Abstract
OBJECTIVES
The purpose of this study was to evaluate the efficacy of the modified Nuss procedure with a subxiphoid incision in correcting recurrent pectus excavatum.
METHODS
From August 2006 to July 2010, 28 patients with recurrent pectus excavatum underwent a secondary repair using the modified Nuss procedure with a subxiphoid incision and bilateral thoracoscopy. Data concerning symptoms, operative course, complications, pulmonary function and early outcome were recorded.
RESULTS
Prior repairs of the reoperation patients included 16 Ravitch, 9 modified Ravitch and 3 sterno-turnover procedures. The median Haller index was 4.52 for the redo patients. Presenting symptoms included decreased endurance, dyspnoea on exertion, chest pain, frequent respiratory infections and palpitations. The median duration of reoperation was slightly longer than that of the primary surgeries. Blood loss and postoperative hospitalization were similar between groups. Complications from pectus reoperations included pneumothorax, pleural effusion, postoperative pain and wound infection in the lateral incision. There were no perioperative deaths or cardiac perforations. Initial postoperative results varied from excellent to good. The patients were followed up for 24–74 months. No steel bar malposition or stabilizer displacement was found in any case.
CONCLUSIONS
The modified Nuss procedure with subxiphoid incision and bilateral thoracoscopy can avoid cardiac injury to the greatest degree. It would be a minimally invasive and safe approach for patients with recurrent pectus excavatum after failed open repair.
Keywords: Pectus excavatum, Recurrence, Reoperation, Minimally invasive, Nuss procedure
INTRODUCTION
Pectus excavatum is the most common congenital chest wall deformity in children, characterized by posterior depression of the sternum and lower costal cartilages. Pectus excavatum occurs in ∼1:400 births [1]. The traditional open procedure for pectus excavatum repair includes Ravitch and modified Ravitch procedures, which involve the sub-perichondrial resection of abnormal costal cartilages, and the sterno-turnover procedure. Approximately 2–11% of patients have unsatisfactory results after initial chest wall correction and require reoperation [2]. Reoperative open surgery for pectus excavatum has been associated with extensive dissection, substantial blood loss, long surgical times and poor outcomes [3].
The Nuss procedure has become increasingly popular over recent years because of its minimally invasive approach. This method avoids extensive dissection and involves using a precurved metal bar to elevate the costal cartilages and sternum through two lateral chest wall incisions [4]. It is associated with short operating times, minimal blood loss and good cosmetic results. In general, the best candidates for the Nuss procedure are young patients with symmetrical deformities. However, the reoperative procedure is complicated and dangerous because of adhesions between the sternum and mediastinal structures. Since August 2006, we have repaired recurrent pectus excavatum using the modified Nuss procedure and achieved satisfactory results.
MATERIALS AND METHODS
A retrospective review was conducted concerning the records of all patients who undertook primary or recurrent pectus excavatum repair in our hospital from August 2006 to July 2010. Twenty-eight patients had recurrent pectus excavatum that had been previously open-repaired at other institutions. Evaluation before surgical repair included a chest computed tomographic scan to calculate the Haller index (defined as a ratio of the transverse diameter of the chest and the antero-posterior diameter between the sternum and spine), pulmonary function tests, electrocardiograph (ECG) and echocardiogram if necessary. Patient's medical records were reviewed for demographics, medical history, symptoms, degree of deformity, operative course, complications and initial outcomes. We took another 65 patients with pectus excavatum, who underwent primary Nuss procedure in our hospital during this period, as control. Data are expressed as mean ± SD. Statistical analysis was performed using the Student's t-test and a P-value <0.05 was considered significant.
Operative technique
The routine Nuss procedure required two lateral skin incisions (length: 2–3 cm), subcutaneous tunnelling and intrathoracic placement of a substernal convex bar (Walter Lorenz, Co., Jacksonville, FL, USA) with thoracoscopy. With regard to reoperation, in addition to bilateral axillary incisions, a small vertical subxiphoid anterior chest wall incision was crested (Fig. 1), which allowed the surgeon to pass his finger under the lower sternum. The surgeon's finger was used to bluntly dissect the retrosternal adhesions and push the pericardium away from the chest wall through the subxiphoid incision. After that, adhesions between lung with chest wall and pericardial was dissected with bilateral thoracoscope. Then the bar passer was placed through the interspace under the sternum onto the surgeon's finger, which was positioned in the retrosternal space via the subxiphoid incision to guide the passer. Finally, the pectus bar was pulled through the anterior mediastinum with a tape to the other incision, and again the surgeon's finger was used to protect the heart in the retrosternal space.
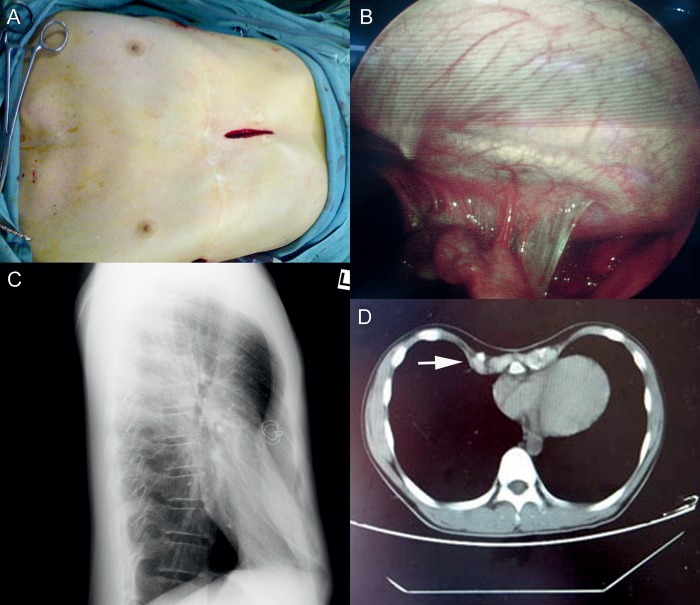
Figure 1:
(A) A small vertical subxiphoid incision was made in the anterior chest wall. (B) Thoracoscopy shows adhesions behind the sternum. (C) Chest X-ray shows depressed sternum after first operation; (D) CT scan shows abnormal ossification of the costal cartilages after Ravitch procedure.
The bar was secured on each lateral chest wall with two stabilizers that are fixed with a steel wire. The lung was inflated, and air was vented before closing the incisions. Postoperative pain was controlled by intravenous analgesia pumps for 48 h and by oral medications thereafter. Physical activity was restricted for 12 weeks. The bars were left in place for 2 years for first-time patients and >3 years for recurrent patients.
RESULTS
Patient characteristics
All patients with recurrent pectus were male, mean age was 14.6 years (range: 11–17 years) at reoperation and 8.5 years (range: 1–12 years) at the time of original operation. The primary operation of the recurrent patients included 16 Ravitch procedures, 9 modified Ravitch procedures and 3 sterno-turnover procedures (Table 1). All recurrent patients were classified with moderate-to-severe deformity with mean Haller index 4.7 (range: 3.6–5.9) at the time of reoperation. CT scan also showed abnormal ossification of the costal cartilages after open procedure in 23 (82.1%) patients (Fig. 1).
Table 1:
Characteristics of the 28 patients with recurrent pectus excavatum
| Male:female ratio | 25:3 |
| Primary operation | |
| Ravitch procedure | 16 |
| Modified Ravitch procedure | 9 |
| Sterno-turnover procedures | 3 |
| Mean age at initial surgery (years) | 8.5 (range: 1–12) |
| Mean age at reoperation (years) | 14.6 (range: 11–17) |
| Median Haller index | 4.7 (range: 3.6–5.9) |
Accompanying symptoms include decreased endurance, dyspnoea on exertion, chest pain, frequent respiratory infections and palpitations. CT scans and physical examination revealed cardiac compression and cardiac displacement. Some patients had ECG changes such as right bundle branch block and atrial premature beats. Pulmonary function tests showed impaired pulmonary function and mild-to-moderate restrictive lung disease in 20 patients (Table 2).
Table 2:
Presenting symptoms of patients
| Symptoms | Recurrent (n = 28) | Primary (n = 65) |
|---|---|---|
| Decreased endurance or dyspnoea on exertion | 17 (60.7%) | 29 (44.6%) |
| Chest pain | 8 (28.6%) | 15 (23.1%) |
| Palpitations | 6 (21.4%) | 13 (20%) |
| Frequent upper respiratory infections | 8 (28.6%) | 18 (27.7%) |
| Cardiac compression or displacement by CT | 25 (89.3%) | 49 (75.4%) |
| ECG anomalies | 10 (35.7%) | 24 (36.9%) |
| Abnormal pulmonary function | 20 (71.4%) | 19 (29.2%) |
The primary data were the control group.
Operative features
Operative features are shown in Table 3. All operations for recurrent patients were performed with lateral and subxiphoid incisions. No intraoperative complications occurred. Operating time was 66.5 min for the primary repair group and 85.5 min for the reoperative group (P < 0.01). Blood loss was 53.1 ml for the primary repair group and 64.6 ml for the reoperative group (P > 0.05). No patients required blood transfusion. The duration of postoperative hospital stay was similar between groups, with 3.8 days for the primary repair group and 4.2 days for the reoperative group (P > 0.05).
Table 3:
Operative results of patients
| Operative results | Recurrent (n = 28) | Primary (n = 65) | P-value |
|---|---|---|---|
| Operating time (min) | 85.5 ± 19.4 | 66.5 ± 15.4 | 0.001 |
| Blood loss (ml) | 64.6 ± 30.5 | 53.1 ± 30.4 | 0.150 |
| Hospital stay (days) | 4.2 ± 1.3 | 3.8 ± 1.1 | 0.163 |
The primary data were the control group.
Complications
The early complications included pneumothorax requiring chest tube, pleural effusion requiring drainage, wound infection, pericardium injury and postoperative pain. None of the patients developed pericarditis or pneumonia (Table 4). The complications rate was 13.8% for primary repair patients and 21.4% for reoperative patients. However, the complication of reoperative cases was minor. There were no perioperative deaths or cardiac perforations.
Table 4:
Complications of patients
| Complications | Recurrent (n = 28) | Primary (n = 65) |
|---|---|---|
| Pneumothorax (requiring chest tube) | 1 (3.6%) | 2 (3.1%) |
| Pleural effusion (requiring drainage) | 2 (7.1%) | 2 (3.1%) |
| Wound infection | 2 (7.1%) | 2 (3.1%) |
| Pericardium injury | 1 (3.6%) | 2 (3.1%) |
| Cardiac injury | 0 | 0 |
| Bar shifts (requiring revision) | 0 | 1 (1.5%) |
| Complications rate | 21.4% | 13.8% |
The primary data were the control group.
Postoperative follow-up
The postoperative assessments evidenced the disappearance of preoperative symptoms and the indication of patient satisfaction in all cases (Figs 2 and 3). The postoperative follow-up ranged from 24 to 74 months (mean: 42 months). Exercise tolerance increased in 16 patients and chest discomfort improved in 13. Pulmonary function tests 2 years after reoperation showed improvement in FEV1, FVC and FEF25–75% (Table 5). There was no stabilizer displacement and bar removal. Nineteen patients have had bar removal with no recurrence.
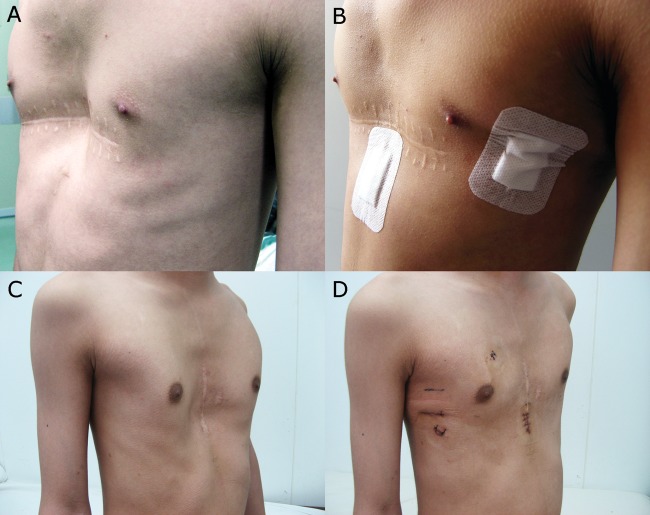
Figure 2:
Two patients with recurrent pectus after Ravitch procedure and postoperative appearance after reoperation. (A and B) The appearance of a 17-year old patient with recurrent pectus before (A) and after (B) reoperation. (C and D) The appearance of a 15-year old patient with recurrent pectus before (C) and after (D) reoperation.
Figure 3:
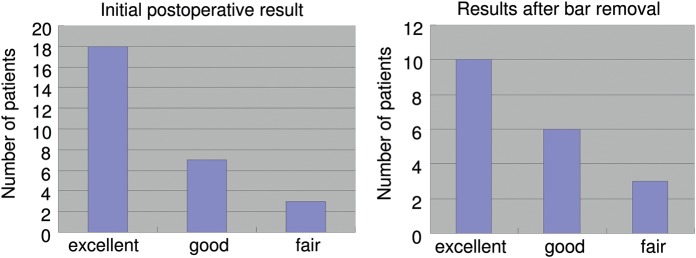
(A) Postoperative results for patients who underwent reoperation; (B) postoperative results after bar removAl.
Table 5:
Pulmonary function of recurrent patients before reoperation and 2 years after reoperation (xˉ ± s)
| Pulmonary function parameter (% predicted) | Before reoperation (% predicted) | 2 years after reoperation (% predicted) | P-value |
|---|---|---|---|
| VC | 81.3 ± 5.6 | 83.6 ± 4.5 | 0.088 |
| MVV | 87.9 ± 5.4 | 90.1 ± 5.2 | 0.114 |
| FEF25–75% | 80.2 ± 5.7 | 83.8 ± 4.9 | 0.012 |
| TLC | 88.2 ± 4.9 | 90.5 ± 4.5 | 0.078 |
| FRC | 97.7 ± 8.8 | 95.8 ± 8.5 | 0.117 |
| FVC | 73.3 ± 6.6 | 78.6 ± 5.5 | 0.002 |
| FEV1 | 75.5 ± 6.3 | 79.4 ± 6.6 | 0.017 |
VC: vital capacity; MVV: maximum minute ventilation; FEF25–75%: forced expiratory flow; TLC: total lung capacity; FRC: functional residual capacity; FVC: forced vital capacity; FEV1: forced expired volume in 1 s.
For follow-up classifications, the surgeon, the patient and his parents reached consensus regarding postoperative results using the following criteria: excellent if preoperative symptoms were resolved and the chest appearance was normal; good if preoperative symptoms were resolved and the chest appearance was improved; fair if preoperative symptoms were improved but the appearance was not completely normal; and failed if symptoms were worse and the chest appearance was not improved or if the deformity reoccurred.
DISCUSSION
Surgical repair of pectus deformities is most commonly performed during childhood and early adolescence in order to minimize cardiac or respiratory impairment, as well as to diminish significant psychological consequences. The standard open repair for pectus excavatum has been the Ravitch technique for decades [5], which involves extensive resection of deformed costal cartilages, anterior osteotomy and stabilization of the sternum. Despite the success of the techniques described above, some patients still face recurrent deformity or failure of the primary procedure [6].
The reasons for recurrence were varied. Age at the time of operation may be a principal factor in recurrence [7]. Extensive resection of ribs is another factor [8], which may cause injury to the growth centre of the rib and costal cartilage. Most recurrences are seen within the first 1–3 years after operation and a small depression can become severe during growth spurt [8]. Patients with connective tissue disorders such as Marfans' syndrome have a higher incidence of recurrence after repair [9]. Other factors associated with recurrence include displacement or premature removal of the metal support bar and local infection [10, 11].
We found it important to evaluate the presenting symptoms and chest wall configuration. Most of our patients required reoperation because they presented with symptoms such as chest pain, dyspnoea on exertion, frequent upper respiratory infections and palpitations, which were accompanied by cardiac compression, cardiac displacement and decreased values in pulmonary function tests. Many patients also have psychosocial reasons for repair, which should be considered. Antonoff's found that the median time until need for reoperation was 4.1 years, and they recommended that repair for both primary and recurrent pectus deformities be delayed until age 10 [12]. We thought the indications for reoperation should be based on the patient's age, symptoms, chest wall appearance and psychosocial impairments.
Correction of recurrent pectus is much more difficult and complex than primary repair. With the first open procedure, the irregular fusion and ossification of the regenerated costal cartilages often adhered to the pericardium and lung. The intrathoracic dense adhesions between the sternum and mediastinal structures increase both the difficulty and the potential risks of reoperative procedures [13]. When the bar passes through the mediastinum, it could produce fatal cardiac injury [14]. We applied thoracoscopy to prevent cardiac injury as the bar passed through the mediastinum and took down the pleural adhesions between lung and the anterior chest wall.
Thoracoscopy can assure that the course of the bar is satisfactory but does not assure safety as the bar passes across the mediastinum [15]. In order to assure safe passage of the bar, we add a subxiphoid incision to avoid injury to the heart. Through the incision, the surgeon's finger is used to dissect the pericardium safely off the substernal. Then the bar can pass safely through the mediastinum by guiding the surgeon's finger. Previous reports also advocate using a subxiphoid incision to decrease mediastinal injuries [16]. We feel that this modification diminishes the chance of serious injury to the heart as the bar passes through the mediastinum. The subxiphoid incision is a simple, safe and reproducible method, which was performed in all patients undergoing reoperation without difficulty.
Redo Nuss procedures require slightly more operating time compared with primary Nuss repairs, but this operating time is significantly shorter than the traditional open procedure [11]. Blood loss and postoperative hospitalization were similar between groups. The disadvantage is the location, being a cosmetic visible anterior incision, but the subxiphoid incision is small and worthwhile compared with the cardiac complications. Croitoru et al. [17] reported a slight increase in postoperative complications in 50 recurrent pectus excavatum patients who had undergone the Nuss procedure. There were no recurrences after removal of the bars in their study. Postoperative pulmonary function tests showed that FEF25–75%, FVC, FEV1 improved at 2 years of follow-up; but FVC and FEV1 were still <80%. This finding may be related to the abnormal ossification of the chest wall after open surgery [18].
The results of our study showed that patients with recurrent pectus excavatum after open repair can safely undergo reoperation by modified Nuss procedure, and achieve satisfactory results. Reoperation for recurrent pectus defects is a challenging undertaking. For patients undergoing failed prior open repair, the Nuss procedure is a viable alternative approach, which can be performed safely with minimal blood loss and a relatively short operating time. We believe the modified Nuss procedure with a subxiphoid incision and bilateral thoracoscopy is a promising alternative for patients with recurrent pectus deformities after previous open repair.
Conflict of interest: none declared.
REFERENCES
- 1.Jaroszewski DE, Fonkalsrud EW. Repair of pectus chest deformities in 320 adult patients: 21 year experience. Ann Thorac Surg. 2007;84:429–33. doi: 10.1016/j.athoracsur.2007.03.077. doi:10.1016/j.athoracsur.2007.03.077. [DOI] [PubMed] [Google Scholar]
- 2.Colombani PM. Recurrent chest wall anomalies. Semin Pediatr Surg. 2003;12:94–9. doi: 10.1016/s1055-8586(02)00018-5. doi:10.1016/S1055-8586(02)00018-5. [DOI] [PubMed] [Google Scholar]
- 3.Miller KA, Ostlie DJ, Wade K, Chaignaud B, Gittes GK, Andrews WM, et al. Minimally invasive bar repair for ‘redo’ correction of pectus excavatum. J Pediatr Surg. 2002;37:1090–2. doi: 10.1053/jpsu.2002.33883. doi:10.1053/jpsu.2002.33883. [DOI] [PubMed] [Google Scholar]
- 4.Nuss D, Kelly RE, Croitoru DP, Katz ME. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg. 1998;33:545–52. doi: 10.1016/s0022-3468(98)90314-1. doi:10.1016/S0022-3468(98)90314-1. [DOI] [PubMed] [Google Scholar]
- 5.Ravitch MM. The operative treatment of pectus excavatum. Ann Surg. 1949;129:429–44. doi: 10.1097/00000658-194904000-00002. doi:10.1097/00000658-194904000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willital GH, Meier H. Cause of funnel chest recurrences—operative treatment and long-term results. Prog Pediatr Surg. 1977;10:253–6. [PubMed] [Google Scholar]
- 7.Ishimaru T, Kitano Y, Uchida H, Kawashima H, Gotoh C, Satoh K, et al. Growth spurt-related recurrence after Nuss procedure. J Pediatr Surg. 2009;44:E13–6. doi: 10.1016/j.jpedsurg.2009.04.014. doi:10.1016/j.jpedsurg.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Haller JA, Jr, Colombani PM, Humphries CT, Azizkhan RG, Loughlin GM. Chest wall constriction after too extensive and too early operations for pectus excavatum. Ann Thorac Surg. 1996;61:1618–24. doi: 10.1016/0003-4975(96)00179-8. doi:10.1016/0003-4975(96)00179-8. [DOI] [PubMed] [Google Scholar]
- 9.Ellis DG, Snyder CL, Mann CM. The ‘re-do’ chest wall deformity correction. J Pediatr Surg. 1997;32:1267–71. doi: 10.1016/s0022-3468(97)90299-2. doi:10.1016/S0022-3468(97)90299-2. [DOI] [PubMed] [Google Scholar]
- 10.Willital GH, Meier H. Cause of funnel chest recurrences-operative treatment and long-term results. Prog Pediatr Surg. 1977;10:253–6. [PubMed] [Google Scholar]
- 11.Kasagi Y, Wada J, Nakajima H, Irie T, Kondo K, Ikeda T. Re-operation of pectus excavatum. Nippon Kyobu Geka Gakkai Zasshi. 1989;37:540–5. [PubMed] [Google Scholar]
- 12.Antonoff MB, Saltzman DA, Hess DJ, Acton RD. Retrospective review of reoperative pectus excavatum repairs. J Pediatr Surg. 2010;45:200–5. doi: 10.1016/j.jpedsurg.2009.10.036. doi:10.1016/j.jpedsurg.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 13.Lodge AJ, Wells WJ, Backer CL, O'Brien JE, Jr, Austin EH, Bacha EA, et al. A novel bioresorbable film reduces postoperative adhesions after infant cardiac surgery. Ann Thorac Surg. 2008;86:614–21. doi: 10.1016/j.athoracsur.2008.04.103. doi:10.1016/j.athoracsur.2008.04.103. [DOI] [PubMed] [Google Scholar]
- 14.Bouchard S, Hong AR, Gilchrist BF, Kuenzler KA. Catastrophic cardiac injuries encountered during the minimally invasive repair of pectus excavatum. Semin Pediatr Surg. 2009;18:66–72. doi: 10.1053/j.sempedsurg.2009.02.002. doi:10.1053/j.sempedsurg.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Pisón CJ, González AG, Pérez MA, Martínez Bermejo MA, Conde CJ, Bento BL. Correction of recurrent pectus excavatum post-Ravitch with the Nuss technique. Cir Pediatr. 2009;22:77–80. [PubMed] [Google Scholar]
- 16.St Peter SD, Sharp SW, Ostlie DJ, Snyder CL, Holcomb GW, 3rd, Sharp RJ. Use of a subxiphoid incision for pectus bar placement in the repair of pectus excavatum. J Pediatr Surg. 2010;45:1361–4. doi: 10.1016/j.jpedsurg.2010.02.115. [DOI] [PubMed] [Google Scholar]
- 17.Croitoru DP, Kelly RE, Jr, Goretsky MJ, Gustin T, Keever R, Nuss D. The minimally invasive Nuss technique for recurrent or failed pectus excavatum repair in 50 patients. J Pediatr Surg. 2005;40:181–6. doi: 10.1016/j.jpedsurg.2004.09.038. doi:10.1016/j.jpedsurg.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Zhong H, Zhang FX, Mei J, Li GQ, Xiao HB. Minimally invasive Nuss technique allows for repair of recurrent pectus excavatum following the Ravitch procedure: report of 12 cases. Surgery today. 2011;41:1156–60. doi: 10.1007/s00595-010-4424-8. [DOI] [PubMed] [Google Scholar]