Abstract
OBJECTIVES
In this study, we sought to analyse the incidence of major non-cardiac complications and their impact on survival following cardiac surgery procedures in a contemporary patient cohort. We further determined independent predictors of perioperative mortality and created a logistic regression model for prediction of outcome after the occurrence of these complications.
METHODS
Prospectively collected data of 5318 consecutive adult patients (mean age 68.9 ± 11.0 years; 29.3% [n = 1559] female) undergoing cardiac surgery from January 2009 to May 2012 were retrospectively analysed. Outcome measures were six major non-cardiac complications including respiratory failure, dialysis-dependent renal failure, deep sternal wound infection (DSWI), cerebrovascular accident (CVA), gastrointestinal complications (GIC) and sepsis and their impact on perioperative mortality and hospital length of stay using multivariate regression models. The discriminatory power was evaluated by calculating the area under the receiver operating characteristic curves (C statistic).
RESULTS
A total of 1321 complications were observed in 846 (15.9%) patients: respiratory failure (n = 432; 8.1%), dialysis-dependent renal failure (n = 295; 5.5%), GIC (n = 154; 2.9%), CVA (n = 151; 2.8%), DSWI (n = 146; 2.7%) and sepsis (n = 143; 2.7%). Perioperative mortality was 17.0% in patients with at least one major non-cardiac complication and correlated with the number of complications (single, 9.7%; n = 53/549; double, 24.0%; n = 44/183; ≥3, 41.2%; n = 47/114, P < 0.001). Six preoperative and four postoperative independent predictors of operative mortality were identified (age (odds ratio [OR] 1.8; 95% confidence interval [CI] 1.3–2.4), peripheral vascular disease (OR 2.6; 95% CI 1.6–4.2), pulmonary hypertension (OR 2.7; 95% CI 1.5–4.9), atrial fibrillation (OR 1.5; 95% CI 1.0–2.3), emergency (OR 5.0; 95% CI 3.4–7.2), other procedures than CABG (OR 1.5; 95% CI 1.0–2.1), postoperative dialysis (OR 4.0; 95% CI 2.6–6.1), sepsis (OR 3.4; 95% CI 2.0–5.6), respiratory failure (OR 3.2; 95% CI 2.2–4.9), GIC (OR 3.2; 95% CI 1.9–5.3)) and included in the logistic model, which accurately predicted outcome (C statistic, 0.892; 95% CI 0.868–0.916). Length of hospital stay was significantly increased according to the number of complications (single: median 15 (IQR 10–24) days, double: 16 (IQR 8–28) days, ≥3: 20 (IQR 13–39) days, P < 0.001).
CONCLUSIONS
With a worsening in the risk profile of patients undergoing cardiac surgery, an increasing number of patients develop major complications leading to increased length of stay and mortality, which is correlated to the number and severity of these complications. Our predictive model based on preoperative and postoperative variables allowed us to determine with accuracy the perioperative mortality in critically ill patients after cardiac surgery.
Keywords: Cardiac surgery, Postoperative complications, Mortality, Outcome
INTRODUCTION
In the recent era, cardiac surgery patients become older and often present multiple comorbidities such as diabetes mellitus, renal dysfunction and peripheral or cerebral vascular disease [1]. In addition, during the last decade, many aspects of cardiac surgery procedures have changed. For instance, the rate of patients referred for isolated coronary artery bypass grafting (CABG) is decreasing, whereas an increasing percentage of patients is referred for valve and combined valve CABG procedures [2]. Furthermore, owing to the development of modern interventional treatment, patients are often referred at a later stage of cardiac disease, requiring more complex procedures, or are referred under urgent or emergent conditions [1]. This worsening risk profile has led to an increasing incidence of postoperative complications with consecutive prolongation of intensive care unit stay despite improvements in perioperative care. The impact of single postoperative complications such as renal failure requiring dialysis or respiratory failure on outcome has been reported in numerous studies. However, the cumulative impact of serial complications remains less clear. In a former analysis among a patient population from New York State, we have already shown a correlation between the number of complications and their severity on outcome and developed a predictive model that allowed us to determine operative mortality in critically ill patients following cardiac surgery [3]. The aim of this study was to investigate the incidence of major non-cardiac complications and their impact on perioperative outcome in a recent cohort of patients from a German high-volume center and to identify independent predictors of mortality in these patients. Furthermore, we sought to refine the model for the prediction of survival in these patients.
MATERIAL AND METHODS
Study population
Prospectively collected data of 5714 consecutive adult patients who underwent cardiac surgery between January 2009 and August 2012 at the Department of Cardiothoracic Surgery at the Heart Center of the University Hospital Cologne were retrospectively analysed. The study population consisted of 5318 patients who underwent surgery between January 2009 and May 2012. For validation purposes, a separate cohort of patients was analysed. This validation cohort (n = 396) underwent surgery between June 2012 and August 2012. Patients undergoing cardiac transplantation and ventricular assist device implantation as well as those with preoperative renal failure requiring dialysis were not included in the study population. Patients who died in the operating room were also excluded. Data for all patients' demographics, clinical characteristics, comorbid conditions, perioperative variables and postoperative outcome information were extracted from a computerized database based on the mandatory German Cardiac Surgery Quality Assurance System (http://www.sqg.de/startseite/index.html). Additional medical chart review was carried out to obtain additional information whenever necessary. All variables included into the analysis and their definitions are shown in Table 1.
Table 1:
Variables included in this study and their definitions
| Preoperative variables |
| Age (years) |
| Gender (male/female) |
| Weight (kg) |
| Height (cm) |
| Body mass index (BMI, kg/m2) |
| Hypertension |
| Hyperlipidaemia |
| Diabetes mellitus requiring medication |
| Chronic obstructive pulmonary disease |
| Coronary artery disease |
| Recent myocardial infarction (within 21 days prior surgery) |
| Previous percutaneous coronary intervention |
| Peripheral vascular disease (at least stadium II or previous intervention) |
| Prior cerebrovascular accident |
| Pulmonary hypertension (systolic pulmonary pressure >60 mmHg) |
| Ejection fraction (%) |
| Atrial fibrillation (paroxysmal, persistent, permanent) |
| Prior heart operation |
| Preoperative renal failure without dialysis (creatinine >2 mg/dl) |
| Urgent operation (requiring operation during current hospitalization) |
| Emergent operation (refractory unrelenting cardiac compromise requiring immediate operation) |
| Logistic EuroSCORE |
| Intraoperative variables |
| Type of procedure (isolated coronary artery bypass grafting; isolated valve surgery; aortic surgery, combined valve/CABG procedures and others) |
| Duration of procedure (OP time, min) |
| Cardiopulmonary bypass time (CPB time, min) |
| Cross clamp time (min) |
| Type of cardioplegia (warm blood (Calafiore) or cold blood (Buckberg) cardioplegia) |
| Postoperative variables |
| Perioperative mortality (death during same admission or within 30 day when discharged) |
| Renal failure (need for temporary or permanent dialysis) |
| Respiratory failure (prolonged ventilator therapy (>95 h), reintubation or tracheostomy) |
| Sepsis |
| Stroke (new permanent neurological event-defined interdisciplinary (intensivist and neurologist based on CT scan and/or MRI imaging) |
| Deep sternal wound infection |
| Gastrointestinal complication |
| Length of ICU stay (days) |
| Length of hospital stay (days) |
Outcome analysis
The main outcome measures of our analysis were six major non-cardiac complications including respiratory failure, dialysis-dependent renal failure, deep sternal wound infection (DSWI), cerebrovascular accident (CVA), gastrointestinal complications (GIC) and sepsis and their impact on perioperative mortality and hospital length of stay (LOS). Respiratory failure was defined as prolonged mechanical ventilation (>95 h), or the need for reintubation or tracheostomy during the postoperative course. Dialysis-dependent renal failure was defined as the need for acute haemodialysis regardless of frequency and duration. Deep sternal wound infection was defined as drainage of purulent material from the sternotomy wound or instability of the sternum with proof of infection during reoperation. Cerebrovascular accident was defined as permanent stroke due to ischaemic events or bleeding. Gastrointestinal complications were defined as any gastrointestinal events requiring medical treatment or surgical intervention including bleeding complications, ileus and mesenteric ischaemia. Finally, sepsis was defined as clinical evident infection with positive blood cultures. Perioperative mortality was defined as death during initial hospitalization or within 30 days after surgery when discharged. Patients with at least one non-cardiac complication were allocated to the study group (complication group) whereas patients who did not experience any of the six non-cardiac complications were assigned to the control group (no complication group). Further outcome variables were length of hospital stay and discharge condition (home, rehab facility, long-term care facility).
Intra- and postoperative management
All procedures were performed using standard anaesthetic and surgical techniques adapted to the individual procedures. The majority of procedures were performed through a median (full or partial) sternotomy. Patients referred for minimally invasive mitral valve procedures underwent a right anterior–lateral thoracotomy. Cardiopulmonary bypass was established between the ascending aorta and either the right atrium using a two-stage cannula or both venae cavae. Alternative CPB access sites were the subclavian artery and/or the femoral vessels in selected cases. During CPB, a minimum flow of 2.2 l/min/m2 and a perfusion pressure of 60 mmHg were aspired in all patients. Myocardial protection was achieved using either high potassium cold blood cardioplegia (Buckberg) in an antegrade and/ or retrograde fashion or by means of warm blood cardioplegia (Calafiore). Following surgery, all patients were transferred to the intensive care unit (ICU).
Statistical analysis
Normally distributed continuous variables are presented as mean ± standard deviation (SD) and otherwise as median and interquartile range (IQR). Categorical variables are shown as the percentage of the sample. A P-value <0.05 was considered significant for all statistical methods. The χ2 test, Fisher's exact test, unpaired Student's t-test and Mann–Whitney U-test were used as appropriate to evaluate the relationship between preoperative and postoperative variables and perioperative mortality in univariate analysis. A multivariate logistic regression analysis was then performed to assess the influence of these variables as independent risk factors for perioperative mortality. Variables were selected with the forward stepwise method using the likelihood ratio for variable removal and a cut-off probability value for inclusion and exclusion of 0.10. The calibration of the model was assessed with the Hosmer–Lemeshow goodness-of-fit test [4]. The regression coefficient B, odds ratio (OR), corresponding 95% confidence interval (CI), and the P-value are reported for each independent factor. A logistic equitation was then created using the coefficients of the regression analysis to estimate individual patient's risk of perioperative mortality:
where X × B is coefficient B for each single confounding factor and α is model intercept [3].
After the model was constructed, the probability of dying was calculated for each patient. All patients who underwent surgery before June 2012 were assigned to the study cohort. For validation of the model, we analysed a separate cohort of patients who underwent cardiac surgery between June 2012 and August 2012. The same data elements used for the creation of the model were available for the validation cohort. The probability of dying was also calculated for each individual patient of the validation cohort. To measure and compare the predictive accuracy of the model in the study population and validation cohort, we generated receiver operating characteristic (ROC) curves and compared their area under the curves (AUC, C-statistics). An area under the ROC curve >0.7 was considered accurate [5, 6]. Finally, we compared our score with the logistic EuroSCORE [7]. The statistical analyses were performed with SPSS 20 (IBM, Armonk, NY, USA). The comparison of the ROC AUCs was performed with Sigmaplot 12 (Systat Software, Inc., San José, CA, USA).
RESULTS
The study cohort (cardiac surgery between January 2009 and May 2012) comprised 5318 patients. The mean age was 68.9 ± 11.0 years, and 29.3% (n = 1559) were female. The majority of patients underwent isolated CABG (55.6%, n = 2958). The remaining patients underwent valve procedures (single or multiple valve surgery; 24.0%, n = 1276), combined valve and CABG procedures (13.1%, n = 695), aortic surgery (5.2%, n = 274) and other procedures (2.2%, n = 115). A more detailed description of procedures performed is shown in Table 2.
Table 2:
Surgical procedures performed
| Procedures | n |
|---|---|
| Isolated CABG | 2958 |
| Valve procedures | 1276 |
| Single valve | 1126 |
| AVR | 883 |
| MVP | 140 |
| MVR | 88 |
| TVP/R | 15 |
| Double valve | 143 |
| AVR and MVP | 73 |
| AVR and MVR | 20 |
| AVR and TVP | 4 |
| MVP and TVP | 26 |
| MVR and TVP | 20 |
| Triple vale | 7 |
| Aortic procedures | 274 |
| Bentall or valve sparing | 139 |
| Ascending aorta aortic arch | 135 |
| Combined procedures and other | 810 |
| CABG and MVP | 92 |
| CABG and MVR | 36 |
| CABG and AVR | 464 |
| Other | 218 |
CABG: coronary artery bypass grafting; AVR: aortic valve replacement; MVR: mitral valve replacement; MVP: mitral valve repair; TV: tricuspid valve.
A total of 1321 non-cardiac complications were observed in 846 (15.9%) patients. The most frequent complication was respiratory failure (8.1%, n = 432) followed by renal failure requiring dialysis (5.5%, n = 295), GIC (2.9%, n = 154), CVA (2.8%, n = 151), DSWI (2.7%, n = 146) and sepsis (2.7%, n = 143). The number of complications per patient was distributed as follows: single non-cardiac complication 10.3% (n = 549), two non-cardiac complications 3.4% (n = 183), three or more non-cardiac complications 2.1% (n = 114). The majority of patients with multiple complications had combinations with respiratory failure and renal failure requiring dialysis. Table 3 shows the pattern of complications in detail.
Table 3:
Distribution of major non-cardiac complications and associated perioperative mortality
| Group | Frequency |
Mortality |
||||
|---|---|---|---|---|---|---|
| n | % of all | % in group | % in complications | n | % | |
| No complication | 4472 | 84.1 | 60 | 1.3 | ||
| Single | 549 | 10.3 | 64.9 | 53 | 9.7 | |
| RF | 175 | 3.3 | 31.9 | 20.7 | 20 | 11.4 |
| Dialysis | 121 | 2.3 | 22.0 | 14.3 | 17 | 14.0 |
| DSWI | 100 | 1.9 | 18.2 | 11.8 | 0 | 0.0 |
| CVA | 66 | 1.2 | 12.0 | 7.8 | 4 | 6.1 |
| GIC | 61 | 1.1 | 11.1 | 7.2 | 8 | 13.1 |
| Sepsis | 26 | 0.5 | 4.7 | 3.1 | 4 | 15.4 |
| Double | 183 | 3.4 | 21.6 | 44 | 24.0 | |
| Dialysis + RF | 50 | 0.9 | 27.3 | 5.9 | 21 | 42.0 |
| CVA + RF | 45 | 0.8 | 24.6 | 5.3 | 6 | 13.3 |
| DSWI + RF | 18 | 0.3 | 9.8 | 2.1 | 0 | 0.0 |
| GIC + RF | 18 | 0.3 | 9.8 | 2.1 | 3 | 16.7 |
| Sepsis + RF | 13 | 0.2 | 7.1 | 1.5 | 2 | 15.4 |
| Dialysis + sepsis | 10 | 0.2 | 5.5 | 1.2 | 5 | 50.0 |
| Other double complications | 29 | 0.5 | 15.8 | 3.4 | 7 | 24.1 |
| Three or more complications | 114 | 2.1 | 13.5 | 47 | 41.2 | |
| RF + sepsis + dialysis | 28 | 0.5 | 24.6 | 3.3 | 12 | 42.9 |
| RF + sepsis + dialysis + GIC | 24 | 0.5 | 21.1 | 2.8 | 16 | 66.7 |
| RF + dialysis + GIC | 14 | 0.3 | 12.3 | 1.7 | 5 | 35.7 |
| RF + dialysis + sepsis + CVA | 7 | 0.1 | 6.1 | 0.8 | 3 | 42.9 |
| RF + sepsis + GIC | 6 | 0.1 | 5.3 | 0.7 | 4 | 66.7 |
| RF + dialysis + sepsis + CVA + GIC | 6 | 0.1 | 5.3 | 0.7 | 2 | 33.3 |
| Other complications (≥3) | 29 | 0.5 | 25.4 | 3.4 | 5 | 17.2 |
| Total | 5318 | 100.0 | 204 | 3.8 | ||
RF: respiratory failure; DSWI: deep sternal wound infection; GIC: gastrointestinal complication; CVA: cerebrovascular accident.
The perioperative mortality among the study population was 3.8% (n = 204). An increased mortality was observed in patients with major non-cardiac complications (Table 3). The perioperative mortality rate among patients of the complications group (at least one non-cardiac complication) was 17% (n = 144 of 846) compared with 1.3% (n = 60 of 4472) in patients of the no-complication group. The overall mortality of patients with a single non-cardiac complication was 9.7% (n = 53 of 549). The highest mortality among patients with a single non-cardiac complication was observed in those who experienced renal failure requiring dialysis (14.0%), GIC (13.1%) and respiratory failure (11.4%). Patients with two non-cardiac complications had a more than twofold increased risk of dying compared with those with a single non-cardiac complication (24.0%; n = 44 of 183). Patients with two non-cardiac complications most frequently suffered from respiratory failure in combination with another non-cardiac complication. The highest mortality rate was observed in patients with dialysis-dependent renal failure and sepsis (50.0%), followed by renal failure requiring dialysis and respiratory failure (42.0%), and respiratory failure and GIC (16.7%). The mortality rate further increased among patients with three or more non-cardiac complications (41.2%; n = 47 of 114). Patients with respiratory failure, GIC and sepsis had a 66.7% (n = 16 of 24) mortality rate similar to those with respiratory failure, GIC, sepsis and renal failure requiring dialysis (66.7%, n = 4 of 6).
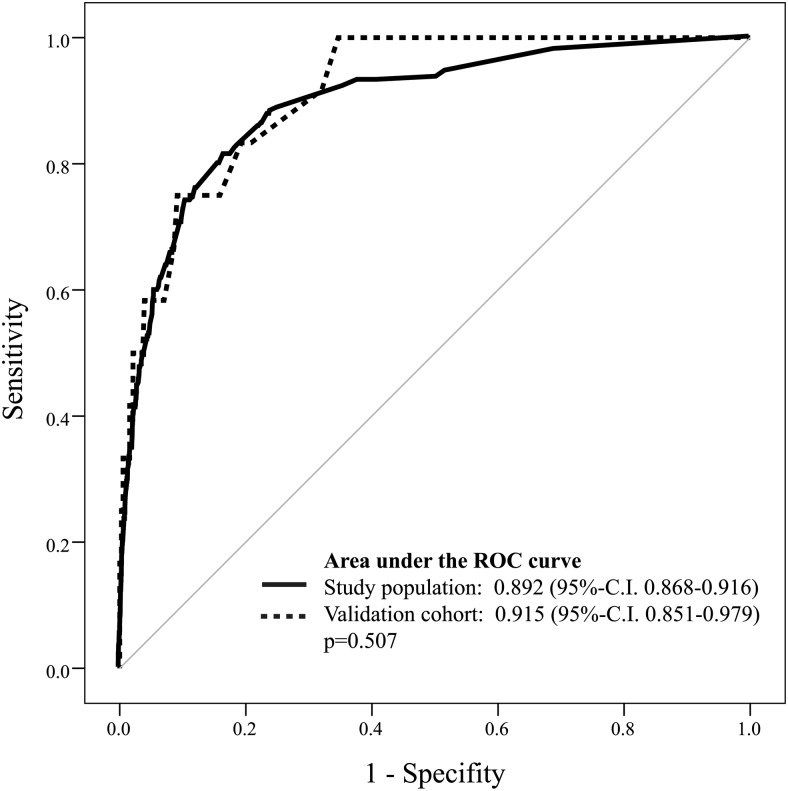
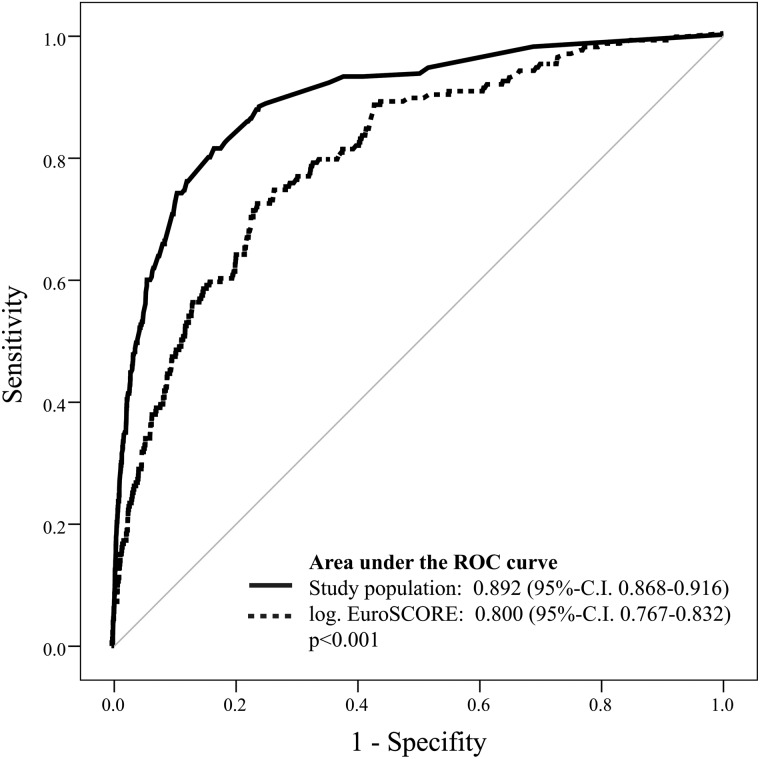
Univariate and subsequent multivariate logistic regression analysis revealed six preoperative and four postoperative independent predictors of perioperative mortality (Tables 4 and 5). The result of the Hosmer–Lemeshow analysis was not statistically significant (P > 0.05), suggesting a good calibration of the model. Based on the 10 variables determined by the multivariate logistic regression analysis, a predictive model was created using a logistic equation and individual patient's risk of hospital mortality was calculated. The predicted mortality risk was compared with the observed mortality rate using C statistics. The ROC area under the curve for the study population was 0.892 (95% CI 0.868–0.916). For validation of the model, the same calculation was performed using data of our validation cohort (n = 396, mean age 6.7 ± 11.8 years, 29.3% (n = 116) female). The ROC area under the curve was 0.915 (95% CI 0.851–0.979) and was not significantly different between the study population and internal verification cohort (P = 0.507) (Fig. 1). We further compared the results of our predictive model to the logistic EuroSCORE [7]. The ROC area under the curve of the latter was 0.800 (95% CI 0.767–0.832) suggesting that our model more accurately predicted mortality (P < 0.001) (Fig. 2).
Table 4:
Predictors of perioperative mortality in univariate analysis
| Factor | Survived, n (%) | Died, n (%) | Univariate |
|||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | ||||
| Preoperative variable | ||||||
| Female gender | 1489 (29.1) | 70 (34.3) | 1.3 | (0.9–1.71) | 0.117 | |
| Age >70 years | 1609 (31.5) | 93 (45.6) | 1.8 | (1.4–2.42) | <0.001 | |
| Obesity | 1262 (24.7) | 57 (27.9) | 1.2 | (0.9–1.62) | 0.284 | |
| Ejection fraction <30% | 205 (4.0) | 18 (8.8) | 2.3 | (1.4–3.83) | 0.003 | |
| Pulmonary hypertension | 133 (2.6) | 23 (11.3) | 4.8 | (3.0–7.59) | <0.001 | |
| Hypertension | 3933 (76.9) | 149 (73.0) | 0.8 | (0.6–0.12) | 0.205 | |
| Atrial fibrillation | 607 (11.9) | 39 (19.1) | 1.8 | (1.2–2.51) | 0.003 | |
| Diabetes | 1420 (27.8) | 44 (21.6) | 0.7 | (0.5–1.00) | 0.055 | |
| Peripheral vascular disease | 294 (5.7) | 30 (14.7) | 2.8 | (1.9–4.24) | <0.001 | |
| Stroke | 342 (6.7) | 20 (9.8) | 1.5 | (0.9–2.44) | 0.088 | |
| Renal failure | 171 (3.3) | 26 (12.7) | 4.2 | (2.7–6.55) | <0.001 | |
| Chronic obstructive pulmonary disease | 100 (2.0) | 6 (2.9) | 1.5 | (0.7–3.51) | 0.301 | |
| Coronary artery disease | 4045 (79.1) | 157 (77.0) | 0.9 | (0.6–1.23) | 0.263 | |
| Myocardial infarction | 1248 (24.4) | 77 (37.7) | 1.9 | (1.4–2.51) | <0.001 | |
| Emergent procedure | 424 (8.3) | 70 (34.3) | 5.8 | (4.3–7.84) | <0.001 | |
| Other than CABG | 2244 (43.9) | 116 (56.9) | 1.7 | (1.3–0.24) | <0.001 | |
| Postoperative morbidities | ||||||
| Respiratory failure | 333 (6.5) | 99 (48.5) | 13.5 | (10.1–18.20) | <0.001 | |
| Sepsis | 85 (1.7) | 58 (28.4) | 23.5 | (16.2–34.10) | <0.001 | |
| Stroke | 135 (2.6) | 16 (7.8) | 3.1 | (1.8–5.38) | <0.001 | |
| Dialysis | 208 (4.1) | 87 (42.6) | 17.5 | (12.9–23.92) | <0.001 | |
| Deep sternal wound infection | 140 (2.7) | 6 (2.9) | 1.1 | (0.5–2.47) | 0.826 | |
| Gastrointestinal complication | 109 (2.1) | 45 (22.1) | 13.0 | (8.9–19.03) | <0.001 | |
CABG: coronary artery bypass grafting; CI: confidence interval; OR: odds ratio.
Table 5:
Predictors of perioperative mortality in multivariate analysis
| Coefficient B | OR | 95% CI | P-value | |
|---|---|---|---|---|
| Preoperative variables | ||||
| Emergent procedures | 1.6 | 5.0 | (3.4–7.2) | <0.001 |
| Pulmonary hypertension | 1.0 | 2.7 | (1.5–4.9) | 0.001 |
| Peripheral vascular disease | 0.9 | 2.6 | (1.6–4.2) | <0.001 |
| Age >70 years | 0.6 | 1.8 | (1.3–2.4) | 0.001 |
| Atrial fibrillation | 0.4 | 1.5 | (1.0–2.3) | 0.050 |
| Other than CABG | 0.4 | 1.5 | (1.0–2.1) | 0.026 |
| Postoperative morbidities | ||||
| Renal failure (dialysis) | 1.4 | 4.0 | (2.6–6.1) | <0.001 |
| Sepsis | 1.2 | 3.4 | (2.0–5.6) | <0.001 |
| Respiratory failure | 1.2 | 3.2 | (2.2–4.9) | <0.001 |
| GIC | 1.2 | 3.2 | (1.9–5.3) | <0.001 |
| Intercept (α)* | −4.9 | |||
CABG: coronary artery bypass grafting; GIC: gastrointestinal complication; OR: odds ratio; CI: confidence interval.
*See logicstic equation.
Figure 1:
Validation of the logistic regression model for predicting perioperative mortality using receiver operating characteristic (ROC) curves.
Figure 2:
Comparison of our logistic regression model with the logistic EuroSCORE [7] using receiver operating characteristic (ROC) curves.
The median length of stay in hospital of the study cohort was 11 (IQR 9–14) days. The hospital LOS was 10 (IQR 9–13) days for patients without any non-cardiac complication compared with 16 (IQR 10–26) days in the complications group (P < 0.001). When patients with any non-cardiac complication were analysed, the median LOS was 15 (IQR 10–24) days, 16 (IQR 8–28) days and 20 (IQR 13–39) days for patients with one, two and three or more non-cardiac complications, respectively. Among patients who survived, 28% (n = 39 of 139) of patients with two non-cardiac complications and 40% (n = 27 of 67) of patients with three or more non-cardiac complications had a LOS of >30 days.
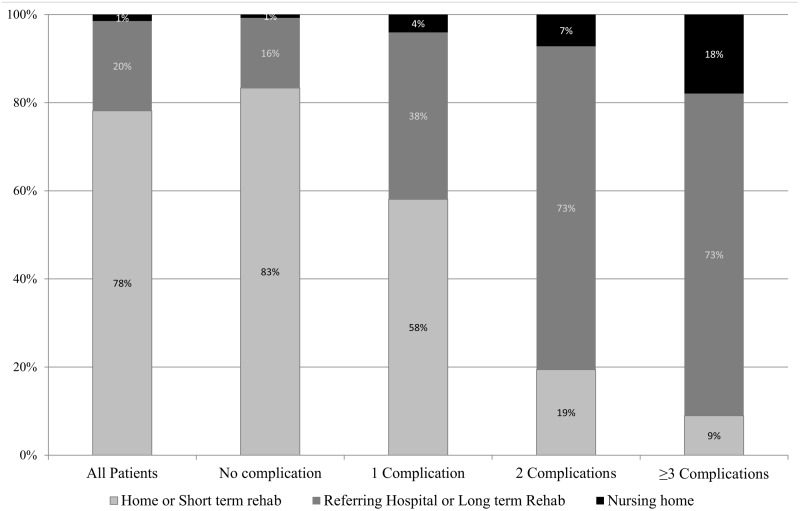
Finally, the impact of non-cardiac complications on discharge condition was analysed. Among surviving patients without any major non-cardiac complication (n = 4472) 83% (n = 3676) were discharged home or to a short-term rehab facility. The remaining patients were transferred back to their referring hospital, required long-term rehabilitation or were sent to a nursing home. Surviving patients with one (n = 496), two (n = 139) or three or more (n = 67) non-cardiac complications were discharged home or to short-term rehab in only 58% (n = 288), 19% (n = 27) and 9% (n = 6, P < 0.001) of cases, respectively (Fig. 3).
Figure 3:
Discharge condition stratified by the number of non-cardiac complications.
DISCUSSION
In this study, we sought to determine the impact of major postoperative non-cardiac complications in a very recent patient cohort undergoing the whole spectrum of cardiac surgery procedures. We observed 1321 complications in 846 (15.9%) patients. The most frequent complications were respiratory failure (8.1%) and renal failure requiring dialysis (5.5%). These findings are in accordance with other studies focusing on single postoperative complications that have reported similar results. The rate of respiratory failure following cardiac surgery procedures reported in the literature varies from 5 to 20% [8], with a correlation to the complexity of underlying procedures [3]. The reported incidence of postoperative acute renal failure varies within a wide range, depending on the definition of this complication and the composition of studied populations. An increase of creatinine during the postoperative course is observed in up to 40% of patients, whereas the incidence of dialysis-dependent renal failure has been reported with a rate of 0.5–15% [9]. Similar to respiratory failure, there is a correlation between the complexity of surgical procedures and the incidence of dialysis-dependent renal failure [10]. The rate of the remaining four non-cardiac complications analysed in our study, namely DSWI, sepsis, CVA and GIC, are also in the range reported by previous studies [3, 11–13].
Another important finding of our study is the fact that severe postoperative complications often occurred sequentially during prolonged ICU or hospital stay. In the group of patients with major non-cardiac complications, two-third suffered from a single complication whereas two or three or more complications were seen in one-third of patients. The majority of patients with multiple complications experienced combinations with respiratory failure or dialysis-dependent renal failure. This finding might be related to the circumstance that every single non-cardiac complication reflects an acute injury leading to a prolonged ICU stay and increases the risk of patients for the development of sequential classic ICU complications. Therefore, patterns of complications such as sepsis and respiratory failure or sepsis and dialysis-dependent renal failure may be a result of mutual interaction between each other. Another pattern of complications, namely stroke, ischaemic GIC or dialysis-dependent renal failure are probably related to atherosclerotic disease reflected by preoperative risk factors such as peripheral vascular disease or history of CVA. This atherosclerotic burden potentially increases the risk of organ hypoperfusion and thromboembolic events, which represent the two main pathophysiological mechanisms of ischaemic organ injury [13]. Renal failure requiring dialysis was seen in most patients with three or more complications reflecting the severity of patients' illness. It was further associated with the development of multiorgan system failure and was the strongest predictor of mortality among postoperative non-cardiac complications.
Several risk factors for the development of complications following cardiac surgery have been described in the literature. These risk factors include comorbid conditions, such as advanced age, impaired left ventricular function, atherosclerotic disease and renal insufficiency, which are seen increasingly frequently in recent patients cohorts referred for cardiac surgery [13–17]. Therefore, it is increasingly important to determine the outcome of patients following the development of these complications as well as their impact on an increased use of resources in a contemporary cohort [18, 19]. In this study, we have shown that perioperative mortality correlates with the type and the number of complications, a finding that was very similar to the results of a former study of the author [3]. The highest mortality rate, for example was observed in patients suffering from postoperative sepsis (40%) and renal failure requiring dialysis (30%). On the other hand, the perioperative mortality following isolated deep sternal wound infection without any other complication was zero, again similar to the results of the former study [3]. Obviously, DSWI leads to relevant resource consumption due to reoperation and prolonged ICU and hospital stay, but is not life threatening for most patients. Another important finding of our study was the correlation of perioperative mortality with the number of non-cardiac complications. The overall perioperative mortality in patients with two and three or more major non-cardiac complications was more than twofold (24.0 vs 9.7%) and fourfold (40.2 vs 9.7%), respectively, higher than in patients with a single non-cardiac complication. In these groups, the highest mortality was observed in patients presenting with respiratory failure or dialysis-dependent renal failure combined with other major non-cardiac complications. In patients requiring dialysis, the addition of one or two non-cardiac complications increased the perioperative mortality from 14 to 39 and 41%, respectively. These findings are in concordance with other studies that have confirmed the significant impact of renal failure alone or in combination with other complications and reported a perioperative mortality ranging from 30 to 80% [10, 20].
The association of most major non-cardiac complications following cardiac surgery with a negative outcome may lead to a situation where a decision has to be made about continuing, escalating, or de-escalating intensive care therapy in these critically ill patients. Several scoring systems such as the Acute Physiology and Chronic Health Evaluation score II (APACHE score II) or the Sequential Organ Failure Assessment score (SOFA score) have been developed for these purposes. However, most of these scoring systems were developed based on general and medical ICU patient populations and not specifically in cardiac surgery patients. This is important, because the latter often present particular risk factors such as advanced atherosclerotic disease or impaired left ventricular function [21]. Furthermore, the few studies that developed risk models specifically for cardiac surgery patients were based on preoperative characteristics only and did not take into account postoperative morbidities that have their own additional impact on postoperative mortality [22, 23]. The former study of the author was one of the first to include postoperative variables for the prediction of outcomes of critically ill patients following cardiac surgery [3]. In our recent study, which involves a large and contemporary cohort of patients, we confirmed our former findings and were able to generate and validate a predictive model, including 10 independent predictors of perioperative mortality: six preoperative and four postoperative variables. Most preoperative variables identified by our analysis, such as advanced age, peripheral vascular disease and emergency, have also been shown by other studies to negatively impact surgical outcome [3, 14, 24]. In addition, we included postoperative variables, which, once they occur, have an independent additional effect on perioperative mortality.
Our logistic model enabled us to predict perioperative mortality of individual patients after the occurrence of major non-cardiac complications with good accuracy as shown by the C-statistic, which indicated similar areas under the ROC curves for both the study population and the validation cohort. When our model was compared with the logistic EuroSCORE [7], it predicted mortality also more precisely. Despite the effectiveness of our model, it is important to recognize that even the most accurate scoring system generally only estimates, and misclassification rates may be up to 15% when using predictive models [25]. Furthermore, owing to time-related changes in patient demographics and improvements in medical therapy, clinical scoring systems need to be readjusted frequently. Nonetheless, we believe that predictive models such as ours, if applied with caution, can serve as a useful tool to provide an estimate of hospital survival in critically ill patients, which may be helpful in the interaction with the patient and his or her family and in the decision making about the length and invasiveness of ICU therapy in particularly difficult situations.
It was not the aim of this study to determine the risk factors for the development of the analysed major non-cardiac complications. However, the potentially worse outcome of patients experiencing these complications should also lead to a focus on the risk reduction for their occurrence. Patients at high risk for the development of major non-cardiac complications should be accurately identified based on known predictors reported by previous studies [10, 12, 13] and aggressively optimized preoperatively whenever possible. In addition, high-risk patients may require individual treatment strategies. For example, patients with complex valvular disease with or without concomitant coronary artery disease may benefit from hybrid and/or novel therapeutic approaches, such as transcatheter aortic valve replacement [10], particularly if presenting with other risk factors, such as advanced age, peripheral vascular disease or impaired left ventricular function.
Strengths and limitations
The study includes a large and heterogeneous group of patients who underwent the whole spectrum of cardiac surgical procedures, and therefore, the findings are applicable to the general population of adult cardiac surgery patients. Furthermore, the data analysed in this study were obtained from the mandatory and audited German Cardiac Surgery Quality Assurance System, which therefore provide very accurate information about perioperative variables. However, the set of included variables remains limited and some potential unknown confounding factors may not be addressed. Furthermore, this was a retrospective observational study, and therefore, conclusions are necessarily limited in their application. Moreover, our study did not examine the chronology of occurrence of non-cardiac complications, and we have not analysed the impact of primary cardiac complications such as low cardiac output syndrome, perioperative myocardial infarction or prolonged catecholamine requirement. Furthermore, clinical outcome analysis focused on postoperative mortality and morbidity, and we were not able to provide information on late complications, quality of life and cause of death following discharge.
CONCLUSIONS
Cardiac surgery today faces a patient population with a worsening risk profile, requiring more complex procedures than a decade ago and resulting in an increasing number of patients experiencing major postoperative complications. Advances in intensive care management of these complications help a growing number of patients to survive acute incidence. However, some patients develop serial complications and become chronically ill. Our study showed that these patients required prolonged hospitalization and presented with increased mortality, which was correlated with the number and severity of these complications. Furthermore, a significant number of patients with major morbidities were discharged to long-term rehab facilities or nursing homes. The development of a scoring system based on preoperative and postoperative variables allowed us to determine, with accuracy, the perioperative mortality in critically ill patients after cardiac surgery.
Conflict of interest: none declared.
APPENDIX. CONFERENCE DISCUSSION
Dr L. Noyez (Nijmegen, Netherlands): In the last 10 to 15 years we have seen a shift away from cardiac-related mortality in cardiac surgery, to what we can call morbidity and complication-related mortality. Until now, most published studies have dealt with the relationship between comorbidity and mortality/survival. In this regard your paper is interesting because you are studying the relationship between major adverse events and mortality and the impact of these complications on perioperative outcome. You identified independent predictors, and you constructed a model for prediction of perioperative mortality. So far I think it is a very excellent study.
However, I am a little bit disappointed in your end point of hospital or 30-day mortality. I think most of us know that if you have a patient with a major complication post cardiac surgery, he has a risk for a long hospital stay and a higher risk for perioperative mortality. So my first question is, why did not you study as an end point, six-month or one-year mortality or survival in relation to the complications? I think it is a very interesting subject. And a second question concerns the clinical use of your prediction model. If you have a patient with a major complication and a predictive risk of more than 90%, is this for you a moment that you decide not to treat these patients anymore?
Dr Rahmanian: I would like to answer the second question first. We just wanted to show that serious complications have a huge impact on survival, so we will not use just one model to calculate a patient's risk of dying and then decide to stop or go further with the therapy. That is not the case, and I think no one will do this during ICU therapy. But what is important is to know that the risk of patients not surviving the ICU stay increases with every additional serious complication that occurs. And if you know these numbers, you do not need to know the formula.
But if you go to your data in your own institution and try to analyse them in the same manner, then you will see the impact that complications will have on the survival of your patients. And then you know the percentages and can talk to the relatives and be able to say that things are going in a good or in a bad way. You can talk to them, and you can involve the family in deciding what to do with the patient. I think it is important to know the impact these complications have on survival. It is not only the model where you put numbers in and then you decide what to do; there is always a discussion with the family, in other words.
Dr H. Kamiya (Düsseldorf, Germany): I have a question. The goal of this study is to calculate postoperative mortality, yes?
Dr Rahmanian: Yes.
Dr Kamiya: Why did you exclude cardiac complications? For example, perioperative myocardial infarction also has a very big impact on postoperative mortality, right?
Dr Rahmanian: That is a good question. The aim of our study was to analyse the impact of complications on ICU stay and then to identify predictors that may be correlated with adverse outcome postoperatively. So we included typical ICU complications. Cardiac complications were excluded because they usually occur in the OR, in the short term after surgery. And most patients do not survive the cardiac complications of a cardiac arrest, or they may not survive the cardiac complication. The complications we analysed are following cardiac complications, and if you want to assess the impact of respiratory failure, then it does not matter if it occurs because of the weakness of the patient or if it occurs because the patient had a myocardial infarction during the operative procedure. We did not want to analyse this during our study.
Dr Kamiya: I said that because non-cardiac complications are often expressions of cardiac complications. For example, if a patient had low output syndrome, then he will have renal insufficiency, and he will have bowel ischaemia and so on. And so I think if the cardiac complications were to be involved, the study would be better.
Dr Rahmanian: We wanted to analyse the impact of non-cardiac complications because cardiac complications, first of all, are studied in many other reports. For example, perioperative myocardial infarction; there are a lot of studies assessing the impact of this complication on outcome. But if a patient survives myocardial infarction and becomes a long-term ICU patient, then he develops these complications which we analysed afterwards.
So the aim of the study was particularly not to analyse the cardiac complications but the non-cardiac complications. It is true that there are patients, of course, who had the cardiac complication as the reason for their worse perioperative outcome, but it was not the aim of our study to analyse this subgroup of patients.
Dr P. Gerometta (Milan, Italy): May I ask you just one question? It is really a wonderful score, and I realize how interesting it is for judging patients postoperatively. I was wondering whether you found correlation with preoperative scores, like the EuroSCORE or similar?
Dr Rahmanian: There is, of course, a correlation. The EuroSCORE, for example, can also predict survival of patients postoperatively, and we analysed the EuroSCORE or made a comparison of our score with the EuroSCORE, because I was thinking maybe these questions might be asked.
Dr Gerometta: You are then prepared.
Dr Rahmanian: But the EuroSCORE is weak in predicting survival postoperatively because the variables that are included in our score occur postoperatively and the EuroSCORE has no chance to catch them up. EuroSCORE includes risk factors for our complications, of course - combined procedures which means prolonged bypass time associated with respiratory failure. So EuroSCORE can also catch this, but if the complication occurs, then the score is much different. And when we compared the EuroSCORE with our score, EuroSCORE was 0.8. It is quite good also, but it is about 10% less accurate than our score.
REFERENCES
- 1.ElBardissi AW, Aranki SF, Sheng S, O'Brien SM, Greenberg CC, Gammie JS. Trends in isolated coronary artery bypass grafting: an analysis of the Society of Thoracic Surgeons adult cardiac surgery database. J Thorac Cardiovasc Surg. 2012;143:273–81. doi: 10.1016/j.jtcvs.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 2.O'Brien SM, Shahian DM, Filardo G, Ferraris VA, Haan CK, Rich JB, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2—isolated valve surgery. Ann Thorac Surg. 2009;88:S23–42. doi: 10.1016/j.athoracsur.2009.05.056. [DOI] [PubMed] [Google Scholar]
- 3.Rahmanian PB, Adams DH, Castillo JG, Carpentier A, Filsoufi F. Predicting hospital mortality and analysis of long-term survival after major noncardiac complications in cardiac surgery patients. Ann Thorac Surg. 2010;90:1221–9. doi: 10.1016/j.athoracsur.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Hosmer DW, Lemeshow S. Applied logistic regression. New York: Wiley; 1989. [Google Scholar]
- 5.Lemeshow S, Le Gall JR. Modeling the severity of illness of ICU patients. A systems update. JAMA. 1994;272:1049–55. [PubMed] [Google Scholar]
- 6.Bouch DC, Thompson JP. Severity scoring systems in the critically ill. Contin Educ Anaesth Crit Care Pain. 2008;8:181–85. [Google Scholar]
- 7.Roques F, Michel P, Goldstone AR, Nashef SA. The logistic EuroSCORE. Eur Heart J. 2003;24:881–2. doi: 10.1016/s0195-668x(02)00799-6. [DOI] [PubMed] [Google Scholar]
- 8.Pappalardo F, Franco A, Landoni G, Cardano P, Zangrillo A, Alfieri O. Long-term outcome and quality of life of patients requiring prolonged mechanical ventilation after cardiac surgery. Eur J Cardiothorac Surg. 2004;25:548–52. doi: 10.1016/j.ejcts.2003.11.034. [DOI] [PubMed] [Google Scholar]
- 9.Mehta RH, Grab JD, O'Brien SM, Bridges CR, Gammie JS, Haan CK, et al. Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation. 2006;114:2208–16. doi: 10.1161/CIRCULATIONAHA.106.635573. quiz 08. [DOI] [PubMed] [Google Scholar]
- 10.Rahmanian PB, Kwiecien G, Langebartels G, Madershahian N, Wittwer T, Wahlers T. Logistic risk model predicting postoperative renal failure requiring dialysis in cardiac surgery patients. Eur J Cardiothorac Surg. 2011;40:701–7. doi: 10.1016/j.ejcts.2010.12.051. [DOI] [PubMed] [Google Scholar]
- 11.Filsoufi F, Castillo JG, Rahmanian PB, Broumand SR, Silvay G, Carpentier A, et al. Epidemiology of deep sternal wound infection in cardiac surgery. J Cardiothorac Vasc Anesth. 2009;23:488–94. doi: 10.1053/j.jvca.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Filsoufi F, Rahmanian PB, Castillo JG, Bronster D, Adams DH. Incidence, imaging analysis, and early and late outcomes of stroke after cardiac valve operation. Am J Cardiol. 2008;101:1472–8. doi: 10.1016/j.amjcard.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 13.Filsoufi F, Rahmanian PB, Castillo JG, Scurlock C, Legnani PE, Adams DH. Predictors and outcome of gastrointestinal complications in patients undergoing cardiac surgery. Ann Surg. 2007;246:323–9. doi: 10.1097/SLA.0b013e3180603010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canver CC, Chanda J. Intraoperative and postoperative risk factors for respiratory failure after coronary bypass. Ann Thorac Surg. 2003;75:853–7. doi: 10.1016/s0003-4975(02)04493-4. discussion 57–8. [DOI] [PubMed] [Google Scholar]
- 15.Mangi AA, Christison-Lagay ER, Torchiana DF, Warshaw AL, Berger DL. Gastrointestinal complications in patients undergoing heart operation: an analysis of 8709 consecutive cardiac surgical patients. Ann Surg. 2005;241:895–901. doi: 10.1097/01.sla.0000164173.05762.32. discussion 01–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salazar JD, Wityk RJ, Grega MA, Borowicz LM, Doty JR, Petrofski JA, et al. Stroke after cardiac surgery: short- and long-term outcomes. Ann Thorac Surg. 2001;72:1195–201. doi: 10.1016/s0003-4975(01)02929-0. discussion 201–2. [DOI] [PubMed] [Google Scholar]
- 17.Rajakaruna C, Rogers CA, Angelini GD, Ascione R. Risk factors for and economic implications of prolonged ventilation after cardiac surgery. J Thorac Cardiovasc Surg. 2005;130:1270–7. doi: 10.1016/j.jtcvs.2005.06.050. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson TB, Jr, Hammill BG, Peterson ED, DeLong ER, Grover FL. A decade of change—risk profiles and outcomes for isolated coronary artery bypass grafting procedures, 1990–1999: a report from the STS National Database Committee and the Duke Clinical Research Institute. Society of Thoracic Surgeons. Ann Thorac Surg. 2002;73:480–9. doi: 10.1016/s0003-4975(01)03339-2. discussion 89–90. [DOI] [PubMed] [Google Scholar]
- 19.Rankin JS, Hammill BG, Ferguson TB, Jr, Glower DD, O'Brien SM, DeLong ER, et al. Determinants of operative mortality in valvular heart surgery. J Thorac Cardiovasc Surg. 2006;131:547–57. doi: 10.1016/j.jtcvs.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 20.Bove T, Calabro MG, Landoni G, Aletti G, Marino G, Crescenzi G, et al. The incidence and risk of acute renal failure after cardiac surgery. J Cardiothorac Vasc Anesth. 2004;18:442–5. doi: 10.1053/j.jvca.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 21.Ryan TA, Rady MY, Bashour CA, Leventhal M, Lytle B, Starr NJ. Predictors of outcome in cardiac surgical patients with prolonged intensive care stay. Chest. 1997;112:1035–42. doi: 10.1378/chest.112.4.1035. [DOI] [PubMed] [Google Scholar]
- 22.Tu JV, Jaglal SB, Naylor CD. Multicenter validation of a risk index for mortality, intensive care unit stay, and overall hospital length of stay after cardiac surgery. Steering Committee of the Provincial Adult Cardiac Care Network of Ontario. Circulation. 1995;91:677–84. doi: 10.1161/01.cir.91.3.677. [DOI] [PubMed] [Google Scholar]
- 23.Hein OV, Birnbaum J, Wernecke KD, Konertz W, Jain U, Spies C. Three-year survival after four major post-cardiac operative complications. Crit Care Med. 2006;34:2729–37. doi: 10.1097/01.CCM.0000242519.71319.AD. [DOI] [PubMed] [Google Scholar]
- 24.Scott BH, Seifert FC, Grimson R, Glass PS. Octogenarians undergoing coronary artery bypass graft surgery: resource utilization, postoperative mortality, and morbidity. J Cardiothorac Vasc Anesth. 2005;19:583–8. doi: 10.1053/j.jvca.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 25.Bashour CA, Yared JP, Ryan TA, Rady MY, Mascha E, Leventhal MJ, et al. Long-term survival and functional capacity in cardiac surgery patients after prolonged intensive care. Crit Care Med. 2000;28:3847–53. doi: 10.1097/00003246-200012000-00018. [DOI] [PubMed] [Google Scholar]