1.0 mm. Using 3D volume rendering, a solid image was constructed from 1.25-mm data slices of the contrast-enhanced CT images. These digital imaging and communication in medicine data were transferred to a workstation with the Synapse Vincent volume-rendering reconstruction software, which includes a novel 3D image-processing system for the preoperative simulation of segmentectomy. The procedures for 3D reconstruction of the pulmonary vessels and bronchial trees were performed automatically, then further corrected by a thoracic surgeon, taking anatomical variability into account. These procedures took only 5–10 min (Fig. 1).
Figure 1:
Interface of the lung resection analysis function packaged in Synapse Vincent (Fujifilm).
Preoperative segmentectomy simulation, virtual segmentectomy
Image analysis for the preoperative segmentectomy simulation called virtual segmentectomy was performed using the novel 3D CT image-processing software, including a lung resection analysis function, packaged in Synapse Vincent, developed in collaboration between our department and Fujifilm Corporation. This software has been commercially available since April 2012 and is now being used routinely in our institution. This particular process consisted of the following steps: (i) transfer of CT images to the 3D imaging system; (ii) 3D reconstruction of the tumour, pulmonary vessels, tracheo-bronchial tree and lung parenchyma (Fig. 1); (iii) definition of the segmental bronchi based on the location of the tumour; (iv) calculation of the bronchial ventilation area using an algorithm based on the direction and diameter of the bronchi (Figs 2B and 3B); (v) determination of the sites of resection of the pulmonary vessels, bronchi and inter-segmental veins (Figs 2C and 3E); (vi) calculation of the extent of the surgical margin (Fig. 2D and E) and (vii) visualization of the appropriate segmentectomy surface (Figs 2E and 3E). The 3D CT-guided segmentectomy was initiated by detaching the pulmonary arteries and veins from the pulmonary parenchyma along the shortest route to the target segmental bronchi, according to the preoperative virtual segmentectomy and intraoperative visualization guidance, with an appropriate inter-segmental plane surface (Fig. 3C–E). We performed those operations with double monitor guidance: one was a thoracoscopy television monitor, and the other was a 3D imaging system allowing 3D lung structure and virtual segmentectomy, with which we could reconstruct 3D imaging during operations (Fig. 4).
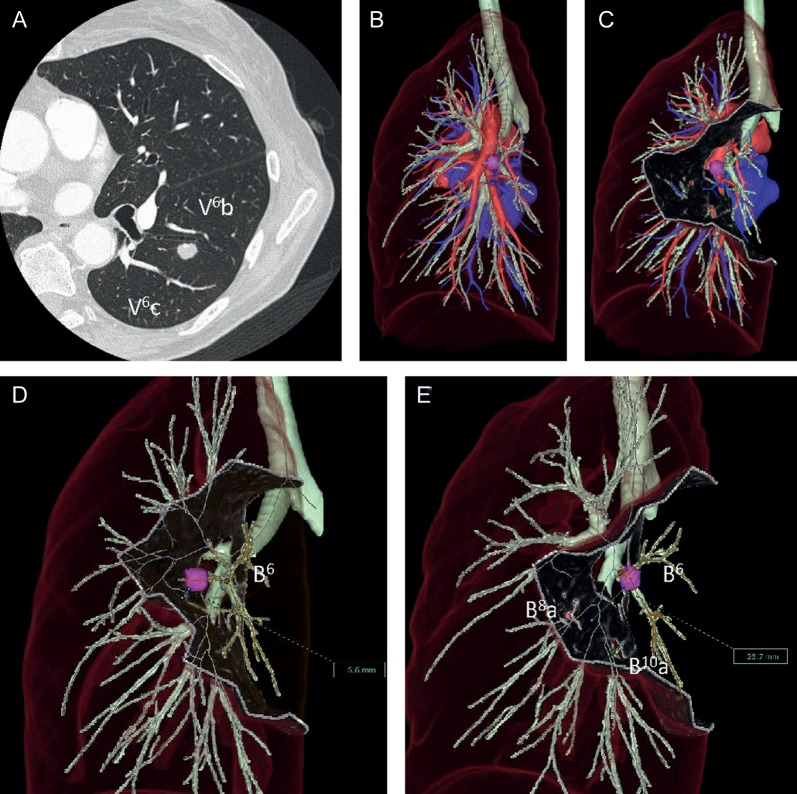
Figure 2:
Case 1 (A and B). According to the virtual S6 segmentectomy, the surgical margin would be estimated to be only 6.6 mm (D). While in the virtual segmentectomy of left S6 + S8a + S10a, the appropriate surgical margin was 25.7 mm (C and E). V6: superior lower vein, B6: superior lower bronchus, B10: posterior basal bronchus.
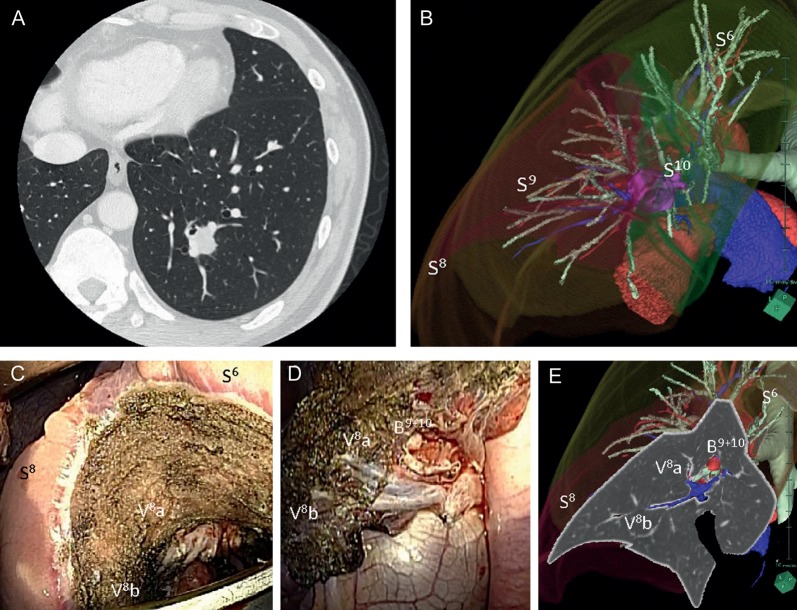
Figure 3:
Case 2 (A and B). Left S9 + S10 segmentectomy under visual guidance of the appropriate virtual segmentectomy (C–E). S6: superior, lower lobe, S8: anteromedial basal segment, S9: lateral basal segment, S10: posterior basal segment, V8: anterior basal pulmonary vein, B9 + 10: lateral and posterior basal bronchi.
Figure 4:
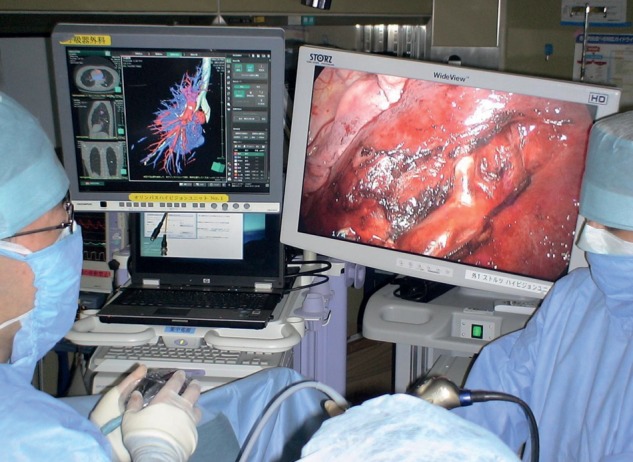
Thoracoscopic surgery using the double monitor guidance system. The thoracoscopy television monitor (right) and the 3D imaging system (left).
The simulation system was implemented as a plug-in in the processing workstation (Dell Precision T5500, Windows 7 Professional, 64-bit, 12GB, DDR3 RDIMM).
3D CT-guided segmentectomy
We performed 3D CT-guided segmentectomy for video-assisted thoracoscopic surgery (VATS)-assisted mini-thoracotomy under guidance from both a television monitor and visualization of virtual segmentectomy. Basically, we applied a surgical approach with a combination of muscle-sparing mini-thoracotomy (incision, 4–10 cm) and another incision for an access port of a thoracoscope for video assistance and used mainly direct visualization for the resection of lung parenchyma. The surgeon directly observed the proximal region of the target lobe and individually ligated and dissected the pulmonary arteries and veins from the pulmonary parenchyma along the shortest route to the intended segmental bronchi, according to preoperative virtual segmentectomy and intraoperative 3D CT guidance (Figs 2E, 3C–E). After the segmental bronchi involved with the diseased segment were isolated and divided, the lung was ventilated using selective jet ventilation through a double-lumen tube or directly through mini-thoracotomy into the orifice of the targeted segmental bronchi. The diseased segment was inflated while the preserved segments appeared to collapse and a line formed between the inflated and deflated lung parenchyma, showing the anatomical inter-segmental plane. After the segmental bronchi were isolated and dissected using staples or 2-0 silk sutures. Electrocauterization was used to divide the inter-segmental plane along the inflation–deflation line from the peripheral site to the hilum. At the central portion around the hilum, the inter-segmental plane was approached along the inter-segmental vein. We also guided the visualization of 3D CT imaging, including the segmental surface based on virtual segmentectomy simulation to obtain accurate surgical margins. Staplers were only used to divide the inter-segmental plane when the lung was emphysematous, to minimize air leakage. The dissected raw parenchymal surface was sealed with a commercially available fibrin sealant and a bioabsorbable polyglycolic acid felt.
CLINICAL EXPERIENCE
When first using this surgical simulation and navigation system for segmentectomy, we attempted this novel technique on 3 cases of pulmonary metastases and 1 of multiple lung cancer (Table 1). Here, we describe 2 representative cases.
Table 1:
Patient characteristics and surgical outcomes
| Case | Age (years) | Sex | Diagnosis | Location | Tumour size (mm) | Operation procedure | Inter-segment | Operation time | Blood loss (ml) | Duration of chest tube placement (POD) | Duration of hospitalization | Morbidity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 69 | F | PM from mesopharyngeal carcinoma | Rt S6 | 8.8 | Lt S6 segmentectomy | Electrocautery | 3 h 39 m | 120 | 3 | 7 | None |
| 2 | 43 | M | PM from thymic carcinoma | Lt S9 | 21.2 | Lt S9 + S10 segmentectomy | Electrocautery | 3 h 09 m | 24 | 3 | 9 | None |
| 3 | 77 | F | Multiple lung cancer | Rt S8 | 13.0 | Rt S7 + S8 segmentectomy | Electrocautery | 3 h 42 m | 68 | 3 | 5 | None |
| 4 | 59 | M | PM from rectal cancer | Rt S9 | 19.8 | Rt basal segmentectomy | Stapler | 3 h 52 m | 70 | 3 | 5 | None |
Lt: left; PM: pulmonary metastasis; POD: postoperative days; Rt: right.
Case 1 was a 69-year old woman with a small solitary pulmonary nodule. Her previous history was mesopharyngeal carcinoma treated with surgical resection followed by chemo-radiotherapy 1 year previously. Chest CT scanning revealed a suspicious metastatic pulmonary tumour located in the superior segment of the left lower lobe (S6) (Fig. 2A). This tumour was very close to pulmonary veins V6b and V6c, which are supposed to be inter-segmental pulmonary veins between S6 and the basal segment of the left lower lobe (Fig. 2A). If only the superior segmentectomy of the left lower lobe was performed in this case, this surgical margin was estimated at 6.6 mm, which is <2.0 cm or the maximum tumour size based on the result of the virtual segmentectomy of the superior segment of the left lower lobe (Fig. 2D). Therefore, we performed the extended superior segmentectomy of the left lower lobe according to the preoperative simulation of the virtual segmentectomy of left S6 + S8a + S10a, obtaining an appropriate surgical margin (25.7 mm), >2.0 cm or the maximum diameter of this tumour (8.8 mm) (Fig. 2E) [6].
Case 2 was a 43-year old man with a 21.2-mm solitary pulmonary nodule in the left S9 + 10 basal segment of the left lower lobe. He had undergone thyroidectomy and thymectomy for thyroid carcinoma and thymic carcinoma 3 and 2 years previously, respectively. This tumour was located on the exact line between S9 and S10 according to the 3D imaging reconstruction of each segmental area of the left lower lobe (Fig. 3B). Therefore, we performed a left S9 + S10 segmentectomy under visual guidance of the appropriate virtual segmentectomy. We used an electrocautery Bovie to dissect the lung parenchyma along the line between inflated and deflated lung, visually guided by the visualization of 3D CT imaging, based on virtual segmentectomy simulation to obtain accurate surgical margins (Fig. 3C–E).
Table 1 shows the preliminary results of surgical outcomes in our 4 consecutive cases. We performed three complex segmentectomies: an extended left S6 segmentectomy, a left S9 + S10 segmentectomy and a right S7 + S8 segmentectomy. We also performed one moderate segmentectomy: a basal segmentectomy of the right lower lobe. After the lung parenchyma was dissected, we sealed it with a commercially available fibrin sealant and a bioabsorbable polyglycolic acid felt in 3 of 4 cases. Operation times were all within 4 h, blood loss volumes ranged from 24 to 120 ml, and chest tubes could be removed on POD 3 in all patients. The durations of hospitalization were from POD 5 to POD 9 without moderate or severe postoperative complications. According to comparisons between 3D CT imaging and pathological findings (Table 2), the extents of preoperative predicted surgical margins by Synapse Vincent tended to be shorter than those of pathological surgical margins, because of deflated resected lung lobes. However, there were no residual tumour cells at the surgical resection margins (confirmed pathologically) and no local recurrence could be detected in any of the 4 cases during the short follow-up period (15–17 months).
Table 2:
Comparisons between 3D CT imaging and pathological findings
| Case | Vincent tumour size (CT) (mm) | Pathological tumour size (mm) | Vincent surgical margin length (mm) | Pathological surgical margin length (mm) | Pathological surgical margin | Follow-up (months) | Local recurrence |
|---|---|---|---|---|---|---|---|
| 1 | 8.8 | 11.0 | 12.4 | 9.8 | Negative | 14 | None |
| 2 | 21.2 | 20.0 | 15.0 | 10.0 | Negative | 14 | None |
| 3 | 13.0 | 13.0 | 20.6 | 12.0 | Negative | 13 | None |
| 4 | 19.8 | 18.0 | 30.0 | 25.0 | Negative | 12 | None |
COMMENT
The efficacy of 3D CT angiography using MDCT for preoperative assessment for thoracic surgery has been described. Accuracy in depicting individual anatomies of pulmonary vessels and bronchi and preoperative simulation using 3D CT could play an important role in facilitating a safe and complete VATS lobectomy procedure, as has been suggested in some previous reports. Fukuhara et al. reviewed 49 consecutive patients with clinical stage I lung cancer and compared intraoperative findings of the pulmonary artery branching pattern with 3D CT angiography images preoperatively obtained using MDCT. According to the results, 95.2% of the pulmonary artery branches were precisely identified on preoperative 3D CT angiography and conversion to open thoracotomy was not needed. Watanabe et al. also reviewed 14 lung cancer cases and stated that 3D CT pulmonary artery navigation may have the potential to increase the safety of surgical procedures [7, 8]. Recently, preoperative identification of the inter-segmental pulmonary vessels and bronchi involved with the resected segment using 3D CT angiography and establishment of a virtual bronchial tree (virtual bronchography) have been demonstrated to be useful tools, enabling the surgeon to perform an accurate anatomical segmentectomy [9, 10]. However, to the best of our knowledge, identifying the inter-segmental surface and calculating appropriate surgical margins by this method have never been established for preoperative procedures for segmentectomy. This article is apparently the first report demonstrating the efficacy of virtual segmentectomy.
It is critical to identify the inter-segmental veins as the boundary lines of the pulmonary segments to determine the lateral surgical margins and to identify the target segmental bronchi as the vertical surgical margins before segmentectomy. However, given the many anatomical variations of pulmonary veins and bronchi, it is difficult to identify the intra-segmental and inter-segmental veins and target segmental bronchi, even by using 3D CT angiography and virtual bronchography. Moreover, it is technically difficult to perform a typical segmentectomy because of the tumour location (Cases 1 and 2). Recently, we have occasionally encountered cases which required multi-segmentectomy for several tumours. Therefore, we have developed the novel segmentectomy simulation system based on high-quality 3D lung modelling from MDCT imaging using the specially developed Synapse Vincent software in collaboration with Fujifilm Corporation.
This particular system consist of the following three novel functions: (i) calculation of the bronchial ventilation area using the algorithm based on the direction and diameter of the bronchi; (ii) visualization of the segmental surface resulting in determination of resected pulmonary artery, vein, bronchi and inter-segmental veins and (iii) calculation of the length of the surgical margin. Based on the preliminary results of our cases, we believe that it could be useful for appropriate anatomical segmentectomy with curative resection. It would enable one to obtain accurate preoperative information regarding not only the relationships among the tumour, the intra-segmental and inter-segmental veins, the target segmental bronchi and pulmonary arteries, but also to visualize the inter-segmental plane surface and secure surgical margins. Moreover, intraoperative visualization guidance of the inter-segmental surface, including intra-segmental and inter-segmental veins, target pulmonary arteries, and bronchi could help thoracic surgeons perform accurate anatomical segmentectomy and curative resection. Additional applications of this novel system include preoperative simulation of multi-segmentectomy for synchronous or metachronous multiple pulmonary tumours and visual guidance for robotic surgeries.
In this preliminary study, we first attempted to demonstrate the usefulness of virtual segmentectomy based on high-quality 3D lung modelling from conventional multidetector CT images to compare intraoperative findings of the pulmonary artery, pulmonary vein and bronchial branching patterns and visualization of the segmental surface. Therefore, we basically performed hybrid VATS to make an accurate assessment not only on a television monitor that provides a 2D view, but also for direct observation using mini-thoracotomy with a real 3D view. In our next study, we will perform complete VATS segmentectomy using this novel technology.
It is critical to obtain an adequate surgical margin for pulmonary malignant tumours, particularly for primary lung cancer. The Vincent surgical margins are overestimated, as evidenced by the pathological margins (Table 2). As this is a preliminary study with a small number of sample cases, we are currently evaluating more cases and assessing detailed information. Because we are more familiar with using this novel technology and performing segmentectomy in many cases with much more inflated target segments, the discrepancies between the Vincent surgical margin and the pathological surgical margin is decreasing.
We understand that a major limitation remains in this system. Virtual visualization of the segmentectomy resection line consisted of the border of the inflated lung, whereas the lung was, in reality, deflated during actual surgical procedures. Further technical development is needed to improve this limitation.
We describe the benefit of virtual segmentectomy, a novel surgical simulation system based on high-quality 3D lung modelling, including vascular and bronchial structures. Preoperative determination of the branches of the pulmonary vessels and segmental bronchi branches and the anatomical inter-segmental surface estimated by the inflated area of segmental bronchi is possible by visualization. Moreover, a sufficient surgical margin from the tumour can be estimated. This new technology appears useful for thoracic surgeons to perform appropriate anatomical segmentectomy and curative resection. Furthermore, we are currently performing a prospective clinical study to demonstrate the efficacy of this novel system for small peripheral primary lung cancer lesions.
FUNDING
This study was supported by a Grant-in-Aid for Scientific Research, Japan Society for the Promotion of Science (24592104), and Ministry of Education, Culture, Sports, Science and Technology, Japan.
Conflict of interest: Hisashi Saji and Norihiko Ikeda have given remunerated lectures for Fujifilm. No author received research funding and all had full control of the study design, methods used, outcome parameters, data analysis and production of the written report.
ACKNOWLEDGEMENTS
We are indebted to Clifford A. Kolba, Edward F. Barroga and J. Patrick Barron, Department of International Medical Communications of Tokyo Medical University, for their editorial review of the English manuscript.
REFERENCES
- 1.Sakata R, Fujii Y, Kuwano H. Thoracic and cardiovascular surgery in Japan during 2009: annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg. 2011;59:636–67. doi: 10.1007/s11748-011-0838-5. [DOI] [PubMed] [Google Scholar]
- 2.Yamanaka J, Saito S, Fujimoto J. Impact of preoperative planning using virtual segmental volumetry on liver resection for hepatocellular carcinoma. World J Surg. 2007;31:1249–55. doi: 10.1007/s00268-007-9020-8. [DOI] [PubMed] [Google Scholar]
- 3.Saito S, Yamanaka J, Miura K, Nakao N, Nagao T, Sugimoto T, et al. A novel 3D hepatectomy simulation based on liver circulation: application to liver resection and transplantation. Hepatology. 2005;41:1297–304. doi: 10.1002/hep.20684. [DOI] [PubMed] [Google Scholar]
- 4.Lang H, Radtke A, Hindennach M, Schroeder T, Fruhauf NR, Malago M, et al. Impact of virtual tumor resection and computer-assisted risk analysis on operation planning and intraoperative strategy in major hepatic resection. Arch Surg. 2005;140:629–38. doi: 10.1001/archsurg.140.7.629. discussion 638. [DOI] [PubMed] [Google Scholar]
- 5.Yamanaka J, Saito S, Iimuro Y, Hirano T, Okada T, Kuroda N, et al. The impact of 3-D virtual hepatectomy simulation in living-donor liver transplantation. J Hepatobiliary Pancreat Surg. 2006;13:363–9. doi: 10.1007/s00534-005-1075-z. [DOI] [PubMed] [Google Scholar]
- 6.Sawabata N, Ohta M, Matsumura A, Nakagawa K, Hirano H, Maeda H, et al. Optimal distance of malignant negative margin in excision of nonsmall cell lung cancer: a multicenter prospective study. Ann Thorac Surg. 2004;77:415–20. doi: 10.1016/S0003-4975(03)01511-X. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe S, Arai K, Watanabe T, Koda W, Urayama H. Use of three-dimensional computed tomographic angiography of pulmonary vessels for lung resections. Ann Thorac Surg. 2003;75:388–92. doi: 10.1016/s0003-4975(02)04375-8. discussion 392. [DOI] [PubMed] [Google Scholar]
- 8.Fukuhara K, Akashi A, Nakane S, Tomita E. Preoperative assessment of the pulmonary artery by three-dimensional computed tomography before video-assisted thoracic surgery lobectomy. Eur J Cardiothorac Surg. 2008;34:875–7. doi: 10.1016/j.ejcts.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Oizumi H, Endoh M, Takeda S, Suzuki J, Fukaya K, Sadahiro M. Anatomical lung segmentectomy simulated by computed tomographic angiography. Ann Thorac Surg. 2010;90:1382–3. doi: 10.1016/j.athoracsur.2009.11.062. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu K, Nakano T, Kamiyoshihara M, Takeyoshi I. Segmentectomy guided by three-dimensional computed tomography angiography and bronchography. Interact CardioVasc Thorac Surg. 2012;15:194–6. doi: 10.1093/icvts/ivs202. [DOI] [PMC free article] [PubMed] [Google Scholar]