Abstract
OBJECTIVES
This study was conducted to investigate the prognostic factors of pulmonary metastases, focusing on the time of detection of pulmonary metastases related to adjuvant chemotherapy in patients with colorectal cancer (CRC).
METHODS
Between June 2003 and December 2010, 84 patients underwent pulmonary metastasectomy for pulmonary metastasis (PM) from CRC. The clinicopathological data of colorectal surgery and pulmonary metastasectomy were analysed retrospectively. Disease-free intervals (DFIs) were specifically classified into the following three groups related to adjuvant chemotherapy after colorectal operation: Group 1, metastasis detected at initial presentation; Group 2, metastasis detected during adjuvant chemotherapy; Group 3, metastasis detected after completion of chemotherapy. The prognostic influence of these variables on disease-free survival was analysed using the log-rank test for univariate analysis and the Cox proportional hazards model for multivariate analysis.
RESULTS
The median follow-up durations for patients after curative resection of CRC and pulmonary metastasectomy were 48.6 and 28.8 months, respectively. After pulmonary metastasectomy, recurrence was seen in 49 (58.3%) patients—pulmonary recurrence in 37 and extrathoracic recurrence in 12. Young age (<54 years old) at CRC operation, more than one PM, a DFIs of <12 months, detection synchronously or under adjuvant chemotherapy, and high CEA level before metastasectomy were worse prognostic factors by univariate analysis. From multivariate analysis, the number of pulmonary metastases (multiple metastases, HR = 2.121, 95% confidence interval 1.081–4.159, P = 0.029) and DFIs related with adjuvant chemotherapy (Group 1+2, HR = 1.982, 95% confidence interval 1.083–3.631, P = 0.027) were found to be independent predictors of disease-free survival.
CONCLUSIONS
Disease-free intervals in association with the time of adjuvant chemotherapy and number of metastases were independent poor prognostic factors for pulmonary metastases from colorectal cancer.
Keywords: Pulmonary metastasis, Colorectal carcinoma, Prognostic factor.
INTRODUCTION
Colorectal cancer (CRC) is one of the most common types of cancer in developed countries and its incidence rate has been increasing very rapidly in the last decade in Korea. Approximately 10% of patients with CRC develop pulmonary metastases (PM), and only 1–2% of them will be candidates for pulmonary metastasectomy [1]. Pulmonary metastasectomy is able to significantly improve the overall survival rate in patients with PM, and various prognostic indicators have been suggested in order to increase the success rate of surgical treatment [2–4]. However, due to the heterogeneity of the selection criteria and variations in the multimodal treatment provided, various overall survival rates and prognostic factors have been reported according to the study design and size of the study population. The resectability, disease-free interval (DFI) and number of metastases are well known as prognostic factors from large-scaled studies [5]. Among the three factors, DFIs represent the biology of the tumour; therefore, aggressive tumours reappeared in the lung within a short time and patients with short DFIs had a worse prognosis. However, it is difficult to exactly define DFIs because the time of detection, rather than time when the metastasis reappears, is the only information available. If a patient were to undergo intensive surveillance in the postoperative follow-up programme, recurrence would be found earlier and DFIs would be shorter than patients who underwent less intensive surveillance. After a CRC operation, most patients with pulmonary metastasis (PM) underwent adjuvant chemotherapy due to the advanced stage of the initial primary cancer. Therefore, in the process of treatment for CRC, time of identification of PM related with the process of treatment for CRC might be more important and meaningful than only dichotomized DFIs.
Therefore, the purpose of this study was to investigate the prognostic factors of PM and focus on the prognostic value of the time of identification of PM related to adjuvant therapy in patients after CRC surgery.
METHODS
Between June 2003 and December 2010, after curative resection of CRC, the patients regularly received follow-up care with physical examinations, biochemistry tests and abdominal computed tomography (CT) to check for local recurrence, and chest CT was performed every 6 months for the first 2 years and once a year thereafter.
Among the patients who underwent curative resection of CRC, 107 underwent pulmonary metastasectomy. The selection criteria for pulmonary resection were as follows: (i) no evidence of uncontrolled primary site, (ii) no evidence of extrathoracic metastasis except liver metastasis, (iii) chest CT demonstrating that complete resection could be warranted regardless of the number of lesions, and (iv) tumours in the bilateral thorax not considered as a contraindication. As for patients who developed additional pulmonary metastases after pulmonary resection, further resections were performed according to the above-mentioned criteria. Principally, wedge resection through thoracotomy or video-assisted thoracic surgery (VATS) was the procedure of choice, although lobectomy was performed when the metastatic lesion was located deep in the pulmonary parenchyma. Mediastinal lymph node dissection was not routinely undertaken. Among patients who underwent surgery for CRC, those who had pathological stage III or higher stage cancer all received adjuvant chemotherapy about 1 month after surgery. The choice of the chemotherapy agent was 5-FU, and irinotecan or oxaliplatin was added to complete the dual therapy. The average period for the chemotherapy was 5 months.
In each patient, clinicopathological data of the CRC operation and pulmonary metastasectomy were analysed retrospectively. As for CRC factors, age, gender, smoking status, origin (colon vs rectum), body mass index (BMI) and serum carcinoembryonic antigen (CEA), albumin level before CRC resection, pathological stage and differentiation were evaluated. The pulmonary factors of size, number of pathologically confirmed metastases, maximum standard uptake value (mSUV) of pulmonary metastatic lesions in positron emission tomography/CT (PET/CT), CEA, albumin level before metastasectomy and laterality of metastasis distribution were included. The DFIs comprised the period between the last curative treatment for CRC and the appearance of PM. In this study, the traditional classification of the DFIs was used where they are divided into those appearing before or after 12 months. The DFIs were also more specifically classified into the following three groups related to adjuvant chemotherapy after CRC resection: Group 1 (synchronous), patients whose PM were simultaneously identified with CRC; Group 2 (during chemotherapy), those whose PM were identified during adjuvant chemotherapy; Group 3 (after chemotherapy), those whose PM were identified after the completion of adjuvant treatment. The disease-free survival (DFS) and overall survival rates from pulmonary metastasectomy were analysed.
Statistical methods
DFS was estimated by the Kaplan–Meier method. The prognostic influence of variables on DFS was analysed using the log-rank test for univariate analysis and the Cox proportional hazards model for multivariate analysis. A value of P < 0.05 was regarded as significant. SPSS statistical software version 14.0 (SPSS, Inc., Chicago, IL, USA) was used for all statistical analyses.
RESULTS
One hundred and seven patients (64 males and 43 females) underwent curative pulmonary metastasectomy between June 2003 and December 2010. Twenty-three patients (21.5%) were excluded from this study due to incomplete pre-CRC resection and adjuvant chemotherapy data because their surgery was performed outside of our hospital; as a result, the study population consisted of 84 patients.
Thirteen (15.5%) patients were ever-smokers and the mean BMI before CRC resection was 24.0 (range 16.7–36.4) kg/m2. The CRC origin was the colon in 50 (59.5%) patients and the rectum in 34 (40.5%). The preoperative CEA was abnormal (≥5 ng/ml) in 34 patients and normal (<5 ng/ml) in 50 patients, and the mean albumin level was 4.1 (range 2.5–4.9) g/dl before CRC resection. The mSUV of the primary mass was evaluated in only 19 (25.0%) patients, and the mean mSUV was 8.0 (range 2.8–16.6). In terms of differentiation, 7 patients presented well-differentiated lesions, 75 presented moderately differentiated lesions, and 2 patients presented poorly differentiated lesions. All patients except 7 underwent adjuvant chemotherapy after curative resection of CRC. The median DFIs between the curative resection of CRC and appearance of PM was 14 (range 0–72) months. The DFIs were <12 months in 36 patients (42.9%) and >12 months in 48 (57.1%). When the DFIs were classified according to the adjuvant chemotherapy used, 21 patients (27.3%) were in Group 1 (synchronous), 12 (15.6%) in Group 2 (during chemotherapy) and 44 (57.1%) in Group 3 (after chemotherapy).
The average mass size of the PM was 14 (range 3–35) mm and the average number of pulmonary metastases was 2.6 (range 1–16). The mSUV of PM in the PET/CT was obtained in 71 (84.5%) patients and the mean mSUV was 2.7 (range 0.6–16.6). The mean CEA value and albumin level were 7.4 ng/ml (range 1.0–203.2) and 4.3 g/dl (range 3.1–4.9), respectively. Sixty-two (73.8%) patients had unilateral lesions and 22 (26.2%) had bilateral lesions. Among the 22 patients with bilateral lesions, 17 underwent bilateral resection simultaneously and 5 had staged surgery.
Regarding the surgical approach, 49 (58.3%) and 35 (41.7%) patients underwent VATS and an open procedure, respectively. The extent of surgery was as follows: wedge resection in 69 (82.1%), segmentectomy in 5 (6.0%) and lobectomy in 10 (11.9%).
As for node metastasis, none of the patients included in this study received mediastinal lymph node dissection. However, 10 patients who received lobectomy presented negative intrapulmonary node from pathological tests.
There were no major complications or operative mortality. The median follow-up for patients after curative resection of CRC and pulmonary resection was 48.6 and 28.8 months, respectively. Seventeen patients (20.2%) died during the follow-up period. The cause of death was disease-related for all patients. The median survivals after the pulmonary resection and the curative resection of CRC were 31.1 (range 1.5–88.0) months and 50.8 (range 15.2–104.5) months, respectively. After pulmonary metastasectomy, recurrence was seen in 49 (58.3%) patients; pulmonary recurrence in 37 and extrathoracic recurrence in 12. Among the 37 patients with pulmonary recurrence, 9 had recurrences at previous resection sites, 6 had ipsilateral recurrences, 13 had contralateral recurrences, and 9 had recurrences bilaterally. In terms of frequencies or locations of pulmonary recurrences, there was no difference observed between VATS and thoracotomy. There were 19 patients who received surgery, and they presented 1.7 (1–4) metastasized lesions. On the other hand, 36 patients could not receive surgery due to the multiple numbers of lesions; 7 presented <10 metastasized lesions, and the rest presented 10 or more lesions. Among those 19 patients who received surgery, VATS was chosen as the surgical method in 13 patients.
The 1-, 3- and 5-year DFS rates after pulmonary metastasectomy were 63.2, 43.9, and 41.3%, respectively. The DFS rates according to various clinical factors were determined by univariate analysis. Young age (<54 years old) at CRC operation, more than one PM, a DFIs of <12 months, detection synchronously or under adjuvant chemotherapy, and high CEA level before PM were worse prognostic factors (Table 1). The mean duration of DFS was 20.5 months in Group 1 (synchronous), 25.7 months in Group 2 (during chemotherapy) and 48.7 months in Group 3 (after chemotherapy). There was a significant difference between Groups 1 + 2 and Group 3 (P = 0.005); however, there was no difference between Group 1 and Group 2 (Fig. 1). From multivariate analysis, the number of pulmonary metastases (multiple metastases, HR = 2.121, 95% confidence interval 1.081–4.159, P = 0.029) and DFIs related with adjuvant chemotherapy (Group 1+2, HR = 1.982, 95% confidence interval 1.083–3.631, P = 0.027) were found to be independent predictors of overall survival (Table 2). Twenty-two patients who had liver metastasis combined with PM presented mean survival of 26.3 months, which was statistically significantly different from the mean survival of 45.1 months in patients who solely had PM (P = 0.0249).
Table 1:
Univariate analysis for disease-free survival
| No. of patients | DFS | P-value | |
|---|---|---|---|
| Age (years) | 0.035 | ||
| ≤54 | 27 | 24.8 | |
| >54 | 57 | 46.3 | |
| Sex | 0.891 | ||
| Male | 47 | 41.9 | |
| Female | 37 | 34.8 | |
| Tumour location | 0.274 | ||
| Colon | 50 | 36.2 | |
| Rectum | 34 | 48.5 | |
| BMI before CRC (kg/m2) | 0.261 | ||
| <23.8 | 34 | 49.2 | |
| >23.8 | 50 | 30.7 | |
| Smoking | 0.599 | ||
| Never | 71 | 39.8 | |
| Ever | 13 | 49.2 | |
| Albumin level before CRC (mg/dl) | 0.131 | ||
| >4.0 | 42 | 32.2 | |
| <4.0 | 42 | 49.4 | |
| CEA level before CRC (ng/ml) | 0.141 | ||
| <6.0 | 50 | 37.0 | |
| >6.0 | 34 | 41.0 | |
| Number | 0.001 | ||
| 1 | 35 | 58.5 | |
| >1 | 49 | 29.0 | |
| Disease-free interval (months) | 0.027 | ||
| >12 | 48 | 52.3 | |
| <12 | 36 | 23.6 | |
| DFI, CTx related | 0.012 | ||
| Synchronous | 21 | 20.5 | |
| During CTx | 12 | 25.7 | |
| After CTx | 44 | 48.7 | |
| DFI | 0.005 | ||
| Synchronous + during CTx | 33 | 22.9 | |
| After CTx | 44 | 48.7 | |
| Size of PM (cm) | 0.163 | ||
| <1.8 | 65 | 44.8 | |
| >1.8 | 19 | 32.8 | |
| mSUV of PM | 0.172 | ||
| <1.2 | 18 | 43.9 | |
| >1.2 | 53 | 37.1 | |
| BMI before PM (kg/m2) | 0.129 | ||
| >24.1 | 33 | 43.2 | |
| <24.1 | 51 | 35.3 | |
| CEA level before PM (ng/ml) | 0.020 | ||
| <6.1 | 71 | 47.7 | |
| >6.1 | 13 | 17.4 | |
| Albumin level before PM (mg/dl) | 0.588 | ||
| >4.3 | 52 | 35.8 | |
| <4.3 | 32 | 35.8 |
BMI: body mass index; CRC: colorectal cancer; mSUV: maximum standard uptake value; CEA: carcinoembryonic antigen; DFI: disease-free interval; CTx: chemotherapy; PM: pulmonary metastasis; DFS: disease-free survival.
Figure 1:
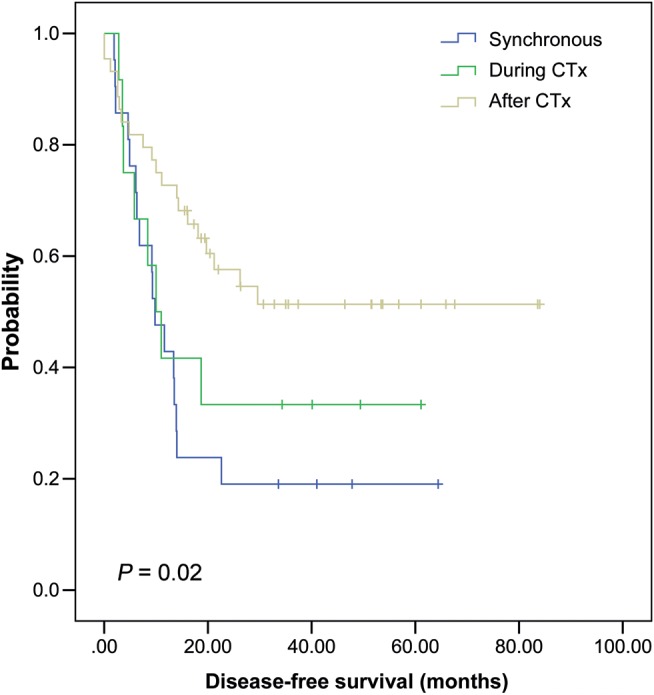
Disease-free survival according to the disease-free interval related to adjuvant chemotherapy after colorectal cancer resection.
Table 2:
Multivariate analysis for disease-free survival
| OR | 95% CI | P-value | |
|---|---|---|---|
| Age, <54 years old | 1.734 | 0.952–3.160 | 0.072 |
| Multiple metastases | 2.121 | 1.081–4.159 | 0.029 |
| Synchronous/during chemotherapy | 1.982 | 1.083–3.631 | 0.027 |
CI: confidential intervals.
The patients were divided into the synchronous and metachronous groups, and the number of patients were 23 (27.4%) and 61 (72.6%), respectively. The mean DFSs were 19.5 and 50.4 months, respectively, which showed a statistically significant difference between the groups (P = 0.004).
DISCUSSION
Advances in surgical techniques and adjuvant therapy have resulted in better surgical outcomes and longer term survival of patients with CRC in recent years. Among the long-term survivors, many patients experienced PM and more of them had the opportunity to undergo pulmonary metastasectomy. The number of pulmonary metastasectomies appears to be increasing, and has now reached over 10%. In our institution, on comparing the number of cases of PM in 2004–2007 with those of 2008–2011, many more pulmonary metastasectomies had been performed during the latter period. This phenomenon resulted from the widening of the indications for pulmonary metastasectomy because surgical resection showed better outcomes than chemotherapy for PM from CRC. However, retrospective studies and those with small sample sizes have not shown constant prognostic factors. A report of the International Registry of Lung Metastases (IRLM) that collated results from 18 major centres in Europe and North America analysed 5206 cases and found that the following parameters showed prognostic significance: completeness of resection, a DFI over 36 months and a solitary metastasis at the time of resection [5].
The number of pulmonary metastases is one of the most common prognostic factors found in the IRLM study. In their study, 2169 patients with single metastasis showed 43% and 342 patients with more than 10 metastases showed 26% 5-year survival rate [5]. Yedibela et al. [1] reported that patients with a solitary metastasis showed a higher 5-year survival rate than those with multiple metastases in pulmonary metastasectomy from CRC (44.4 vs 24.4%). In accordance with these reported results, the number of pulmonary metastases was found to be an independent predictor of overall survival (HR = 2.121, 95% confidence interval 1.081–4.159, P = 0.029). However, the prognostic value of the number of pulmonary metastases seemed to be more dependent on associated resectability than on the number per se. It is the author's opinion that it is highly probable that patients with multiple metastases had additional hidden nodules that were not resected at the initial operation; therefore, complete resection has not been accomplished at the initial operation. In case of multiple small metastases, it would be more beneficial if pulmonary metastasectomy is withheld for at least 3 months from the first detection. It will allow sufficient time to clarify if there is no additional PMs as Tanaka mentioned [6]. Although the survival rate clearly tends to decrease proportionally with the number of resected lesions, a firm cut-off point beyond which resection is useless has not been defined.
A shorter DFI indicated more aggressive biology of the primary tumour and ultimately determined poorer overall survival. In our study, 42.9% of patients had short DFIs (<12 months) and shorter DFSs than those with long DFIs. Such a cut-off value of 12 months was calculated by using receive of characteristic (ROC) from the patients enrolled in this study. However, even though applying ROC curve to dichotomize continuous value is considered as the most common and appropriate method, it can be problematic because the cut-off values vary in each study. Instead of using the conventional DFIs classification, we might approach the time from CRC operation to pulmonary recurrence from a different angle. As most of the enrolled patients have adjuvant chemotherapy due to advanced stage of initial CRC, the new classification which was the relation of the CRC operation and the time of adjuvant chemotherapy has been applied. Tsuchiya [7] also explained this concept by showing the relationship between the PM from osteosarcoma and perioperative chemotherapy. Therefore, we classified the DFIs into three groups based on when the PM was detected: first, synchronous (detected simultaneously with CRC); second, within 2 months after adjuvant chemotherapy and third, after 2 months of adjuvant chemotherapy. When the DFIs are considered in light of this classification, a question may arise regarding why this new classification is meaningful since most of the patients in the first 2 cases can be clustered in the shorter DFI group (<12 months). First, it settled the cut-off value, which can be changed in each research, and second, it was not necessary to change continuous values to dichotomized values. Therefore, DFI in association with the time of adjuvant chemotherapy, in the present author's opinion, is more valuable than DFI alone as a prognostic factor for recurrence.
Age is frequently associated with the prognosis of primary cancer and PM. It is generally believed that young patients with CRC have a worse survival rate. Young patients are more likely to present with late-stage disease and also have higher grade tumours [8]. About 60–67% of young patients with CRC have an advanced stage (III/IV) disease, most of which are poorly differentiated or mucinous tumours indicating a very poor prognosis [9, 10]. Iizasa et al. [11] reported that patients <60 years old had a worse 5-year survival rate than those 60 or older, although age was not an independent prognostic factor. This explained that the biology of tumour cells in young patients is much more aggressive than that in older patients, and it gives a reason why young patients with PM show such poor prognosis. In our study, univariate analysis revealed a statistically significant lower DFS rate in patients younger than 54 years than in patients aged 54 years or older, but multivariate analysis did not show any statistically significant result. Selection bias may exist because those young patients with PM were often excluded from surgery due to their extensive recurrence.
Recently, VATS has become a very popular method of minimally invasive surgery, and it is increasingly being applied to pulmonary metastasectomy. Although its efficacy for pulmonary metastasectomy is controversial, in our study, 58.3% of patients underwent VATS metastasectomy and showed a comparable survival rate to those undergoing open surgery. The main disadvantages of VATS metastasectomy are localization of small nodules and loss of non-visualized additional nodules. However, in terms of loss of non-visualized nodules, Nakas et al. [12] reported no difference in the incidence of missed lesions and concluded that VATS metastasectomy in conjunction with multidetector CT was justified, and the insertion of a surgical digit was not mandatory. Therefore, if complete resection of PMs by VATS is promising and no additional detection of nodules during open surgery are guaranteed due to the precise CT results, metastasectomy by VATS can certainly be an option.
The role of PET/CT in pulmonary metastasectomy is controversial, and there have been no reports on the prognostic impact of the mSUV of PM. The mSUV of PET/CT was obtained in 71 (84.5%) patients in our study; this high proportion arose from our routine use of PET/CT before pulmonary metastasectomy for excluding extrathoracic or primary site recurrence. Although mSUV was influenced by tumour size, cell type and differentiation of primary cancer, the role of mSUV of PM as a prognostic factor needed to be clarified. The DFS was longer in patients whose mSUV was <1.2 than in those whose value was >1.2 (43.9 vs 37.1 months, P = 0.172). There were significant borderline trends with a P-value of 0.067 in the overall survival between the high mSUV and low mSUV groups (17.1 vs 22.2 months) [13].
This study had some limitations. First, it used a small sample size. Twenty-three patients who received surgery for CRC from other institutes were excluded from this study because the indications for adjuvant chemotherapy could be incompatible with treatment schedules. All of them presented metachronous cancer without PM at the time of CRC surgery. The median follow-up durations for patients after curative resection of CRC and pulmonary metastasectomy were 68.8 and 31.2 months, respectively. This duration was slightly longer than that of enrolled patients, and recurrence was seen in 10 (43.5%) patients after pulmonary metastasectomy. Based on the given data, the characteristics of this group of patients were not believed to be different from those of enrolled patients.
Second, there was no standard protocol of adjuvant chemotherapy. During a long-term period, the schedule and regimen of chemotherapy were changed; as a result, the duration of Group 2 was different in each patient. Third, we divided post-CRC periods into within 2 months after chemotherapy and after 2 months of chemotherapy. A follow-up chest CT was performed within 2 months after adjuvant chemotherapy; therefore, metastatic lesions that were detected with this chest CT were included in Group 2 (during chemotherapy).
CONCLUSIONS
After pulmonary metastasectomy, DFIs in association with the time of adjuvant chemotherapy and number of metastases were independent poor prognostic factors for pulmonary metastases from CRC. Considering that this is a single-centre study with limited sample size, further prospective studies might be suggested with larger numbers of samples.
Conflict of interest: none declared.
REFERENCES
- 1.Yedibela S, Klein P, Feuchter K, Hoffmann M, Meyer T, Papadopoulos T, et al. Surgical management of pulmonary metastases from colorectal cancer in 153 patients. Ann Surg Oncol. 2006;13:1538–44. doi: 10.1245/s10434-006-9100-2. doi:10.1245/s10434-006-9100-2. [DOI] [PubMed] [Google Scholar]
- 2.Inoue M, Ohta M, Iuchi K, Matsumura A, Ideguchi K, Yasumitsu T, et al. Benefits of surgery for patients with pulmonary metastases from colorectal carcinoma. Ann Thorac Surg. 2004;78:238–44. doi: 10.1016/j.athoracsur.2004.02.017. doi:10.1016/j.athoracsur.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 3.Girard P, Ducreux M, Baldeyrou P, Rougier P, Le Chevalier T, Bougaran J, et al. Surgery for lung metastases from colorectal cancer: analysis of prognostic factors. J Clin Oncol. 1996;14:2047–53. doi: 10.1200/JCO.1996.14.7.2047. [DOI] [PubMed] [Google Scholar]
- 4.Okumura S, Kondo H, Tsuboi M, Nakayama H, Asamura H, Tsuchiya R, et al. Pulmonary resection for metastatic colorectal cancer: experiences with 159 patients. J Thorac Cardiovasc Surg. 1996;112:867–74. doi: 10.1016/S0022-5223(96)70085-5. doi:10.1016/S0022-5223(96)70085-5. [DOI] [PubMed] [Google Scholar]
- 5.Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. The International Registry of Lung Metastases. J Thorac Cardiovasc Surg. 1997;113:37–49. doi: 10.1016/s0022-5223(97)70397-0. doi:10.1016/S0022-5223(97)70397-0. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka Y, Maniwa Y, Nishio W, Yoshimura M, Okita Y. The optimal timing to resect pulmonary metastasis. Eur J Cardiothorac Surg. 2008;33:1135–8. doi: 10.1016/j.ejcts.2008.03.002. doi:10.1016/j.ejcts.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Tsuchiya H, Kanazawa Y, Abdel-Wanis ME, Asada N, Abe S, Isu K, et al. Effect of timing of pulmonary metastases identification on prognosis of patients with osteosarcoma: the Japanese Musculoskeletal Oncology Group study. J Clin Oncol. 2002;20:3470–7. doi: 10.1200/JCO.2002.11.028. doi:10.1200/JCO.2002.11.028. [DOI] [PubMed] [Google Scholar]
- 8.O'Connell JB, Maggard MA, Liu JH, Etzioni DA, Ko CY. Are survival rates different for young and older patients with rectal cancer? Dis Colon Rectum. 2004;47:2064–9. doi: 10.1007/s10350-004-0738-1. doi:10.1007/s10350-004-0738-1. [DOI] [PubMed] [Google Scholar]
- 9.Bedikian AY, Kantarjian H, Nelson RS, Stroehlein JR, Bodey GP. Colorectal cancer in young adults. South Med J. 1981;74:920–4. doi: 10.1097/00007611-198108000-00007. doi:10.1097/00007611-198108000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Minardi AJ, Jr, Sittig KM, Zibari GB, McDonald JC. Colorectal cancer in the young patient. Am Surg. 1998;64:849–53. [PubMed] [Google Scholar]
- 11.Iizasa T, Suzuki M, Yoshida S, Motohashi S, Yasufuku K, Iyoda A, et al. Prediction of prognosis and surgical indications for pulmonary metastasectomy from colorectal cancer. Ann Thorac Surg. 2006;82:254–60. doi: 10.1016/j.athoracsur.2006.02.027. doi:10.1016/j.athoracsur.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 12.Nakas A, Klimatsidas MN, Entwisle J, Martin-Ucar AE, Waller DA. Video-assisted versus open pulmonary metastasectomy: the surgeon's finger or the radiologist's eye? Eur J Cardiothorac Surg. 2009;36:469–74. doi: 10.1016/j.ejcts.2009.03.050. doi:10.1016/j.ejcts.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 13.Kim HR, Kim DJ, Lee WW, Jheon S, Sung SW. The significance of maximum standardized uptake values in patients with stage I pulmonary adenocarcinoma. Eur J Cardiothorac Surg. 2009;35:712–6. doi: 10.1016/j.ejcts.2008.12.030. doi:10.1016/j.ejcts.2008.12.030. [DOI] [PubMed] [Google Scholar]


