Abstract
OBJECTIVES
Bronchopulmonary carcinoid tumours are relatively uncommon primary lung neoplasms. A small proportion of these lesions are predominantly endobronchial and do not extend beyond the bronchial wall. Endoscopic resection can be performed, but carries around a one in three risk of local recurrence and, therefore, mandates long-term surveillance. An alternative is complete surgical resection via bronchoplastic resection. We present our experience of surgical resection in patients with endobronchial carcinoids.
METHODS
From 2000 to 2010, 13 patients (age 45 ± 16 years, 10 males) underwent pure bronchoplastic resection, including systematic nodal dissection, for endobronchial carcinoid tumours, without the resection of lung parenchyma.
RESULTS
There was no significant operative morbidity or mortality. This is a retrospective review of a consecutive case series. The last follow-up for all patients was obtained in 2011. The mean maximum tumour size was 18 ± 8 mm. No lymph node invasion was observed. The median follow-up was 6.3 ± 3.3 years, with no regional recurrence. In 1 case, a tumourlet was identified at 5 years in the contralateral airway and viewed as a metachronous new lesion.
CONCLUSIONS
Bronchial sleeve resection is a safe procedure for suitably located endobronchial carcinoid tumours. Endoscopic resection should be reserved for patients who decline, or are unfit, for surgery.
Keywords: Carcinoid, Bronchoplastic resection, Sleeve resection, Endobronchial
INTRODUCTION
Bronchial carcinoids are rare neuroendocrine tumours, accounting for <5% of all bronchopulmonary tumours [1]. They are categorized as either typical or atypical and have distinctly different prognoses and therapeutic options when compared with non-small-cell carcinomas [1–3]. Typical carcinoids have an excellent prognosis, with a 10-year survival rate of >90%; nevertheless, these tumours can metastasize. In contrast, atypical carcinoids tend to be more aggressive and have a greater potential to metastasize, and thus have a 10-year survival rate of <60% [3, 4]. The natural history of carcinoids is largely related to the presence or absence of lymph node metastases [2, 5], and it is recommended in the 7th staging system to stage them using the TNM classification system [6]. Carcinoids can arise within a bronchus and be suitable for resection with bronchoplastic surgery if appropriately located, and this has been recommended when feasible [7, 8].
Approximately 20% of all carcinoid tumours present as purely intraluminal polyp-like endobronchial lesions without gross radiological detectable involvement of the bronchial wall and lung parenchyma [1]. Until recently, the treatment of choice has remained bronchoplastic surgery. However, some authors have described their experience using different endoscopic resection techniques, such as Nd-YAG laser, diathermy and cryosurgery [9–13].
We present our experience of resection of the central airway without removal of any parenchyma for endobronchial carcinoid tumours.
MATERIALS AND METHODS
Between January 2000 and December 2010, 13 patients underwent a parenchyma-sparing bronchial sleeve resection (i.e. without any parenchymal resection), with systematic nodal dissection for endobronchial carcinoid tumour without extension beyond the bronchial wall. Nodal dissection included principal stations 2, 4, 7, 9, 10 and 11 on the right side, and stations 4, 5, 6, 7, 9, 10 and 11 on the left. Data were collected retrospectively, but follow-up was on a prospective basis through the outpatient clinic and included bronchoscopy and chest X-ray. All patients were followed up within our outpatient clinic for at least 1 year with clinical, radiological follow-up and a bronchoscopy at 1 year. The histopathology reports were reviewed, and the tumours were staged using the 7th TNM staging system [6].
RESULTS
There were 13 patients: 10 were males; the median age was 45 years with a range from 16 to 69 years. Two left-sided, and 11 right-sided procedures were carried out (Table 1) (Fig. 1). Both left-sided procedures were sleeve resections of the left main bronchus. On the right, there were three sleeve resections of the main bronchus, one resection of the main bronchus together with the bronchus intermedius in a case that turned out to be an atypical carcinoid, four resections of the bronchus intermedius and three sleeve resections of the middle lobe bronchus. In one case, an endoscopic disobliteration of the bronchus intermedius was carried out prior to surgery to relieve obstruction. The resection was complete (R0) in all cases. The mean maximum tumour size was 18 ± 8 (range 6–30) mm. No lymph node metastases were observed, and all tumours were pathologically staged as pT1, N0 in that all cases had invasive components limited to the bronchial wall [6] (Table 2).
Table 1:
Resection types for endobronchial carcinoids in the presented patient series
| Right |
Left | ||
|---|---|---|---|
| Main stem bronchus | 3 | 1 | 2 |
| Bronchus intermedius | 4 | – | |
| Middle lobe bronchus | 3 | – | |
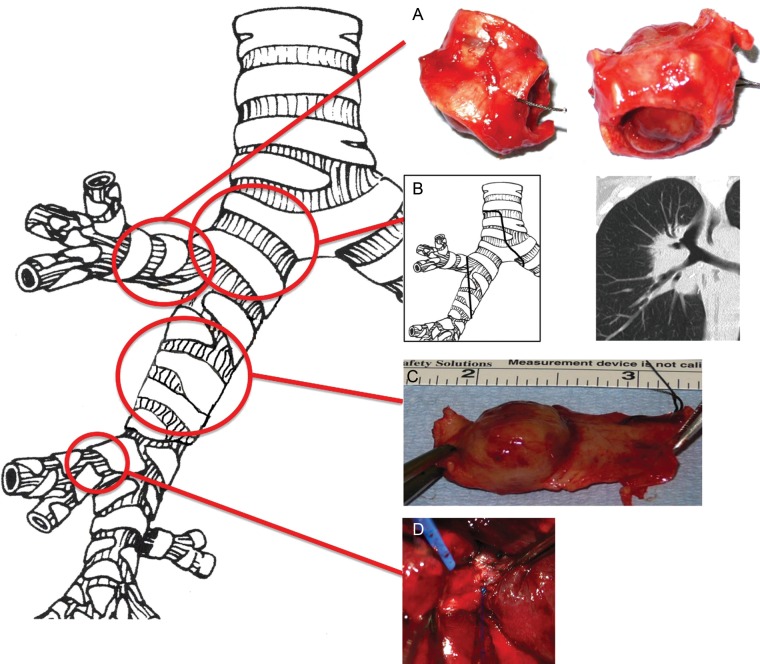
Figure 1:
Right-sided parenchyma-sparing bronchial sleeve resection types for endobronchial carcinoids. (A) Upper lobe division bronchial sleeve resection. (B) Central carinal and right main bronchial sleeve. (C) Bronchus intermedius sleeve resection. (D) Sleeve resection of the middle lobe bronchus.
Table 2:
Patient and tumour characteristics
| Age | Sex | Year of surgery | Operation | Carcinoid | Histology | Size (mm) |
|---|---|---|---|---|---|---|
| 52 | F | 2000 | Bronchial sleeve left main | Typical | pT1a, pN0, R0 | 12 × 12 |
| 23 | M | 2000 | Bronchial sleeve left main | Typical | pT1a, pN0, R0 | 19 × 12 |
| 42 | M | 2001 | Right main bronchial sleeve | Typical | pT1a, pN0, R0 | 8 × 5 × 2 |
| 34 | M | 2003 | Right bronchus intermedius | Tyical | pT1b, pN0, R0 | 30 × 20 × 15 |
| 32 | M | 2003 | Right bronchus intermedius | Ttypical | pT1b, pN0, R0 | 30 × 22 × 10 |
| 56 | M | 2004 | Right main bronchial sleeve | Typical | pT1b, pN0, R0 | 27 × 15 × 15 |
| 42 | M | 2004 | Right bronchus intermedius | Typical | pT1a, pN0, R0 | 19 × 16 |
| 69 | M | 2006 | Right main and bonchus intermedius | Atypical | pT1a, pN0, R0 | 16 × 16 × 10 |
| 16 | M | 2006 | Right main bronchus | Typical | pT1b, pN0, R0 | 22 × 15 × 15 |
| 48 | M | 2007 | Right bronchus intermedius | Typical | pT1a, pN0, R0 | 15 × 10 × 10 |
| 36 | M | 2008 | Right middle lobe bronchus | Typical | pT1a, pN0, R0 | 14 × 10 × 12 |
| 64 | F | 2009 | Right middle lobe bronchus | Typical | pT1a, pN0, R0 | 6 × 6 × 4 |
| 65 | F | 2010 | Right middle lobe bronchus | Typical | pT1a, pN0, R0 | 6 × 6 × 11 |
Twelve tumours were typical carcinoids, and there was one atypical carcinoid. Five years later, the patient with atypical carcinoid was found to have an asymptomatic small endobronchial polyp on the contralateral side at a routine check bronchoscopy. This was resected bronchoscopically and found to be a tumourlet. The median follow-up was 6.3 ± 3.3 years. No recurrence (local, regional or distant) of the primary tumour was observed in any case. There were no early or late surgical (such as anastomotic leak or stenosis) or pulmonary complications in our patient group. The mean hospital stay was 7.3 (minimum: 5 and maximum: 21) days. One patient stayed 21 days in the hospital for further treatment in the department of cardiology.
DISCUSSION
Bronchial sleeve resection without parenchymal resection offers a definitive solution for purely endobronchial carcinoids [14]. This procedure, although it can be technically challenging, can be carried out with very low morbidity and mortality (nil in our group of patients). No regional recurrences were observed, although 1 patient did have a metachronous tumourlet present in a contralateral airway, indicating the importance of long-term follow-up for carcinoids.
Bronchopulmonary carcinoid tumours are rare, accounting for approximately 2% of all pulmonary tumours [1, 12, 13]. About two-thirds arise in the major airways [1], making them potentially amenable to bronchoplastic resection, most of which will be sleeve lobectomies. The prognosis of those tumours is mainly determined by the histological type: for typical carcinoids, the reported 5- and 10-year survivals are between 87–100 and 85–93%, respectively [1, 15–18]. For the atypical variant, the prognosis is worse, with a 5-year survival between 40 and 77% and 10-year survival of about 50% [17, 18]. In the case of typical carcinoids, lymph node metastases can occur in >10% [16, 19]. For endobronchial lesions, pure bronchoplastic procedures (i.e. without parenchymal resection) have been advocated [4, 20, 21].
As carcinoid tumours tend to be indolent, complete resection is mandatory, but margins do not need to be wide. Therefore, bronchoplastic procedures are appropriate [14]. Systematic nodal dissection is an integral part of all resections for carcinoid tumours to ensure complete and accurate staging.
There is also an increasing interest in bronchoscopic treatment of these tumours, and some series have shown very good results also in the mid- and long-term [10, 12, 13, 22]. As pointed out by Brokx et al. [22], an important factor in this setting is whether or not the tumour is growing into or through the bronchial wall, in other words, whether or not a complete resection can be achieved by endoscopy. Finally, there is a real and finite risk of local recurrence (up to one-third) after endoscopic resection [2, 23].
As we could show in our series, despite its limitations (retrospective analysis, small cohort, single institution), surgery appears to offer a safe and definitive treatment option. If the tumour is obstructive, an improvement in postoperative lung function can be expected [14, 24]. We did not perform routine postoperative lung function studies in this series, so we cannot demonstrate this here. Surgical resection has the further advantage of providing complete lymph node staging, which is important, as a number of atypical and typical carcinoids have lymph node metastasis [16, 19].
Endoscopic treatment modalities do, however, have a place in the treatment of these tumours. First, they allow the airway to be disobliterated prior to surgery, so that the distal pneumonia can resolve [22]. They are also a good alternative for patients who are unfit for surgery. They do, however, have some drawbacks: they do not offer lymph node staging, larger tumours may have to be resected piecemeal or with several endoscopies in order to achieve complete resection [13, 22] and follow-up has to be assiduous as the risk of recurrence is higher [2, 23].
Funding
This project was supported by the NIHR Respiratory Research Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London.
Conflict of interest: none declared.
APPENDIX. CONFERENCE DISCUSSION
Dr P. De Leyn (Leuven, Belgium): In this retrospective study, the authors describe their results of 13 patients who underwent bronchoplastic resection for carcinoid tumours without anatomical resection of lung parenchyma, and it is evident that you need the technical skills of sleeve resection, sleeve segmentectomies, to do this type of operation.
I have three brief questions. In your manuscript and also in the presentation, you mentioned that there is no postoperative morbidity at all. This is, of course, very good, but can you tell us what the mean hospital stay of these patients was? And were there really no postoperative problems? It is uncommon in thoracic surgery to have no postoperative problems, even in 13 patients. Can you tell us the mean hospital stay?
Dr Nowak: The range of the mean hospital stay was between 7 and 21 days. We mainly focused on complications related to surgery and on postoperative pulmonary complications such as pneumonia. However, I have to admit that we did not include complications like postoperative tachyarrhythmia.
Dr De Leyn: But I would definitely advise for the paper that, if you are going to compare endoscopic treatment with surgery, you mention the postoperative problems in these patients, because a patient who stays 21 days in the hospital probably will have had some type of complication. You mentioned that you performed systematic nodal dissection. Can you tell us which lymph node stations you routinely dissected in this type of surgery?
Dr Nowak: We perform a standard systematic nodal dissection, so we dissect lymph node stations 2, 4, 7, 10, and then we dissect lymph node stations 9 and 8.
Dr De Leyn: Well, maybe this is also something to mention. And then in your paper, in the patient with atypical carcinoid, you mentioned that it was a local recurrence, the tumourlet. What is this?
Dr Nowak: You are misunderstanding. It was on the contralateral side. There was no recurrence of the tumour at the site of the anastomosis. It was on the contralateral side, and it was a typical carcinoid, not an atypical, as it was before.
Dr De Leyn: So it was a second primary probably?
Dr Nowak: Yes.
Dr K. Moghissi (Goole, United Kingdom): I have a comment and a question. The comment is that sleeve resection has been around, as you well know, for many years. In fact, my own presentation of 101 patients was in the Academie de Chirurgie in 1980-something. However, most people, from the time of Chevalier Jackson (who invented bronchoscopy, or expanded it) have treated typical carcinoid differently than atypical. Atypical carcinoid is a carcinoma, nothing less, nothing more. Typical carcinoids somehow are different. I have treated typical carcinoid with a sleeve resection in those people who, as you said, have not been suitable for surgery, for a number of years, and recently, for the last five or six cases, there has been photodynamic therapy, and the longest survival is about 11 years. The others who didn't survive did not die because of the tumour but died because they were not suitable for surgery to start with. Do you see what I mean? Comorbidity. My question, therefore, is this: In your typical carcinoid, how many patients have had lymphatic involvement?
Dr Nowak: I have shown this. None of the patients had any lymphatic involvement. But it is described in the literature that lymphatic involvement rarely occurs in typical carcinoids.
Dr Moghissi: So you have found it in some of them?
Dr Nowak: No, in that series we didn't find lymphatic involvement.
Dr Moghissi: I have never actually had a typical carcinoid where there has been infiltration of the lymph nodes. I do accept that there can be, but I have never had it. I would suggest that all typical carcinoids can be treated endoscopically, you are right, but not with diathermy.
Dr P. De Leyn (Leuven, Belgium): I have had patients with typical carcinoids who had positive lymph nodes, peribronchial. I think that one of the problems with a centrally-located carcinoid is that, bronchoscopically, you only see the tip of the iceberg. If you see on the CT that it is mainly in the wall of the bronchus and a bit peribronchial, it will be difficult to do it purely bronchoscopically, but that is my own opinion.
Dr M. Dusmet (London, United Kingdom): First of all, I have a slight addition. You asked what lymph node dissection is routinely done. On the right, that will include R2, R4, 7, 8, and 9, as well as the intrafissural, station 11. On the left, it will be at least L4, 5, 6, 7, and 9.
I also wanted to state that, as thoracic surgeons, we all love to tell our patients that they have a typical carcinoid tumour. We are used to dealing with non-small cell lung cancer that has a very poor prognosis. So typical carcinoid sounds fantastic, but I think we do need to be aware that if you look at the non-surgical literature, there is a proportion of typical carcinoids with low mitotic rates, low proliferation indexes, that can present at the outset with distant metastatic disease. Even the typical carcinoids have metastatic and malignant potential.
The other thing that we forget is that these tumours by definition have a very slow and long drawn-out evolution over time, and so if you do an endoscopic procedure, it means that this patient will have to have surveillance bronchoscopies, follow-up scans, and rather intensive follow-up, not for 3, not for 5, but for something like 10, 15, 20 years if they are young patients. That is the other advantage of surgery. It offers a definitive and complete resection.
Finally, as a response to the previous comment, I just recently saw a young woman in whom, at the age of something like 14, somebody did a thoracotomy, opened the airway, resected a typical carcinoid, and when she came back with recurrent disease that involved the lymph nodes, surgery was a real challenge, because, of course, the recurrence involved the whole bronchotomy, which involved the distal trachea, and it was a nightmare.
REFERENCES
- 1.Detterbeck FC. Management of carcinoid tumors. Ann Thorac Surg. 2010;89:998–1005. doi: 10.1016/j.athoracsur.2009.07.097. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Rush W, Flieder DB, Falk R, Fleming MV, Gal AA, et al. Survival analysis of 200 pulmonary neuroendocrine tumors with clarification of criteria for atypical carcinoid and its separation from typical carcinoid. Am J Surg Pathol. 1998;22:934–44. doi: 10.1097/00000478-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Mezzetti M, Raveglia F, Panigalli T, Giuliani L, Lo Giudice F, Meda S, et al. Assessment of outcomes in typical and atypical carcinoids according to latest WHO classification. Ann Thorac Surg. 2003;76:1838–42. doi: 10.1016/s0003-4975(03)01194-9. [DOI] [PubMed] [Google Scholar]
- 4.Hage R, de la Rivie`re AB, Seldenrijk CA, van den Bosch JMM. Update in pulmonary carcinoid tumors: a review article. Ann Surg Oncol. 2003;10:697–704. doi: 10.1245/aso.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Cardillo G, Sera F, Di Martino M, Graziano P, Giunti R, Carbone L, et al. Bronchial carcinoid tumors: nodal status and long-term survival after resection. Ann Thorac Surg. 2004;77:1781–5. doi: 10.1016/j.athoracsur.2003.10.089. [DOI] [PubMed] [Google Scholar]
- 6.Rami-Porta R, Crowley JJ, Goldstraw P. The revised TNM staging system for lung cancer. Ann Thorac Cardiovasc Surg. 2009;15:4–9. [PubMed] [Google Scholar]
- 7.Cooper WA, Thourani VH, Gal AA, Lee RB, Mansour KA, Miller JI. The surgical spectrum of pulmonary neuroendocrine neoplasms. Chest. 2001;119:14–8. doi: 10.1378/chest.119.1.14. [DOI] [PubMed] [Google Scholar]
- 8.Ducrocq X, Thomas P, Massard G, Barsotti P, Giudicelli R, Fuentes P, et al. Operative risk and prognostic factors of typical bronchial carcinoid tumors. Ann Thorac Surg. 1998;65:1410–4. doi: 10.1016/s0003-4975(98)00083-6. [DOI] [PubMed] [Google Scholar]
- 9.Cavaliere S, Foccoli P, Toninelli C. Curative bronchoscopic laser therapy for surgically resectable tracheobronchial tumors. J Bronchol. 2002;9:90–5. [Google Scholar]
- 10.Sutedja TG, Schreurs AJ, Vanderschueren RG, Kwa B, vd Werf TS, Postmus PE. Bronchoscopic therapy in patients with intraluminal typical bronchial carcinoid. Chest. 1995;107:556–8. doi: 10.1378/chest.107.2.556. [DOI] [PubMed] [Google Scholar]
- 11.Luckraz H, Amer K, Thomas L, Gibbs A, Butchart EG. Long-term outcome of bronchoscopically resected endobronchial typical carcinoid tumors. J Thorac Cardiovasc Surg. 2006;132:113–5. doi: 10.1016/j.jtcvs.2006.01.061. [DOI] [PubMed] [Google Scholar]
- 12.Bertoletti L, Elleuch R, Kaczmarek D, Jean-Francois R, Vergnon JM. Bronchoscopic cryotherapy treatment of isolated endoluminal typical carcinoid tumor. Chest. 2006;130:1405–11. doi: 10.1378/chest.130.5.1405. [DOI] [PubMed] [Google Scholar]
- 13.Fuks L, Fruchter O, Amital A, Fox BD, Rahman NA, Kramer MR. Long-term follow-up of flexible bronchoscopic treatment for bronchial carcinoids with curative intent. Diagn Ther Endosc. 2009:782961. doi: 10.1155/2009/782961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Jamal M, Nicholson AG, Goldstraw P. The feasibility of conservative resection for carcinoid tumours: is pneumonectomy ever necessary for uncomplicated cases? Eur J Cardiothorac Surg. 2000;18:301–6. doi: 10.1016/s1010-7940(00)00519-4. [DOI] [PubMed] [Google Scholar]
- 15.Harpole DH, Jr, Feldman JM, Buchanan S, Young WG, Wolfe WG. Bronchial carcinoid tumors: a retrospective analysis of 126 patients. Ann Thorac Surg. 1992;54:50–4. doi: 10.1016/0003-4975(92)91139-z. discussion 54–5. [DOI] [PubMed] [Google Scholar]
- 16.Das-Neves-Pereira JC, Bagan P, Milanez-de-Campos JR, Capelozzi VL, Danel C, Jatene FB, et al. Individual risk prediction of nodal and distant metastasis for patients with typical bronchial carcinoid tumors. Eur J Cardiothorac Surg. 2008;34:473–7. doi: 10.1016/j.ejcts.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Marty-Ané CH, Costes V, Pujol JL, Alauzen M, Baldet P, Mary H. Carcinoid tumors of the lung: do atypical features require aggressive management? Ann Thorac Surg. 1995;59:78–83. doi: 10.1016/0003-4975(94)00630-P. [DOI] [PubMed] [Google Scholar]
- 18.Beasley MB, Thunnissen FB, Brambilla E, Hasleton P, Steele R, Hammar SP, et al. Pulmonary atypical carcinoid: predictors of survival in 106 cases. Hum Pathol. 2000;31:1255–65. doi: 10.1053/hupa.2000.19294. [DOI] [PubMed] [Google Scholar]
- 19.Wurtz A, Benhamed L, Conti M, Bouhindhomme B, Porte H. Curative systematic nodal dissection in typical and atypical carcinoid tumors of the lung. J Thorac Oncol. 2009;4:388–94. doi: 10.1097/JTO.0b013e3181951aa6. [DOI] [PubMed] [Google Scholar]
- 20.Cerfolio RJ, Deschamps C, Allen MS, Trastek VF, Pairolero PC. Mainstem bronchial sleeve resection with pulmonary preservation. Ann Thorac Surg. 1996;61:1458–62. doi: 10.1016/0003-4975(96)00078-1. discussion 1462–3. [DOI] [PubMed] [Google Scholar]
- 21.Yavuzer S, Yüksel C, Kutlay H. Segmental bronchial sleeve resection: preserving all lung parenchyma for benign/low-grade neoplasms. Ann Thorac Surg. 2010;89:1737–43. doi: 10.1016/j.athoracsur.2010.02.060. [DOI] [PubMed] [Google Scholar]
- 22.Brokx HA, Sutedja TG. Cutting edge without cutting corners: bronchoscopic treatment for bronchial carcinoids. Respiration. 2011;81:285–6. doi: 10.1159/000323612. [DOI] [PubMed] [Google Scholar]
- 23.Van Boxem TJ, Venmans BJ, van Mourik JC, Postmus PE, Sutedja TG. Bronchoscopic treatment of intraluminal typical carcinoid: a pilot sudy. J Thorac Cardiovasc Surg. 1998;116:402–6. doi: 10.1016/S0022-5223(98)70005-4. [DOI] [PubMed] [Google Scholar]
- 24.Bagan P, Le Pimpec-Barthes F, Badia A, Crockett F, Dujon A, Riquet M. Bronchial sleeve resections: lung function resurrecting procedure. Eur J Cardiothorac Surg. 2008;34:484–7. doi: 10.1016/j.ejcts.2008.05.051. [DOI] [PubMed] [Google Scholar]