Abstract
OBJECTIVES
Personalized external aortic root support has completed initial evaluation and has technology appraisal in the UK for patients with Marfan syndrome for use as an alternative to root replacement. Its long-term success in preventing aortic dissection remains uncertain. Here, we report a study in sheep to establish whether the externally supporting mesh, as used clinically, is biologically incorporated. The strength of the resulting mesh/artery composite has been tested.
METHODS
The carotid artery of growing sheep (n = 6) was enclosed in a mesh sleeve made of a polymer, polyethylene terephthalate. After a predefined interval of 4–6 months, a length of the artery was excised, including the sleeved and unsleeved portions, and was stress tested and examined histologically.
RESULTS
One animal died of pneumonia 7 days after implantation. Comparing sleeved with normal segments, the overall thickness was increased and there was a fibrotic sheet in the periarterial space. The overall vessel wall architecture was preserved in all specimens. Although media thickness of ensleeved arteries was smaller and in one animal mild oedema was found in one quadrant of the outer part of the media. There was a significant increase in stiffness and maximum tensile strength of the supported segments compared with normal arterial tissue.
CONCLUSIONS
Polyethylene terephthalate mesh, as used for the external support of the dilated aortic root in Marfan syndrome, becomes incorporated in the periadventitial tissue of the carotid artery of sheep. Limited thinning of the media, without any signs of inflammation or medial necrosis, was visible. There was a significantly greater tensile strength in the carotid artery/mesh composite compared with the unsleeved carotid artery.
Keywords: Experimental, Thoracic aortic aneurysm, Blood vessel prosthesis
INTRODUCTION
Aortic root replacement, either with valve replacement or valve conservation, is the standard means of reducing the risk of aortic dissection in people with Marfan syndrome [1]. In 2004, an alternative to root replacement was proposed in which an external support of a pliant mesh is computer designed and custom made, as shown in Fig. 1. This fits the individual patient's ascending aorta from the aortoventricular junction to beyond the brachiocephalic artery [2]. The polymer of which the support is manufactured is polyethylene terephthalate, familiar to the polymer used in Dacron vascular grafts. However, these are woven to make a low-porosity vascular replacement with a relatively rigid, non-compliant wall structure. When knitted as a mesh, the material is soft and macroporous.
Figure 1:
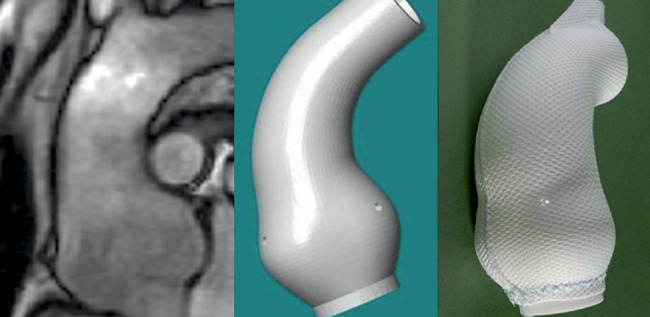
Computed tomography images and computer reconstruction of the ascending aorta of a Marfan patient leading to a custom-made, porous, pliable mesh.
The technical details of the manufacture and placement of the external support were reported for the first 10 consecutive patients, all operated on with the same protocol. They had stable aortic root and arch dimensions on magnetic resonance imaging after 1–3 years of follow-up [3]. The early perioperative and procedural advantages (avoidance of bypass and much reduced blood product usage) were confirmed in the first 20 consecutive patients compared with 20 patients of similar age and aortic root size having root replacement surgery in the same time frame [4].
It is noteworthy that the material used and its method of placement bear scant resemblance to the ‘wrap’ technique as proposed by Robicsek and Thubrikar [5]. This difference merits attention because reports of histological changes in the aorta underlying external support with Dacron grafts [6] and aortic erosion due to dislocation of a supporting Dacron Graft [7] have raised serious concerns in discussion of this novel form of personalized external aortic root support. To specifically address these questions, a study in sheep was performed.
MATERIALS AND METHODS
Operation
Approval was obtained from the Animal Ethics Committee of KUL, Leuven, Belgium (EC no.: P144-2010). Growing sheep were fasted for 24 h prior to surgery and sedated with intramuscular injection of ketamine (15 mg/kg). Anaesthesia was induced with isoflurane (5%), the animals were intubated and an oral-gastric tube was placed. Anaesthesia was maintained with Isoflurane (2–4%). Peripheral venous and arterial access were established. Heart rate, blood pressure, end-tidal CO2 and blood O2 saturation were monitored continuously. The animals were positioned in a right lateral position. After scrubbing and skin preparation of the neck, sterile towels and drapes were applied. A longitudinal incision was made to expose the common carotid artery. Polyethylene terephthalate (Dacron® mesh, Exstent Ltd., Tewkesbury, UK) was fitted around the carotid artery to form a sleeve over a length of 3 cm and closed with a running suture Prolene 4/0 (Fig. 2A). The incision was closed in layers. Explantation was performed between 4 and 6 months later. The animals were anaesthetized as described above. The incision was reopened and the carotid artery exposed. After the administration of heparin (100 U/kg), the entire carotid artery was removed (Fig. 2B). Thereafter, samples from sleeved and unoperated parts of the same carotid artery were taken for macroscopic, histological and mechanical evaluation.
Figure 2:
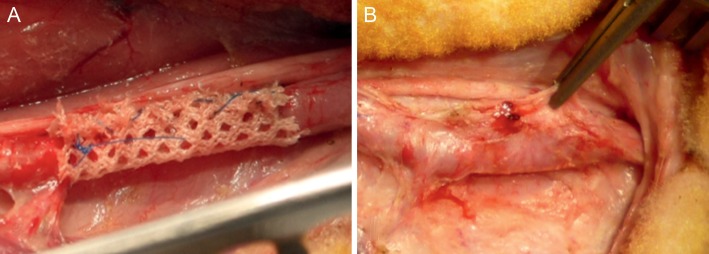
Exstent mesh material at the time of implantation (A) and explantation (B).
Macroscopic evaluation
Excised samples were photographed, opened longitudinally, and the thickness of samples was assessed with callipers. From the wrapped segments, the thickness of the graft material (0.60 mm) was subtracted from the measured thickness to obtain the corrected thickness.
Histological evaluation
Samples were fixed in a 6% formaldehyde solution and embedded in paraffin. Slices were prepared and stained with H&E and elastica staining. All samples were evaluated by an experienced pathologist (E.V.). Inflammation and fibrosis of the arterial and periarterial tissue were evaluated. The diameter of the lumen, media thickness, adventitial thickness and thickness of the fibrotic sheet were measured. From each sample, three slides were evaluated and six measurements per slide were performed.
Mechanical evaluation
From each retrieved graft and control carotid segment, a circumferentially oriented strip of approximately 10 by 5 mm was cut. All samples were retained in a physiological saline solution at 4°C and tested within 24 h of explantation. The segments under test were chosen to exclude the suture line of the mesh sleeve. Before being mounted on a computerized uniaxial test bench (Zwick/Roell Z0.5, Ulm, Germany), with a 200-N load cell, the initial width and thickness of each sample were measured with callipers. A cross-head speed of 0.5 mm/s was applied. The specimens were first subjected to five preconditioning cycles of loading, up to a standard force of 6.67 N/cm2. This was followed by a continuous stretch to failure while recording the load and gauge length. The breaking point of the sample was defined as the first point where failure occurred, easily identified in the tensile curve by a sudden drop of the load. Results were discarded if the specimen slipped in the grips during the test, or if specimen failure occurred close to the grips.
The data were processed, with the Zwick/Roelll testXpert II V2.2 software, to obtain the tensile strength, which is the tensile force normalized to the cross-sectional area at that point. To calculate the appropriate cross-sectional area, the assumption of material incompressibility was made [8]. The stiffness was obtained in the zone with physiological values of applied stress. Therefore, the first derivative of the tensile strength—logarithmic strain curve, was obtained.
Statistics
Statistical analysis was conducted using SPSS (IBM SPSS statistics, Sun Microsystems, USA). Data are presented as mean ± standard deviation (SD). Independent samples t-test was used. An α-value of <0.05 was considered significant.
RESULTS
Six sheep with a mean weight of 16 ± 0.9 kg were operated on. One animal died 7 days after the operation because of pneumonia and was excluded from further analysis. At the time of explantation, the mean body weight was 27 ± 1.6 kg. We obtained supported and unsupported samples from each of the five carotid arteries. All samples were macroscopically and histologically examined.
Several segments were tensile tested from each specimen giving a mean value for each sheep. The results of the tensile tests of one of the normal carotid arteries were excluded, due to slippery of the specimen and/or failure within the grips.
Macroscopic evaluation
Macroscopic evaluation of all samples showed loose tissue between the mesh and vessel wall (Fig. 3). In three of the samples, the mesh material was visible through the arterial wall. Measurements of the overall thickness, including the perivascular tissue, showed a significantly greater dimension of the externally supported samples, compared with the sham-operated carotid arteries (Table 1).
Figure 3:
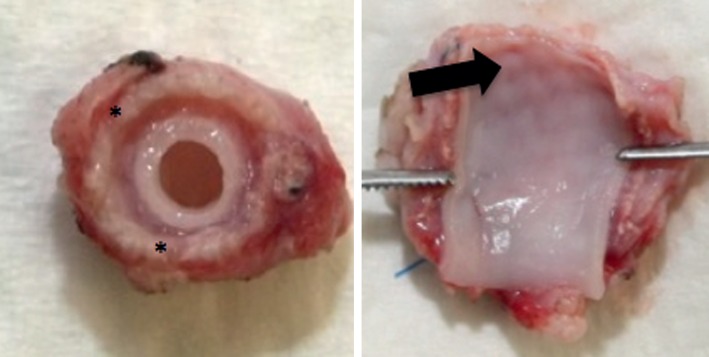
Mesh (*) grafted carotid artery; in some samples graft material shined through the vessel wall (arrow).
Table 1:
Corrected thickness, stiffness and tensile strength for the normal and supported carotid arteries
| Sheep | Corrected thickness (mm) |
Stiffness |
Tensile strength (N/cm2) |
|||
|---|---|---|---|---|---|---|
| Normal | Supported | Normal | Supported | Normal | Supported | |
| 1 | 0.66 | 1.13 | 1.11 | 2.83 | 206.29 | 1359.94 |
| 2 | 0.69 | 1.62 | 1.61 | 2.58 | 211.43 | 1057.21 |
| 3 | 1.66 | 3.27 | 1128.94 | |||
| 4 | 0.64 | 1.20 | 1.33 | 1.38 | 170.22 | 370.21 |
| 5 | 0.74 | 1.68 | 1.20 | 4.53 | 167.21 | 863.44 |
| Mean | 0.68 | 1.34 | 1.31 | 2.92 | 188.79 | 955.95 |
| P-value | <0.001 | 0.029 | 0.010 | |||
Histological evaluation
The graft material in all samples was located within the periarterial tissue; on several places, it touched the adventitia. Figure 4 illustrates the intact vessel wall architecture as it was retrieved in four of five wrapped specimens. The surrounding graft material caused a fibrotic reaction, but no inflammation, of the periarterial tissue in all samples. In all samples, the intima was well preserved. In four of the five vessels, the media was intact. In one, we found oedema of the outer part of the media in one quadrant of the circumference. In sham-operated samples, there was an intact arterial and periarterial structure without evidence of fibrosis or inflammation.
Figure 4:
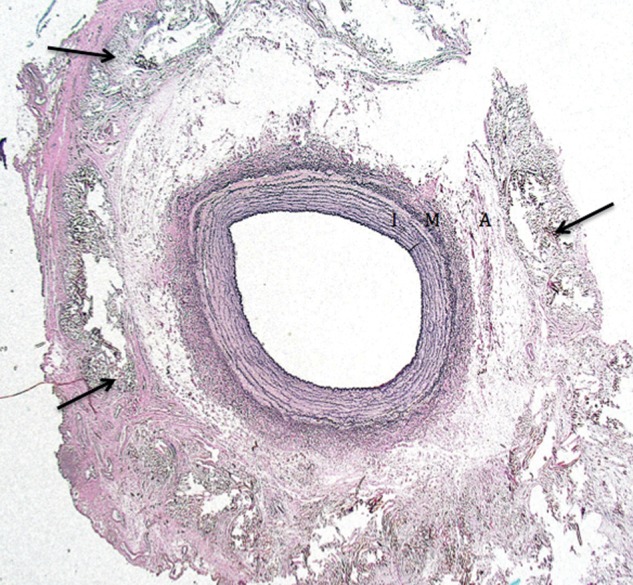
Elastica staining of the wrapped carotid artery with preserved vessel architecture (I: intima; M: media; A: adventitia) and fibrotic incorporated mesh material (arrow) in periarterial tissue.
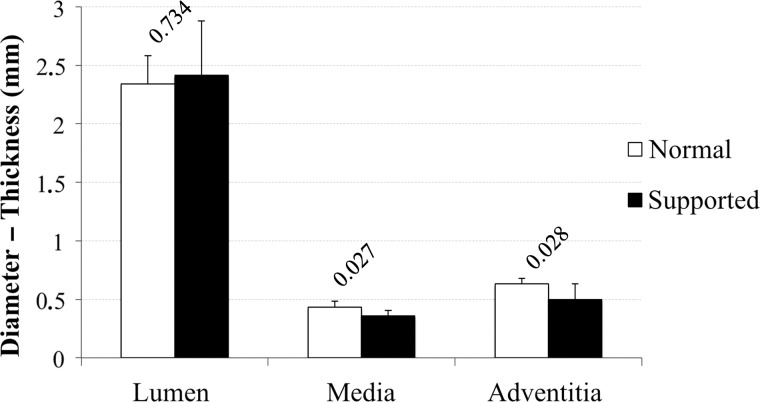
As shown in Fig. 5, the luminal diameters of the normal (2.34 ± 0.24 mm) and supported arteries (2.42 ± 0.46 mm) were comparable. The mean media thickness was significantly lower in the supported segments (0.38 ± 0.04 mm), compared with control segments (0.43 ± 0.05 mm). The adventitia thickness in both groups was similar. The perivascular circular fibrotic sheet, in the operated segments, had an average size of 1.98 mm (±0.57).
Figure 5:
Mean, with SD, of lumen diameter, media thickness and adventitia thickness of the control and supported samples (label: P-value).
Mechanical evaluation
As shown in Table 1, wrapping an artery caused a significant increase in stiffness of 2.92 ± 1.10 compared with 1.31 ± 0.22 in a non-wrapped artery. Accordingly, there was a significant increase in tensile strength in the treated artery 955.95 ± 372.5 vs 188.79 ± 23.30 N/cm2, respectively.
DISCUSSION
Our principle finding is that implantation of mesh material around the carotid arteries of growing sheep causes a strong fibrotic reaction, an increased stiffness and tensile strength of the tissue, without a great effect on the arterial wall architecture. The material used in aortic support is chemically the same polymer, polyethylene terephthalate, as used in Dacron grafts. However, the textile made from it, and used in our clinical work [2, 4, 9], is very different. It is soft, pliant and has a pore size of 0.7 mm. There is very strong similarity between these observations in sheep and two clinical reports in patients operated on between 1970 and 2003 [10, 11]. In total, 162 patients were operated on, using a similar highly porous textile, made of the same polymer. Polyethylene terephthalate is attractive, because it has been found to be biologically tolerated after many years of implantation. Over 30 years ago, Tanabe reported on 60 patients in whom they used highly porous Dacron mesh to reinforce aortic suture lines throughout the 1970's without problems [11]. Five patients died of cancer in the years following surgery, and the authors retrieved specimens of the aorta and were able to report ‘excellent attachment of the mesh to the aortic wall and minimal structural changes in the wrapped aortic wall’. Apart from one early death, not related to any misbehaviour of the mesh, all the other patients remained well [11]. In 2007, Laks' group reported on a series of 102 patients over a 20-year period in whom mesh was used for supporting, or as nicely expressed ‘girdling the ascending aorta’ if it was found to be aneurysmal in the course of surgery. Aortic valve disease was present in 81 of 102, but it was elected to not replace the aorta. The authors established that there had been no deaths related to the aorta in the 88 of 102 patients they were able to trace. Of note was that in two of these patients ‘aneurysmal dilatation of the sinuses developed below the wrap and reoperation was required. No patient in whom the mesh wrap was anchored to the aortic annulus required reoperation’ [10]. A false comparison has been made with aortic ‘wrapping’ as described by Robicsek in the 1990's. Following the report of his clinical experience [5], he published a further paper with his engineer colleague Thubrikar [12], providing a theoretical explanation invoking the Law of Laplace to argue that if the aortic dimensions are fixed, wall tension will not further increase. It was hoped that by wrapping the dilated vessel segment further expansion would be physically prevented, thereby diminishing the risk of fatal consequences, such as dissection and rupture dissection. Although the evidence is sparse, to the level of anecdotal, there have been seriously concerning reports. Neri et al. [6] argued that it was not just a ‘mechanical matter’. Two patients whose aortas had been supported with Dacron at the time of aortic valve replacement when aged 58 and 66 came to surgery 7 and 11 years later with aortic aneurysms. The aortic wall underlying the reinforcement was extremely thin compared with the unwrapped adjacent aorta. Histological examination showed that the normal layers were no longer present and there was extreme atrophy where the aorta was in direct contact with the Dacron cuff. Aortic tissue available from the time of their aortic valve replacement had been normal [6]. Another anxiety often expressed is that the external support might dislocate and erode the aorta. This has been recorded by Hetzer's team in 2003, but the material was again Dacron vascular graft [7]. While the reports of thinning [6] and dislocation [7] are well remembered, the favourable clinical results, in much larger numbers of patient where a porous mesh was used [10, 11], may be more applicable to personalized aortic root support as used in Golesworthy's proposal [2–4].
Our findings in sheep are remarkably similar to the findings of Tanabe's group in the way the mesh is incorporated. What is achieved by the intimate ensleeving of the aorta with a porous mesh is that a textile/biological composite ensues. In sheep, the mesh had been incorporated to make macroscopically a composite vessel wall, 4–6 months after implantation. The corrected thickness of this substrate was remarkably larger than the control sample due to the formation of a periaterial fibrotic sheet. Histological examination in five surviving sheep (of six) showed that the arterial wall architecture is well preserved in nearly all specimens. The graft material was located in the outer part of the adventitia and periarterial tissue and induced a strong fibrotic reaction. Histological measurements showed a mild decrease in media thickness of the supported arteries. The reason for this remains unclear, as there was no clear inflammation or necrosis of media. In one vessel there was some oedema over about a quarter of its circumference. This should be controlled in larger series and after longer implantation times. The microscopic finding of increased fibrosis is reflected in the tensile tests as the tensile strength and the stiffness of wrapped arteries are significantly higher than non-wrapped arteries. Increased tensile strength might be in favour of preventing aortic rupture, as this occurs when the wall stress exceeds the ultimate tensile strength of aortic wall tissue. It is known that the wall stress in aneurysms is increased and the aortic wall is weakened [13]. In these circumstances, a physiological range of blood pressure may exhibit wall stress that equals or exceeds the tensile strength of aortic aneurysm tissue [14]. Therefore, reinforcing the aortic wall with mesh material increases the tensile strength and will prevent aortic rupture. Theoretically, the increased stiffness may influence the working load of the heart, arterial pressures and mechanical effects on the aortic valve [15, 16]. The ascending aorta acts as a windkessel, an elastic buffering chamber, beyond the heart [17]. In theory, changed elasticity may also have a local effect by potentially inducing dilatation beyond the supported portion [18]. However, the same theoretical risk applies with graft replacement [19]. In fact, it was not seen when specifically looked for in a carefully constructed study where measurements of the aortic arch were made before and after external support without knowledge of the patients identity and whether it was a before or after image [20]. Ideally, different types of mesh should be compared to obtain an ideal material, which allows compliance of the textile/biological composite while increasing sufficiently the tensile strength after implantation.
Of course, care must be taken in extrapolating animal data to a Marfan patient population. Apart from the fact that we only describe the results of mesh implantation in a small number of animals, this model does not directly address the prevention of dissection. The effects of a mesh on a healthy artery might be different from its influences on an artery with defects in its major structural components and specific regulatory pathways [21, 22]. Evaluation of external aortic support in a bovine Marfan model [23], with phenotypical characteristics comparable to those of human Marfan patients, might be interesting, but bovine models are extremely expensive and difficult to handle in laboratory conditions. Another limitation is the use of carotid arteries and not the ascending aorta, the reason being that the ascending aorta of quadrupeds is very short and divides quickly in a common carotid artery and descending thoracic aorta. Therefore, the long and easy accessible carotid artery was believed to be a valid alternative.
To conclude, external aortic root support polyethylene terephthalate mesh, as used for the personalized external support of dilated aortic roots in Marfan syndrome, becomes incorporated in the periadventitial tissue of the carotid artery of sheep. Limited thinning of the media, without any signs of inflammation or media necrosis, was visible. The significance of this finding should be controlled in larger series and after longer implantation times. The graft material reinforces the vessel wall when stress tested.
Conflict of interest: none declared.
REFERENCES
- 1.Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, Jr, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Catheter Cardiovasc Interv. 2010;76:E43–86. doi: 10.1002/ccd.22537. doi:10.1002/ccd.22537. [DOI] [PubMed] [Google Scholar]
- 2.Golesworthy T, Lamperth M, Mohiaddin R, Pepper J, Thornton W, Treasure T. The Tailor of Gloucester: a jacket for the Marfan's aorta. Lancet. 2004;364:1582. doi: 10.1016/S0140-6736(04)17308-X. doi:10.1016/S0140-6736(04)17308-X. [DOI] [PubMed] [Google Scholar]
- 3.Pepper J, Golesworthy T, Utley M, Chan J, Ganeshalingam S, Lamperth M, et al. Manufacturing and placing a bespoke support for the Marfan aortic root: description of the method and technical results and status at one year for the first ten patients. Interact CardioVasc Thorac Surg. 2010;10:360–5. doi: 10.1510/icvts.2009.220319. doi:10.1510/icvts.2009.220319. [DOI] [PubMed] [Google Scholar]
- 4.Treasure T, Crowe S, Chan KM, Ranasinghe A, Attia R, Lees B, et al. A method for early evaluation of a recently introduced technology by deriving a comparative group from existing clinical data: a case study in external support of the Marfan aortic root. BMJ Open. 2012;2:e000725. doi: 10.1136/bmjopen-2011-000725. doi:10.1136/bmjopen-2011-000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robicsek F, Thubrikar MJ. Conservative operation in the management of annular dilatation and ascending aortic aneurysm. Ann Thorac Surg. 1994;57:1672–4. doi: 10.1016/0003-4975(94)90160-0. doi:10.1016/0003-4975(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 6.Neri E, Massetti M, Tanganelli P, Capannini G, Carone E, Tripodi A, et al. Is it only a mechanical matter? Histologic modifications of the aorta underlying external banding. J Thorac Cardiovasc Surg. 1999;118:1116–8. doi: 10.1016/S0022-5223(99)70111-X. doi:10.1016/S0022-5223(99)70111-X. [DOI] [PubMed] [Google Scholar]
- 7.Bauer M, Grauhan O, Hetzer R. Dislocated wrap after previous reduction aortoplasty causes erosion of the ascending aorta. Ann Thorac Surg. 2003;75:583–4. doi: 10.1016/s0003-4975(02)04338-2. doi:10.1016/S0003-4975(02)04338-2. [DOI] [PubMed] [Google Scholar]
- 8.Famaey N, Vander Sloten J. Soft tissue modelling for applications in virtual surgery and surgical robotics. Comput Methods Biomech Biomed Eng. 2008;11:351–66. doi: 10.1080/10255840802020412. doi:10.1080/10255840802020412. [DOI] [PubMed] [Google Scholar]
- 9.Treasure T, Pepper J, Golesworthy T, Mohiaddin R, Anderson RH. External aortic root support: NICE guidance. Heart. 2012;98:65–8. doi: 10.1136/heartjnl-2011-301017. doi:10.1136/heartjnl-2011-301017. [DOI] [PubMed] [Google Scholar]
- 10.Cohen O, Odim J, De la Zerda D, Ukatu C, Vyas R, Vyas N, et al. Long-term experience of girdling the ascending aorta with Dacron mesh as definitive treatment for aneurysmal dilation. Ann Thorac Surg. 2007;83:S780–4. doi: 10.1016/j.athoracsur.2006.10.086. discussion S5–90 doi:10.1016/j.athoracsur.2006.10.086. [DOI] [PubMed] [Google Scholar]
- 11.Tanabe T, Kubo Y, Hashimoto M, Takahashi T, Yasuda K, Sugie S. Wall reinforcement with highly porous Dacron mesh in aortic surgery. Ann Surg. 1980;191:452–5. doi: 10.1097/00000658-198004000-00010. doi:10.1097/00000658-198004000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robicsek F, Thubrikar MJ. Hemodynamic considerations regarding the mechanism and prevention of aortic dissection. Ann Thorac Surg. 1994;58:1247–53. doi: 10.1016/0003-4975(94)90523-1. doi:10.1016/0003-4975(94)90523-1. [DOI] [PubMed] [Google Scholar]
- 13.Vorp DA, Schiro BJ, Ehrlich MP, Juvonen TS, Ergin MA, Griffith BP. Effect of aneurysm on the tensile strength and biomechanical behavior of the ascending thoracic aorta. Ann Thorac Surg. 2003;75:1210–4. doi: 10.1016/s0003-4975(02)04711-2. doi:10.1016/S0003-4975(02)04711-2. [DOI] [PubMed] [Google Scholar]
- 14.Koullias G, Modak R, Tranquilli M, Korkolis DP, Barash P, Elefteriades JA. Mechanical deterioration underlies malignant behavior of aneurysmal human ascending aorta. J Thorac Cardiovasc Surg. 2005;130:677–83. doi: 10.1016/j.jtcvs.2005.02.052. [DOI] [PubMed] [Google Scholar]
- 15.Dobson G, Flewitt J, Tyberg JV, Moore R, Karamanoglu M. Endografting of the descending thoracic aorta increases ascending aortic input impedance and attenuates pressure transmission in dogs. Eur J Vasc Endovasc Surg. 2006;32:129–35. doi: 10.1016/j.ejvs.2006.01.020. doi:10.1016/j.ejvs.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 16.Kim SY, Hinkamp TJ, Jacobs WR, Lichtenberg RC, Posniak H, Pifarre R. Effect of an inelastic aortic synthetic vascular graft on exercise hemodynamics. Ann Thorac Surg. 1995;59:981–9. doi: 10.1016/0003-4975(95)00068-v. doi:10.1016/0003-4975(95)00068-V. [DOI] [PubMed] [Google Scholar]
- 17.Belz GG. Elastic properties and windkessel function of the human aorta. Cardiovasc Drugs Ther. 1995;9:73–83. doi: 10.1007/BF00877747. doi:10.1007/BF00877747. [DOI] [PubMed] [Google Scholar]
- 18.Mehigan DG, Fitzpatrick B, Browne HI, Bouchier-Hayes DJ. Is compliance mismatch the major cause of anastomotic arterial aneurysms? Analysis of 42 cases. J Cardiovasc Surg (Torino) 1985;26:147–50. [PubMed] [Google Scholar]
- 19.Tremblay D, Zigras T, Cartier R, Leduc L, Butany J, Mongrain R, et al. A comparison of mechanical properties of materials used in aortic arch reconstruction. Ann Thorac Surg. 2009;88:1484–91. doi: 10.1016/j.athoracsur.2009.07.023. doi:10.1016/j.athoracsur.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 20.Pepper J, John Chan K, Gavino J, Golesworthy T, Mohiaddin R, Treasure T. External aortic root support for Marfan syndrome: early clinical results in the first 20 recipients with a bespoke implant. J R Soc Med. 2010;103:370–5. doi: 10.1258/jrsm.2010.100070. doi:10.1258/jrsm.2010.100070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Hamamsy I, Yacoub MH. Cellular and molecular mechanisms of thoracic aortic aneurysms. Nat Rev Cardiol. 2009;6:771–86. doi: 10.1038/nrcardio.2009.191. doi:10.1038/nrcardio.2009.191. [DOI] [PubMed] [Google Scholar]
- 22.Lindsay ME, Dietz HC. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature. 2011;473:308–16. doi: 10.1038/nature10145. doi:10.1038/nature10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Besser TE, Potter KA, Bryan GM, Knowlen GG. An animal model of the Marfan syndrome. Am J Med Genet. 1990;37:159–65. doi: 10.1002/ajmg.1320370137. doi:10.1002/ajmg.1320370137. [DOI] [PubMed] [Google Scholar]