Abstract
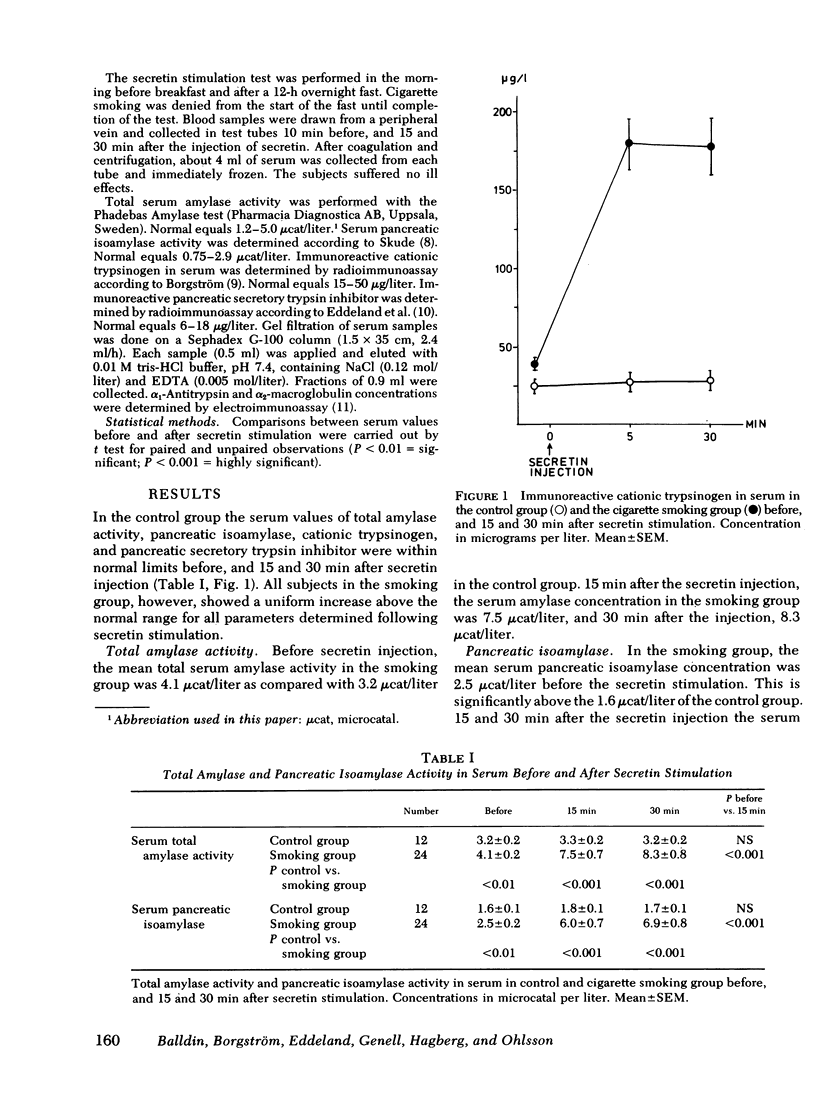
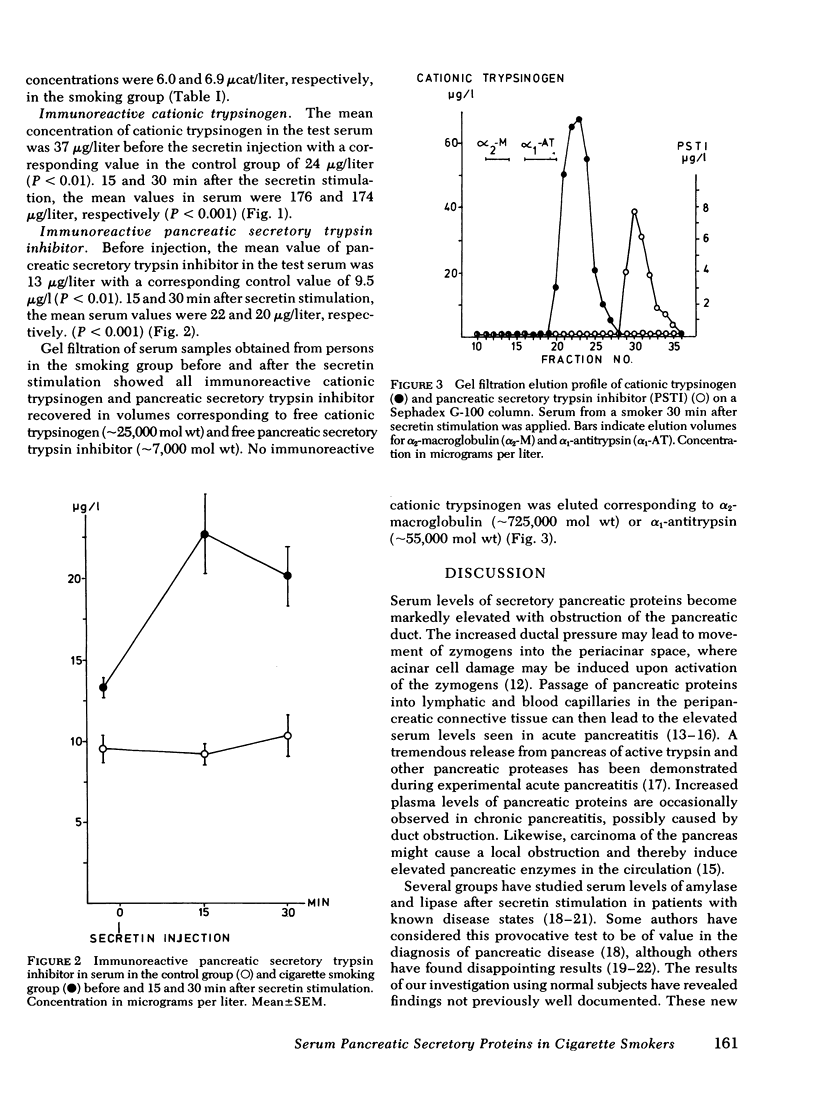
The secretory pancreatic proteins in serum were analyzed in a group of cigarette smokers and a control group of nonsmokers before and after intravenous secretin stimulation. None of these persons had any signs of pancreatic disease. In the control group, serum total amylase activity, pancreatic isoamylase, cationic trypsinogen, and pancreatic secretory trypsin inhibitor concentrations varied within the normal range before and after secretin injection. In contrast, the concentrations of these pancreatic proteins in all the cigarette smokers elevated from normal to abnormally high serum concentrations after secretin stimulation. The results indicate a probable toxic effect of cigarette smoking on the exocrine pancreas.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON P., HAMMOND E. M., HARPER A. A., HOWAT H. T., SCOTT J. E., VARLEY H. Serum amylase and serum lipase levels in man after administration of secretin and pancreozymin. Gut. 1960 Jun;1:125–139. doi: 10.1136/gut.1.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockman D. E., Schiller W. R., Suriyapa C., Mutchler J. H., Anderson M. C. Fine structure of early experimental acute pancreatitis in dogs. Lab Invest. 1973 May;28(5):584–592. [PubMed] [Google Scholar]
- Borgström A., Ohlsson K. Immunoreactive trypsin in serum and peritoneal fluid in acute pancreatitis. Hoppe Seylers Z Physiol Chem. 1978 Jun;359(6):677–681. doi: 10.1515/bchm.1978.359.1.677. [DOI] [PubMed] [Google Scholar]
- Borgström A., Ohlsson K. Radioimmunological determination and characterization of cathodal trypsin-like immunoreactivity in normal human plasma. Scand J Clin Lab Invest. 1976 Dec;36(8):809–814. doi: 10.3109/00365517609081942. [DOI] [PubMed] [Google Scholar]
- Brodrick J. W., Geokas M. C., Largman C., Fassett M., Johnson J. H. Molecular forms of immunoreactive pancreatic cationic trypsin in pancreatitis patient sera. Am J Physiol. 1979 Nov;237(5):E474–E480. doi: 10.1152/ajpendo.1979.237.5.E474. [DOI] [PubMed] [Google Scholar]
- DREILING D. A., RICHMAN A. Evaluation of provocative blood enzyme tests employed in diagnosis of pancreatic disease. AMA Arch Intern Med. 1954 Aug;94(2):197–212. doi: 10.1001/archinte.1954.00250020031002. [DOI] [PubMed] [Google Scholar]
- Eddeland A., Ohlsson K. A radioimmunoassay for measurement of human pancreatic secretory trypsin inhibitor in different body fluids. Hoppe Seylers Z Physiol Chem. 1978 Jun;359(6):671–675. doi: 10.1515/bchm.1978.359.1.671. [DOI] [PubMed] [Google Scholar]
- Elias E., Redshaw M., Wood T. Diagnostic importance of changes in circulating concentrations of immunoreactive trypsin. Lancet. 1977 Jul 9;2(8028):66–68. doi: 10.1016/s0140-6736(77)90066-6. [DOI] [PubMed] [Google Scholar]
- Krain L. S. The rising incidence of carcinoma of the pancreas--real or apparent? J Surg Oncol. 1970;2(2):115–124. doi: 10.1002/jso.2930020206. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Electroimmuno assay. Scand J Clin Lab Invest Suppl. 1972;124:21–37. doi: 10.3109/00365517209102748. [DOI] [PubMed] [Google Scholar]
- Leibow C., Rothman S. S. Enteropancreatic circulation of digestive enzymes. Science. 1975 Aug 8;189(4201):472–474. doi: 10.1126/science.1154022. [DOI] [PubMed] [Google Scholar]
- Lindstedt G., Lundberg P. A., Rolny P. Effect of secretin and cholecystokinin-pancreozymin on plasma CEA concentration in patients with pancreatic carcinoma and pancreatitis. Cancer. 1979 Jun;43(6):2465–2470. doi: 10.1002/1097-0142(197906)43:6<2465::aid-cncr2820430643>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Longnecker D. S. Environmental factors and diseases of the pancreas. Environ Health Perspect. 1977 Oct;20:105–112. doi: 10.1289/ehp.7720105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meites S., Rogols S. Amylase isoenzymes. CRC Crit Rev Clin Lab Sci. 1971 Jan;2(1):103–138. doi: 10.3109/10408367109151305. [DOI] [PubMed] [Google Scholar]
- Ohlsson K., Eddeland A. Release of proteolytic enzymes in bile-induced pancreatitis in dogs. Gastroenterology. 1975 Sep;69(3):668–675. [PubMed] [Google Scholar]
- Ohlsson K. Elimination of 125-I-trypsin alpha-macroglobulin complexes from blood by reticuloendothelial cells in dogs. Acta Physiol Scand. 1971 Feb;81(2):269–272. doi: 10.1111/j.1748-1716.1971.tb04900.x. [DOI] [PubMed] [Google Scholar]
- Pledger R. A., Bates R. R., Saffiotti U. Introduction: national cancer institute pancreatic carcinogenesis program. Cancer Res. 1975 Aug;35(8):2226–2227. [PubMed] [Google Scholar]
- Pour P., Krüger F. W., Althoff J., Cardesa A., Mohr U. A new approach for induction of pancreatic neoplasms. Cancer Res. 1975 Aug;35(8):2259–2268. [PubMed] [Google Scholar]
- SUN D. C., SHAY H. Pancreozymin-secretin test. The combined study of serum enzymes and duodenal contents in the diagnosis of pancreatic disease. Gastroenterology. 1960 Apr;38:570–581. [PubMed] [Google Scholar]
- Sarles H. An international survey on nutrition and pancreatitis. Digestion. 1973;9(5):389–403. doi: 10.1159/000197468. [DOI] [PubMed] [Google Scholar]
- Skude G. Sources of the serum isoamylases and their normal range of variation with age. Scand J Gastroenterol. 1975;10(6):577–584. [PubMed] [Google Scholar]
- Wynder E. L. An epidemiological evaluation of the causes of cancer of the pancreas. Cancer Res. 1975 Aug;35(8):2228–2233. [PubMed] [Google Scholar]


