Abstract
OBJECTIVES
Transcatheter aortic valve implantation (TAVI) is an established intervention for aortic stenosis. While it is known that the requirement for permanent pacing is higher following CoreValve (Medtronic, Inc., Minneapolis, MN, USA) TAVI than after surgical aortic valve replacement (SAVR), it remains uncertain whether pacing is required in the medium-to-long term. We hypothesized that complete heart block following TAVI is more likely to resolve than that following SAVR.
METHODS
A retrospective analysis of prospectively collated data on 528 patients undergoing TAVI or SAVR from May 2008 to December 2010 at a cardiac tertiary referral hospital. Demographic data, timing and indication for pacing post-procedure plus follow-up were recorded. Paced patients were compared and analysed by existing initial indication for pacing.
RESULTS
In total, 31 (5.9%) patients received a pacemaker, and there were limited differences between not paced and paced patient characteristics by procedure type. Of these, a greater proportion were implanted post-TAVI compared with SAVR (17 vs 3.2%, P < 0.001). The mean time to pacemaker follow-up for TAVI and SAVR was 234 and 188 days, P = 0.32, respectively. Fewer patients compared with pacing indication remained in complete heart block at latest follow-up for TAVI (76.5 vs 33.3%, P = 0.02) and SAVR (92.9 vs 58.3%, P = 0.04). Although, there was a trend towards a greater magnitude of TAVI patients regaining atrioventricular nodal conduction, this did not differ significantly from that seen in SAVR patients.
CONCLUSIONS
In keeping with previous reports, this single-centre experience demonstrates that patients undergoing TAVI have higher rates of pacemaker implantation than those following SAVR. However, pacing indication in the short-to-medium term may not persist for all paced patients post-TAVI and -SAVR with the suggestion that a significant proportion recover atrioventricular conduction, which tended to be greatest in TAVI paced patients.
Keywords: Transcatheter aortic valve implantation, Aortic valve implantation, Permanent pacing, Aortic stenosis, Valvular heart disease
INTRODUCTION
The requirement for pacing in the short term following both transcatheter aortic valve implantation (TAVI) and surgical aortic valve replacement (SAVR) for high-grade atrioventricular (AV) block is well recognized [1–14]. Rates of pacemaker implantation post-TAVI range from 6 to 34% [4, 5, 7–9, 11, 12] and post-SAVR from 2 to 7% [3, 6, 10, 13]. However, for TAVI, the rates of pacing are higher in those implanted with a CoreValve (Medtronic, Inc., Minneapolis, MN, USA) than in those with an Edward SAPIEN valve (Edwards Lifesciences Corporation, Irvine, CA, USA), the latter having post-implant pacing rates similar to SAVR [1]. There are well-recognized predictors of pacemaker requirement following TAVI or SAVR [1, 3, 9, 15]. However, the ongoing requirement for ventricular pacing in the short-to-medium term post-pacemaker implantation following CoreValve TAVI or SAVR is less clear. In the present study, we hypothesized that despite the greater incidence of pacing in the TAVI cohort, conduction system damage is more likely to recover in the short-to-medium term.
In the short term, TAVI results in a higher likelihood of conductive tissue damage than SAVR most likely due to both the mechanism of CoreValve deployment [6, 9] and the presence of greater comorbidity in TAVI patients [16]. However, damage to the conduction system following SAVR may result in irreversible damage to the intrinsic conduction system with a greater need for short-to-medium term pacing [2]. We report our pacing experience following TAVI and SAVR implantation, comparing the two populations and the need for pacing requirements in the short-to-medium term.
MATERIALS AND METHODS
A single-centre retrospective analysis of 100 consecutive TAVI patients and 428 consecutive isolated SAVR patients from 29 May 2008 to 3 December 2010 was undertaken. All TAVI procedures were undertaken using the CoreValve device (Medtronic, Inc.). All patients undergoing either TAVI or SAVR received during the procedure a transvenous temporary pacing wire (TPW) or epicardial pacing leads, respectively.
Patients undergoing permanent pacemaker (PPM) implantation during their hospital stay following either TAVI or SAVR were identified from review of prospective databases and formed the study cohort. Patients with a PPM implanted prior to the procedure, including implantable cardiac debrillators and/or cardiac resynchronization therapy, were not included at follow-up.
Patient characteristics and follow-up
Baseline demographics including left ventricular function, pacing indication, type of pacemaker implanted and timing of implant after the TAVI or SAVR procedure were recorded. Pacing follow-up notes were reviewed from several centres across our region. Timing of pacing check, pacemaker settings (AV delays, base rate and mode), percentage ventricular pacing and underlying rhythm were noted. The most recent pacing check data were recorded and used for analysis of pacing need as this helped simplify the collection of pacing data across several hospital sites in our region. The underlying rhythm was recorded as that annotated in the pacing notes.
Statistics
The population was described using crude numerical data and percentages for categorical variables and means (standard deviation) for normally distributed parameters. A χ2 test of significance was used to define significant differences in categorical data except in cells of 5 or fewer observations where Fisher's exact test was applied. A Student's t-test was used in normally distributed parameters. Univariate logistic regression was used to assess the association of patient-level characteristics with PPM implant post-TAVI and -SAVR. All analysis was performed using Stata IC version 11.0 (StataCorp LP, TX, USA).
RESULTS
Patient characteristics
There were significant differences in baseline patient characteristics in those undergoing TAVI compared with those undergoing SAVR (Table 1). TAVI patients were older and had a greater burden of comorbidities, including ischaemic heart disease, hypertension, chronic kidney disease, chronic obstructive pulmonary disease and severe left ventricular systolic dysfunction. Of the 427 isolated SAVR implants, 291 (67.9%) were bioprosthetic and 133 (31.1%) were mechanical valves. In total, 1 SAVR and 8 TAVI patients had a preprocedural pacemaker implanted.
Table 1:
Baseline characteristics of patients undergoing TAVI or SAVR
| Patient characteristics | TAVI (N = 100) | SAVR (N = 428) | P-value |
|---|---|---|---|
| Age (SD), years | 80.5 (5.6) | 66.2 (11.8) | <0.001 |
| Female, n (%) | 52 (52.0) | 164 (38.3) | =0.012 |
| Ischaemic heart disease, n (%) | 61(61.0) | 184 (43.0) | <0.001 |
| Diabetes mellitus, n (%) | 28 (28.0) | 61 (14.3) | <0.001 |
| Hypertension, n (%) | 23 (23.0) | 248 (58.1) | <0.001 |
| Chronic kidney disease, n (%) | 19 (19.0) | 20 (4.7) | <0.001 |
| Atrial fibrillation, n (%) | 29 (29.0) | 1 (0.2) | =0.05 |
| COPD, n (%) | 21 (21.0) | 41 (9.6) | <0.001 |
| Left ventricular ejection fraction, n (%) | |||
| >50% | 55 (55.0) | 275 (64.7) | 0.02 |
| 30–50% | 35 (35.0) | 139 (32.7) | |
| <30% | 10 (10.0) | 11 (2.6) | |
TAVI: transcatheter aortic valve implantation; SAVR: surgical aortic valve replacement; SD: standard deviation; COPD: chronic obstructive pulmonary disease.
Characteristics of patients paced post-TAVI and -SAVR
In total, 31 (5.9%) patients received a PPM. Of these, 17 (17.0%) and 14 (3.2%), P < 0.001, patients underwent TAVI or SAVR, respectively. In the TAVI group, patient characteristics of those not paced compared with those paced did not differ significantly and did not predict the need for a PPM post-TAVI, while chronic kidney failure and left ventricular ejection fraction <30% predicted the need for a PPM post-SAVR (Table 2). There was no significant difference between those paced and not paced by the type of SAVR implanted (proportion of mechanical valves was 21.4 vs 31.5%, P = 0.42, respectively). The average time (mean days, 95% confidence interval [CI]) to pacemaker implantation from procedure by TAVI and SAVR was: 4.5 (2.8–6.2) and 6.6 (3.9–9.3) days, P = 0.14, respectively.
Table 2:
Baseline characteristics of those paced or not paced undergoing TAVI and SAVR
| Patient characteristics | Not paced post-TAVI (N = 83) | Paced post-TAVI (N = 17) | OR (95% CI) | Not paced post-SAVR (N = 414) | Paced post-SAVR (N = 14) | OR (95% CI) |
|---|---|---|---|---|---|---|
| Age (SD), years | 80.4 (5.7) | 80.8 (5.7) | 1.01 (0.92–1.11) | 66.1 (11.7) | 68.1 (13.80) | 1.02 (0.97–1.07) |
| Female, n (%) | 45 (54.2) | 7 (41.2) | 1.69 (0.59–4.87) | 158 (38.2) | 6 (42.9) | 0.82 (0.28–2.42) |
| Ischaemic heart disease, n (%) | 51 (61.5) | 10 (58.8) | 0.90 (0.31–2.59) | 179 (43.2) | 5 (35.7) | 0.73 (0.24–2.21) |
| Diabetes mellitus, n (%) | 23 (27.7) | 5 (29.4) | 1.09 (0.34–3.43) | 57 (13.8) | 4 (28.6) | 2.51 (0.76–8.26) |
| Hypertension, n (%) | 17 (20.5) | 6 (35.3) | 2.12 (0.69–6.55) | 239 (57.9) | 9 (64.30) | 1.31 (0.43–3.98) |
| Chronic kidney disease, n (%) | 16 (19.3) | 3 (17.7) | 0.90 (0.23–3.50) | 17 (4.1) | 3 (21.4) | 6.37 (1.63–24.960) |
| Atrial fibrillation, n (%) | 22 (26.5) | 7 (41.2) | 1.94 (0.66–5.730 | 1 (0.2) | 0 (0.0) | – |
| COPD, n (%) | 18 (21.7) | 3 (17.7) | 0.77 (0.20–2.99) | 39 (9.4) | 2 (14.3) | 1.60 (0.35–7.42) |
| Left ventricular ejection fraction, n (%) | ||||||
| >50% | 46 (55.4) | 9 (52.9) | 0.90 (0.32–2.58) | 268 (65.2) | 7 (50.0) | 0.53 (0.18–1.55) |
| 30–50% | 27 (32.5) | 8 (47.1) | 1.84 (0.64–5.31) | 134 (32.6) | 5 (35.7) | 1.15 (0.38–3.49) |
| <30% | 10 (12.1) | 0 (0.0) | – | 9 (2.2) | 2 (14.3) | 7.44 (1.45–38.23) |
TAVI: transcatheter aortic valve implantation; SAVR: surgical aortic valve replacement; OR: odds ratios; CI: confidence interval; SD: standard deviation; COPD: chronic obstructive pulmonary disease.
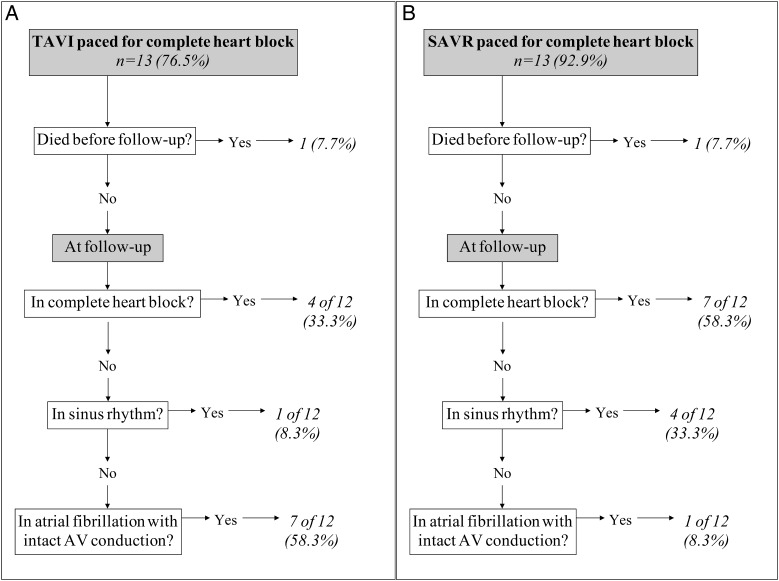
Of those patients receiving a PPM, most (83.9%) were indicated for the presence of complete heart block, which related to 13 (76.5%) patients receiving a TAVI and 13 (92.9%) undergoing SAVR, P < 0.001. The indications for the remaining 4 TAVI patients were: (i) prolonged PR interval and left bundle branch block (LBBB); (ii) second-degree AV nodal block; (iii) prolonged PR interval with left axis deviation and LBBB and (iv) sick sinus syndrome. For SAVR, the remaining patient was paced for second-degree Mobitz Type II AV nodal block.
Biventricular PPM was implanted solely in TAVI patients, while the use of dual chamber devices was significantly lower in TAVI than in SAVR patients (47.1 vs 92.9%, P = 0.01, respectively). There was no significant variation between the type of procedure and the implantation frequency of single-ventricular-lead devices (23.5 vs 7.1%, P = 0.23, respectively).
Pacing follow-up
The mean (95% CI) time to pacing follow-up for TAVI and SAVR was 234.2 (157.3–311.0) and 188.1 (135.8–240.4) days, P = 0.32, respectively. Follow-up for 2 patients (1 TAVI and 1 SAVR) did not occur because they died before their first pacemaker check could be performed. Both these patients were paced for CHB. In total, 16 (94.1%) TAVI and 13 (92.9%) SAVR patients had complete pacing follow-up data. There was no observed significant variation in the frequency of the use of algorithms to manage ventricular pacing between TAVI and SAVR (5.9 vs 14.3%, P = 0.42) or the parameters defining paced (168 vs 201 ms, P = 0.29, respectively) and sensed (132 vs 177 ms, P = 0.06, respectively) AV delay. TAVI patients had significantly lower base rates than SAVR paced patients (57 vs 63 bpm, P = 0.04, respectively), but no significant variation in the percentage of ventricular pacing between the 2 groups was seen (TAVI was 95.5% [95% CI: 34.0–100] and SAVR was 97.0% (11.3–99.0), P = 0.68, respectively).
At final follow-up, fewer patients in both groups were in CHB than when originally paced for TAVI (33.3 vs 76.5%, P = 0.02) and SAVR (58.3 vs 92.9%, P = 0.04), respectively (Fig. 1). However, although this was more likely in the TAVI population, it did not differ significantly from that observed in the SAVR population (33.3 vs 58.3%, P = 0.22, respectively). The pacing indication remained at follow-up for the patient paced post-SAVR for second-degree Mobitz Type II AV nodal block. Of those, TAVI patients paced for prolonged PR interval and LBBB, second-degree AV nodal block, prolonged PR interval with left axis deviation and LBBB; and sick sinus syndrome were found at follow-up to be in CHB, second-degree AV nodal block and sinus rhythm for the later two, respectively.
Figure 1:
Rhythm at final follow-up by those patients paced for complete heart block by (A) TAVI and (B) SAVR.
DISCUSSION
In keeping with previous reports [1, 3–13], the present study demonstrates that patients undergoing TAVI are more likely to need post-procedural implantation of a pacemaker than those undergoing SAVR. However, post-procedural follow-up suggests that the initial indication (CHB) for pacing post-TAVI and -SAVR may not persist in the short-to-medium term; with only 31% of TAVI and 54% of SAVR in CHB at follow-up. On the other hand, although there was a trend towards greater recovery of AV nodal conduction in TAVI patients, this did not appear to significantly differ from the same rates in SAVR paced patients.
CoreValve TAVI is thought to impose greater damage to cardiac conduction tissue than SAVR by compression of the calcified annulus into surrounding tissue, resulting in increased local compressive stress and further radial stress (via the skirt which extends further into the left ventricular outflow tract) on adjacent LBB [9]. The alternative Edwards SAPIEN valve has significantly lower pacing rates (5.4%), which are similar to those seen with SAVR despite similar compression of the AV annulus [1], suggesting that the localized compressive forces of the CoreValve skirt on the LBB may be contributory to the observed increased pacing rates. Indeed, basal interventricular septal necrosis (involving the intrinsic conducting tissue) has been reported following CoreValve implantation and resulted in pacemaker post-procedure [17]. Other pre-procedural predictors of the need for pacing post-TAVI are well reported including: the presence of right bundle branch block, periprocedural AV block, balloon predilatation, increased intraventricular septal diameter, prolonged preimplantation QRS duration, increased annular size and depth of prosthesis implant [1, 4, 9, 18]. During SAVR, conductive tissue damage is thought to occur from excision of adjacent aortic valve and debridement of a calcified annulus, in addition to a time-related ischaemic insult [14]. Risk factors known to increase the likelihood of post-SAVR pacing include annular calcification, bicuspid aortic valve, prolonged QRS, hypertension and ischaemic time [3, 6, 15]. The lower pacing implant rates post-SAVR may also be influenced by the control afforded to the surgeon to avoid damaging the AV conduction system. In addition, the TAVI population were shown to be older with more comorbidity, which may contribute to the greater rates of pacing.
The percentage of ventricular pacing in this study appears at first glance to suggest that the vast majority of implants were needed in the short-to-medium term, demonstrating a high level of ventricular pacing (95.5% for TAVI and 97.0% for SAVR). However, such parameters can be unreliable and depend on pacemaker settings. For instance, only three pacemakers had algorithms activated to reduce ventricular pacing. A more reliable outcome of need for pacemaker is underlying rhythm. The majority of those paced post-TAVI or -SAVR were in CHB. However, at an average follow-up of over 6 months, which did not significantly vary between TAVI and SAVR paced patients, only a third and just over a half of patients, paced initially for CHB, maintained this indication at follow-up following TAVI or SAVR, respectively. This may suggest that a proportion of those patients paced early following TAVI and SAVR for CHB may recover cardiac conduction sufficiently to avoid pacing in the short-to-medium term. Although TAVI patients appeared to show the greatest recovery in AV nodal conduction, this was not significantly different from SAVR patients. Our study might, therefore, suggest that patients may receive a PPM too early post-TAVI or -SAVR. However, clinically withholding a PPM in a patient in CHB post-procedure would be difficult to justify. The average waiting times to PPM implant in our study would not seem unreasonably short, and further waiting time would impact on patient care and service provision.
The current literature is small and contradictory. In one study, 64% of patients paced for CHB, with an average delay of 3 (range 1–7 days) post-TAVI, had complete resolution of AV nodal conduction with a median follow-up of 79 days [12]. However, another study of 15 patients paced post-TAVI for CHB reported that all but 2 remained in CHB at 435-day follow-up, but did not state the time to pacing from TAVI [19]. On the other hand, our study differed from recent findings in 2106 patients undergoing isolated SAVR, in which 6.6% of patients were implanted with a PPM despite having a similar average delay to pacing as our study (7 days, range 1–30 days), but only a small proportion (10%) at a mean follow-up time of 5.3 years were no longer PPM dependent [2]. Similarly, another small series of 22 patients paced post-SAVR for mainly CHB with a mean of 56-month follow-up reported that 21 patients remained 100% ventricular paced and one recovered AV nodal association [20]. However, the majority of patients were implanted with a single ventricular lead device and no reference was made to base rate settings or delay to pacing following the procedure. Our study findings are contrary to previous studies regarding SAVR paced patients but in agreement with studies of TAVI paced patients. The variation from the literature might be explained by the small pacing events analysed, but also that our follow-up time was shorter than those of other studies, which may only capture the transition period of SAVR paced patients to persistent CHB.
Nevertheless, our study highlights an important consideration when deciding on PPM implantation following TAVI or SAVR. There are recognized early and late complications of pacemaker implantation that need to be balanced against justified reasons for device implantation. The findings in our study that most patients paced post-TAVI (and less so post-SAVR) for CHB recovered AV nodal conduction suggests the decision to implant a pacemaker in this situation needs to be considered carefully and patiently. More so, the implantation of biventricular devices (in the absence of significantly impaired left ventricular function) under the assumption that there will be a high burden of ventricular pacing may not be appropriate, given their cost and increased procedural complexity.
Methods to identify individuals who do not require pacing in the long term are yet to be defined, although more recent evidence suggests that a narrow QRS (<120 ms) post-TAVI suggests low rates of pacing in the long term [21]. In this study, patients waited on average 4.5 days for pacing post-TAVI and 6.6 days post-SAVR. It could be argued that the longer waiting time before pacing in the SAVR patients may have led to a more robust pacing indication. However, we can only speculate why there was a difference in the delay to pacing post-TAVI and -SAVR. It is likely that TAVI patients were implanted earlier as they were under direct care of Cardiologists, who have more immediate means to facilitate PPM implantation than Cardiothoracic surgeons. Also, TAVI is a ‘newer’ procedure often leading to increased emphasis on optimizing patient outcomes, which may encourage a more active and earlier approach to pacing than SAVR. In addition, there is considerable concern regarding the risk of infection in keeping a TPW in situ for longer than needed for fear of lead displacement, ventricular perforation and of course infection potentially encouraging earlier PPM implantation. On the other hand, a delay in pacing SAVR patients may occur as such patients often need time on intensive care to stabilize and in such a situation it may be more acceptable to keep epicardial pacing wires in situ, providing a temporary pacing option that reduces the immediate gravity of the pacing indication until a later time point. Indeed, if we could find ways of safely offering more prolonged temporary pacing, this might be preferable. Further work is needed to establish factors that may predict cardiac conduction recovery following TAVI or SAVR and therefore reduce the burden of pacing.
Study limitations
We report findings from practice in a single tertiary cardiac centre in the UK with relatively small numbers, which cannot be generalized to the rest of the UK. This retrospective analysis also depended on what detail of data had been recorded at pacemaker follow-up, and TAVI and SAVR patients were not matched for age or comorbidity. The study also focussed on the pacing requirement at latest follow-up in those patients requiring a PPM before hospital discharge and, therefore, did not observe all patients following TAVI or SAVR. Subsequently, a risk factor analysis of pacing need or AV node recovery was not possible. Furthermore, information pertaining to preprocedural rhythm or QRS morphology was not available for the majority of patients meaning that the comparison of rates of PPM implantation may have been biased, although our reported pacing rates are in keeping with current literature. Moreover, all patients in our cohort had no pre-existing conduction abnormalities; otherwise, they would have received a PPM before undergoing TAVI or SAVR. Moreover, it was not possible to identify those patients initially discharged from the hospital who later required a PPM for AV conduction abnormality nor were we able to evaluate the timing of onset of CHB or those who had transient conduction abnormalities who were not paced. All post-SAVR patients were paced post-procedure, and it was not possible from the data to identify those patients suffering periprocedural CHB in the TAVI group. We could not account for the effect or technique of individual operators for both TAVI and SAVR, which might have biased post-procedural pacemaker implant rates.
CONCLUSION
In keeping with previous reports, this single-centre experience demonstrates that patients undergoing TAVI with the Medtronic CoreValve have higher rates of pacemaker implantation than those following SAVR. However, there was significant recovery in AV nodal conduction in the short-to-medium term for those patients paced initially for CHB following both TAVI and SAVR, although this was more pronounced in TAVI patients. Identification of such individuals remains elusive and warrants further prospective study.
ACKNOWLEDGEMENTS
We thank the pacing department of the Yorkshire Heart Centre at the Leeds General Infirmary for the help and support in obtaining accurate data for this study.
Conflict of interest: none declared.
REFERENCES
- 1.Bates MGD, Matthews IG, Fazal IA, Turley J. Postoperative permanent pacemaker implantation in patients undergoing trans-catheter aortic valve implantation: what is the incidence and are there any predicting factors? Interact CardioVasc Thorac Surg. 2011;12:243–53. doi: 10.1510/icvts.2010.256578. [DOI] [PubMed] [Google Scholar]
- 2.Baraki H, Al AA, Jeng-Singh S, Saito S, Schmitto JD, Fleischer B, et al. Pacemaker dependency after isolated aortic valve replacement: do conductance disorders recover over time? Interact CardioVasc Thorac Surg. 2013;16:476–81. doi: 10.1093/icvts/ivs555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erdogan HB, Kayalar N, Ardal H, Omeroglu SN, Kirali K, Guler M, et al. Risk factors for requirement of permanent pacemaker implantation after aortic valve replacement. J Card Surg. 2006;21:211–5. doi: 10.1111/j.1540-8191.2006.00216.x. [DOI] [PubMed] [Google Scholar]
- 4.Ferreira ND, Caeiro D, Adao L, Oliveira M, Gonçalves H, Ribeiro J, et al. Incidence and predictors of permanent pacemaker requirement after transcatheter aortic valve implantation with a self-expanding bioprosthesis. Pacing Clin Electrophysiol. 2010;33:1364–72. doi: 10.1111/j.1540-8159.2010.02870.x. [DOI] [PubMed] [Google Scholar]
- 5.Godin M, Eltchaninoff H, Furuta A, Tron C, Anselme F, Bejar K, et al. Frequency of conduction disturbances after transcatheter implantation of an Edwards Sapien aortic valve prosthesis. Am J Cardiol. 2010;106:707–12. doi: 10.1016/j.amjcard.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 6.Gordon RS, Ivanov J, Cohen G, Ralph-Edwards AL. Permanent cardiac pacing after a cardiac operation: predicting the use of permanent pacemakers. Ann Thorac Surg. 1998;66:1698–704. doi: 10.1016/s0003-4975(98)00889-3. [DOI] [PubMed] [Google Scholar]
- 7.Haworth P, Behan M, Khawaja M, Hutchinson N, De Belder A, Trivedi U, et al. Predictors for permanent pacing after transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2010;76:751–6. doi: 10.1002/ccd.22457. [DOI] [PubMed] [Google Scholar]
- 8.Jilaihawi H, Chin D, Vasa-Nicotera M, Jeilan M, Spyt T, Ng GA, et al. Predictors for permanent pacemaker requirement after transcatheter aortic valve implantation with the CoreValve bioprosthesis. Am Heart J. 2009;157:860–6. doi: 10.1016/j.ahj.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 9.Khawaja MZ, Rajani R, Cook A, Khavandi A, Moynagh A, Chowdhary S, et al. Permanent pacemaker insertion after CoreValve transcatheter aortic valve implantation: incidence and contributing factors (the UK CoreValve Collaborative) Circulation. 2011;123:951–60. doi: 10.1161/CIRCULATIONAHA.109.927152. [DOI] [PubMed] [Google Scholar]
- 10.Limongelli G, Ducceschi V, D'Andrea A, Renzulli A, Sarubbi B, De Feo M, et al. Risk factors for pacemaker implantation following aortic valve replacement: a single centre experience. Heart. 2003;89:901–4. doi: 10.1136/heart.89.8.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piazza N, Nuis RJ, Tzikas A, Otten A, Onuma Y, Garcia-Garcia H, et al. Persistent conduction abnormalities and requirements for pacemaking six months after transcatheter aortic valve implantation. EuroIntervention. 2010;6:475–84. doi: 10.4244/EIJ30V6I4A80. [DOI] [PubMed] [Google Scholar]
- 12.Roten L, Wenaweser P, Delacrataz E, Hellige G, Stortecky S, Tanner H, et al. Incidence and predictors of atrioventricular conduction impairment after transcatheter aortic valve implantation. Am J Cardiol. 2010;106:1473–80. doi: 10.1016/j.amjcard.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Schurr UP, Berli J, Berdajs D, Hausler A, Zemali O, Emmert M, et al. Incidence and risk factors for pacemaker implantation following aortic valve replacement. Interact CardioVasc Thorac Surg. 2010;11:556–60. doi: 10.1510/icvts.2010.249904. [DOI] [PubMed] [Google Scholar]
- 14.Sinhal A, Altwegg L, Pasupati S, Humphries KH, llard M, Martin P, et al. Atrioventricular block after transcatheter balloon expandable aortic valve implantation. JACC Cardiovasc Interv. 2008;1:305–9. doi: 10.1016/j.jcin.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 15.El-Khally Z, Thibault B, Staniloae C, Theroux P, Dubuc M, Roy D, et al. Prognostic significance of newly acquired bundle branch block after aortic valve replacement. Am J Cardiol. 2004;94:1008–11. doi: 10.1016/j.amjcard.2004.06.055. [DOI] [PubMed] [Google Scholar]
- 16.Appel CF, Hultkvist H, Nylander E, Ahn H, Nielsen NE, Freter W, et al. Transcatheter versus surgical treatment for aortic stenosis: patient selection and early outcome. Scand Cardiovasc J. 2012;46:301–7. doi: 10.3109/14017431.2012.699636. [DOI] [PubMed] [Google Scholar]
- 17.Piazza N, Onuma Y, Jesserun E, Kint PP, Maugenest AM, Anderson RH, et al. Early and persistent intraventricular conduction abnormalities and requirements for pacemaking after percutaneous replacement of the aortic valve. JACC Cardiovasc Interv. 2008;1:310–6. doi: 10.1016/j.jcin.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Erkapic D, Kim WK, Weber M, Möllmann H, Berkowitsch A, Zaltsberg S, et al. Electrocardiographic and further predictors for permanent pacemaker requirement after transcatheter aortic valve implantation. Europace. 2010;12:1188–90. doi: 10.1093/europace/euq094. [DOI] [PubMed] [Google Scholar]
- 19.Rubin J, Avanzas P, del Valle R, Moris C. Long-term follow up of atrioventricular block in transcatheter aortic valve implantation. Am J Cardiol. 2011;107:641–2. doi: 10.1016/j.amjcard.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Sniezek-Maciejewska M, Malecka B, Bednarek J, Machejek J, Kapelak B, Drwila R, et al. Patients history following artificial aortic valve and pacemaker implantation. Przegl Lek. 2004;61:718–21. [PubMed] [Google Scholar]
- 21.Roten AUR, Stortecky STEF, Scarcia FLAV, Kadner ALEX, Tanner HILD, Delacrataz E, et al. Atrioventricular conduction after transcatheter aortic valve implantation and surgical aortic valve replacement. J Cardiovasc Electrophysiol. 2012;23:1115–22. doi: 10.1111/j.1540-8167.2012.02354.x. [DOI] [PubMed] [Google Scholar]