Abstract
OBJECTIVES
Pulmonary metastasectomy for sarcoma is a widely accepted practice. Nevertheless, no previous studies has been reported the outcomes following metastasectomy compared with chemotherapy for patients with resectable and isolated pulmonary metastases. Our aim is to compare these modalities for the subset of patients with resectable metastases. Furthermore, the outcomes for patients with unresectable lung metastases are reported.
METHODS
Sarcoma patients with isolated lung metastases were identified and their computed axial tomography scans were reviewed by a thoracic surgeons' committee. Patients were divided into three groups: A: patients with resectable metastases treated with metastasectomy (n = 29), B: patients with resectable metastases who received systemic therapy (n = 17) and C: patients with unresectable metastases (n = 25). Survival outcomes were plotted and compared through log-rank test for osteosarcoma and non-osteosarcoma patients.
RESULTS
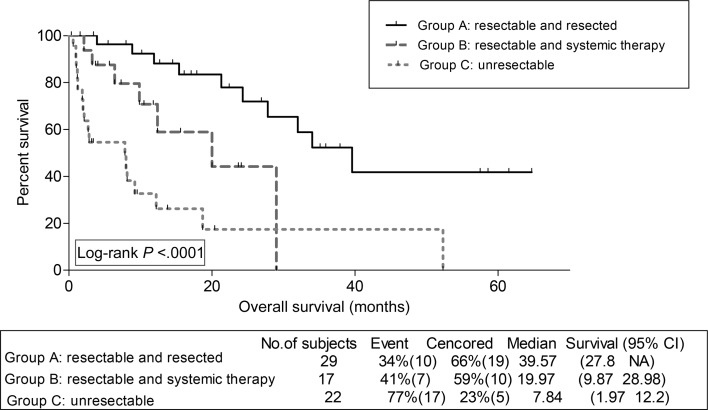
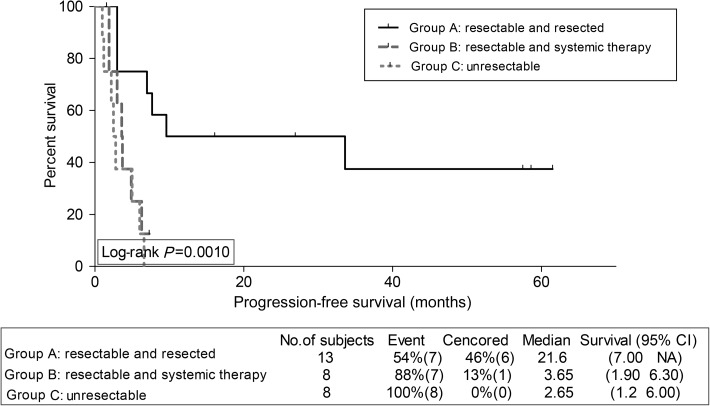
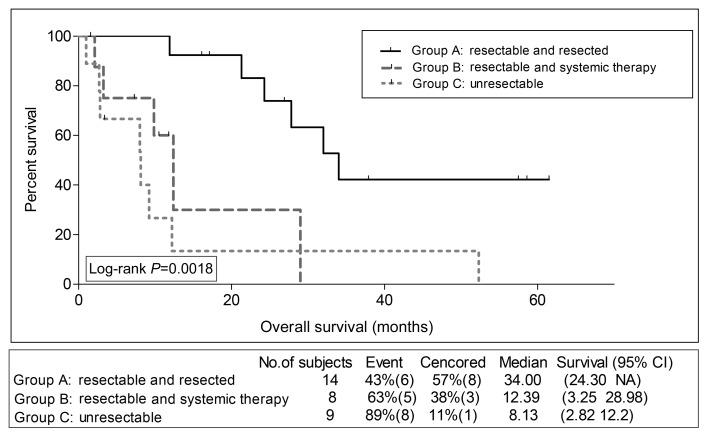
Seventy-one patients (32 with osteosarcoma and 39 with non-osteosarcoma) were eligible. Progression-free survival (PFS) was superior in patients who belonged to Group A compared with Groups B and C (8.0, 4.3 and 2.2 months, respectively, P = 0.0002). Furthermore, overall survival (OS) was superior in patients who belonged to Group A compared with Groups B and C (39.6, 20.0 and 7.8 months, respectively, P < 0.0001). A subanalysis for osteosarcoma patients showed superior PFS and OS for Group A vs B (median PFS 21.6 and 3.65 months, respectively, P = 0.011 and median OS 34.0 and 12.4 months, respectively, P = 0.0044). For non-osteosarcoma patients, there were no such significant survival differences between Groups A and B. Overall, patients who belonged to Group A had significantly lower mean percentage of their follow-up time spent admitted at hospital, and a trend towards lower requirements for home oxygen therapy.
CONCLUSIONS
Pulmonary metastasectomy is associated with improved survival of osteosarcoma patients with resectable lung metastases. For non-osteosarcoma patients, the survival benefit of metastasectomy over chemotherapy is uncertain and warrants further evaluation. Patients with unresectable metastases have poor prognosis.
Keywords: Sarcoma, Lung, Metastasis, Metastasectomy
INTRODUCTION
Pulmonary metastasectomy (PM) for sarcomas is becoming a widely accepted practice, as several retrospective studies have suggested improvement in survival following PM and reported encouraging 5-year survival rates of 23–52% [1–7], which compares favourably with the poor survival for cohorts of patients treated with non-operative therapy [8, 9]. Nevertheless, the apparent favourable survival with PM over chemotherapy could be the result of positive selection of patients treated with PM and the result of including patients with unresectable metastases in the cohorts treated with non-operative therapy: a fact that leads some authors to question the benefit from surgery [10–13].
In the current study, we compare the survival outcomes following PM with those following systemic therapy for the subset of patients who have resectable metastases. Additionally, we compare the outcomes following these two therapeutic strategies for the resectable metastasis with that of sarcoma patients with unresectable metastases.
MATERIALS AND METHODS
Study subjects
We retrospectively reviewed the charts of adult sarcoma patients, excluding Ewing family of tumours, who were treated and followed up at our institution between June 2001 and February 2012, who presented with or developed metastases that were limited to lungs. Patients with concomitant extrapulmonary metastases at the time when the lung metastases were first detected were excluded.
Assessment of resectability of metastases
A committee of three thoracic surgeons was assigned to assess the computed tomography (CT) scans of the patients that were created at the time when metastases were first detected, and to categorize these metastases into resectable and unresectable categories based on predefined criteria, in accordance with the criteria proposed by Thomford et al. [14]; the primary site has to be controlled, and metastases are amenable to surgical resection without residual disease. Surgeons were given information about the patients' demographics, sarcoma diagnosis and therapy to the primary site. However, they were blinded regarding the patients' identities and could not access the patients' names or hospital numbers in order to avoid a bias, which could influence their decision about the respectability. Following correlation of thoracic surgeons' assessment of resectability with the actual treatment each patient received, patients were divided into three groups: A: patients with resectable metastasis who had undergone PM, B: patients with resectable metastases who were treated with systemic therapy alone and C: patients with unresectable lung metastases.
Details of operative or systemic therapy of the metastases were documented. Progression-free survival (PFS), overall survival (OS), days of hospitalization following discharge from the first thoracotomy or following initiation of the first cycle of chemotherapy in patients who did not undergo metastasectomy, and the combined reporting of dyspnoea and any requirements for home oxygen therapy were also documented.
This study was approved by the institutional review board at our institution.
Definitions
Disease-free interval (DFI) was defined as the interval from curative resection of the primary sarcoma site until the first documentation of lung metastases. Progression was defined as the appearance of one or more new lesions or an increase in the size of target measurable lesions of ≥20% of the sum of the longest diameters by response evaluation in solid tumour (RECIST) criteria. OS was defined as the time from first detection of lung metastases until the last follow-up or death. PFS was defined as the time from the initiation of systemic therapy as a treatment of lung metastases in patients who did not undergo metastasectomy, or from first metastasectomy in patients who had undergone metastasectomy, until the first documentation of progression or recurrence, last follow-up or death.
Statistical analysis
PFS and OS were calculated for the entire cohort of patients, per treatment strategy (PM vs systemic therapy) and per patient group (Groups A, B and C). The survival curves were plotted using the Kaplan–Meier method and compared through the log-rank test. P-values of <0.05 were considered statistically significant.
Baseline characteristics of patients and their disease were expressed as proportions and compared using the chi-square (χ2) and Fisher's exact tests.
Analyses were performed to assess the influence of PM on survival outcomes for two subsets of patients; namely, osteosarcoma and non-osteosarcoma patients.
All analyses were performed using SAS version 9.1 (SAS Institute, Inc., Cary, NC, USA).
RESULTS
Screening for eligible patients
Between June 2001 and February 2012, 542 adult sarcoma patients, excluding Ewing family of tumours, were treated and followed up at our institution. Of those, 132 (24%) either presented with or developed lung metastases during the follow-up period. Forty-four (33%) were excluded because of concomitant extrapulmonary metastases. Seven were excluded because extrapulmonary metastatic sites could not be excluded. Of the remaining 81 patients, 1 was excluded for a second lung cancer and 7 because we could not get the films of their CT scans for review. Seventy-three patients remained eligible for resectability assessment by the thoracic surgery committee.
Assessment of resectability and patients' characteristics
Based on thoracic surgeons' assessment, 48 (66%) patients were categorized as having resectable metastases and 25 (34%) as having unresectable metastases. Following correlation of resectability assessment with the actual therapeutic modality administered, patients were divided into three groups: A: patients with resectable metastases who had undergone PM (n = 29), B: patients with resectable metastases who were not treated with PM (n = 17, after excluding 2 patients who refused to receive any kind of therapy) and C: patients with unresectable metastases (n = 25).
None of those 17 patients who belonged within the Group B had documented cardiac or chronic pulmonary disease that would preclude resection, and all of them had an Eastern cooperative oncology group (ECOG) performance status of 0–1 at the time of detection of metastases, and the reason why they were not treated with PM is that the thoracic surgeons were not consulted for opinion about resectability of these patients.
The characteristics of the patients in the 3 groups are summarized in Table 1.
Table 1:
Characteristics of the 71 sarcoma patients with isolated lung metastasis at the time when the metastases were detected.
| Variable | Group A (N = 29) | Group B (N = 17) | Group C (N = 25) | P-value (for comparison between Groups A and B) |
|---|---|---|---|---|
| Gender | ||||
| Males | 17 (58.6%) | 9 (52.9%) | 14 (56.0%) | 0.71 |
| Females | 12 (41.4%) | 8 (47.1%) | 11 (44.0%) | |
| Sarcoma type | ||||
| Osteosarcoma | 14 (48.3%) | 8 (47.1%) | 10 (40.0%) | 0.94 |
| Non-osteosarcoma | 15 (51.7%) | 9 (52.9%) | 15 (60.0%) | |
| Mean age at detection of metastases (years) | 31.9 | 27.6 | 38.4 | 0.28 |
| Site | ||||
| Upper extremity | 8 (27.6%) | 4 (23.5%) | 2 (8.0%) | 1.00 |
| Lower extremity | 17 (58.6%) | 11 (64.7%) | 16 (64.0%) | |
| Trunk | 1 (3.4%) | 1 (5.9%) | 1 (4.0%) | |
| Urogenital | 2 (6.9%) | 1 (5.9%) | 3 (12.0%) | |
| Head and neck | 1 (3.4%) | 0 | 0 | |
| Pelvic | 0 | 0 | 2 (8.0%) | |
| Retroperitoneal | 0 | 0 | 1 (4.0%) | |
| Histological grade | ||||
| ND/not applicable | 1 (3.4%) | 2 (11.8%) | 1 (4.0%) | 0.82 |
| 1 | 2 (6.9%) | 1 (5.9%) | 0 | |
| 2 | 2 (6.9%) | 2 (11.8%) | 4 (16.0%) | |
| 3 | 24 (82.8%) | 12 (70.5%) | 20 (80.0%) | |
| No. of metastatic nodules | ||||
| <3 | 13 (44.8%) | 3 (17.6%) | 0 | 0.014 |
| ≥3 | 14 (48.3%) | 14 (82.4%) | 23 (92.0%) | |
| ND | 2 (6.9%) | 0 | 2 (8.0%) | |
| Size of metastatic nodulesa (cm) | ||||
| <2 | 10 (34.5%) | 9 (52.9%) | 7 (28.0%) | 0.29 |
| ≥2 | 14 (48.3%) | 5 (29.4%) | 14 (56.0%) | |
| ND | 5 (17.2%) | 3 (17.7%) | 4 (16%) | |
| Laterality of nodules | ||||
| Unilateral | 17 (57.1%) | 5 (6.7%) | 2 (8.0%) | 0.001 |
| Bilateral | 12 (42.9%) | 14 (93.3%) | 23 (92.0%) | |
| Mean follow-up time from first detection of lung metastases: days (95% CI) | 764 (590–938) | 340 (229–451) | 257 (144–370) | 0.0049 |
| Mean disease-free interval: months (95% CI) | 12.9 (8.3–17.4) | 8.9 (2.4–15.4) | 4.6 (1.9–7.4) | 0.086 |
ND: no data or data were uncertain.
aThe maximum diameter (cm) of the largest metastatic lung nodule.
Follow-up of the lungs
All the patients were followed up with CT scan of the chest once every 3 months for the first 2 years following definitive therapy of the primary site, then every 6 months for the third through the fifth year. Once lung metastases were detected, the decision regarding treating patients with chemotherapy or referring the patients for PM was left to the judgment of the treating medical oncologist. It is worth noting that not all of these cases were discussed in multidisciplinary setting at our centre. The protocol and schedule of CT scan follow-up did not change at our centre since the year 2000. Apart from 2 patients with unresectable lung metastases who lost the follow-up because they returned back to their country of origin, all other patients continued follow-up at our institution.
Surgical outcomes
Thirty patients were treated with PM, and 22 of them (73%) following preoperative chemotherapy. Two (7%) patients received adjuvant chemotherapy following PM. One of the 30 patients who were treated with PM was considered to have unresectable metastases based on CT scan assessment and had gross residual disease following metastasectomy. Of the Group A patients, 2 had undergone PM with positive margins and 1 with gross residual disease; the other 26 patients (87%) had complete resection of the metastases.
Eleven (37%) patients had repeated PM, and 5 (17%) had at least 3 metastasectomies. Overall, 60 thoracotomies were carried out in all the patients. All the patients had pathologically confirmed metastatic disease. Of 216 resected nodules, 195 (91%) were positive for metastases, 11 (5%) were inflammatory, 7 (3%) were fibrotic and 3 (1%) contained benign lung tissue. The mode of surgical resection was a sub-lobar resection in 48 (80%) thoracotomies, a lobectomy in 10 (17%) and a pneumonectomy in 2 (3%).
One (3.3%) patient died of multilobar pneumonia within 1 month of thoracotomy, corresponding to a post-thoracotomy mortality of 1.7%. Four major postoperative morbidities in 3 (10%) patients were observed following the 60 thoracotomies, 1 had a stroke and peripheral arterial ischaemia and 2 had severe sepsis.
Non-operative therapy for the metastases
Of the 17 patients with resectable metastases who were not treated with PM (Group B), 16 (94%) were treated with chemotherapy and 1 (6%) with imatinib. Ten received their first-line therapy as combination chemotherapy and 6 as single agents. The first-line therapy was doxorubicin based in 10 patients and ifosfamide based in 4.
Of the Group C patients, 19 (76%) received chemotherapy, 3 (12%) received imatinib and 3 (12%) did not receive specific therapy for poor performance status and were not included in the survival analysis. The chemotherapy regimens were single agents for 8 patients and combination chemotherapy for 10. Fourteen patients received doxorubicin-based chemotherapy, 3 received ifosfamide-based chemotherapy and the other received other regimens. It is worth mentioning that the indications of chemotherapy and chemotherapy regimens used at our centre did not change during the period of the study.
Hospitalizations and home oxygen therapy
Patients who belonged to Group A had significantly lower mean percentage of their follow-up days spent admitted in hospital (4.2, 15.2 and 25.2%, for Groups A, B and C, respectively, P = 0.0004). The combined reporting of shortness of breath and any requirements for home oxygen therapy did not differ significantly between the groups (10.3, 24 and 20% of patients who belonged to Groups A, B and C, respectively, P = 0.17).
Survival outcomes
The entire cohort of patients had a median PFS and OS of 4.9 and 24.3 months, respectively.
Patients who were treated with PM had a significantly favourable PFS compared with those treated with systemic therapy; 8.0 vs 3.0 months, respectively, P = 0.0009. Furthermore, OS was significantly superior in patients treated with PM; 39.6 and 9.9 months, respectively, P < 0.0001.
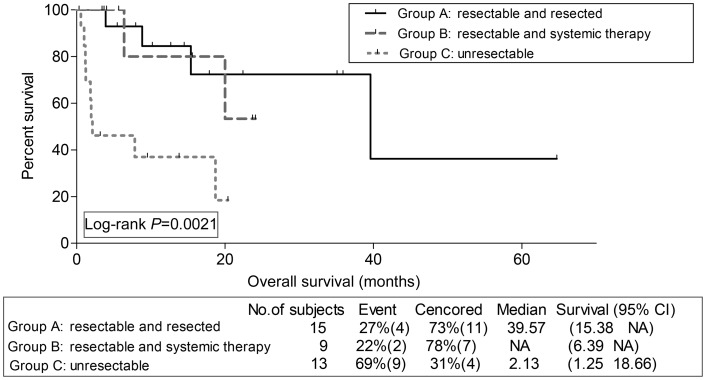
Assessment of survival outcomes by groups revealed favourable PFS for Group A compared with Groups B and C; 8.0, 4.3 and 2.2 months, respectively; P = 0.0002. Similarly, patients who belonged to Group A had favourable OS compared with Groups B and C (Fig. 1).
Figure 1:
Kaplan–Meier overall survival estimation for sarcoma patients per group.
Subanalysis for osteosarcoma and non-osteosarcoma
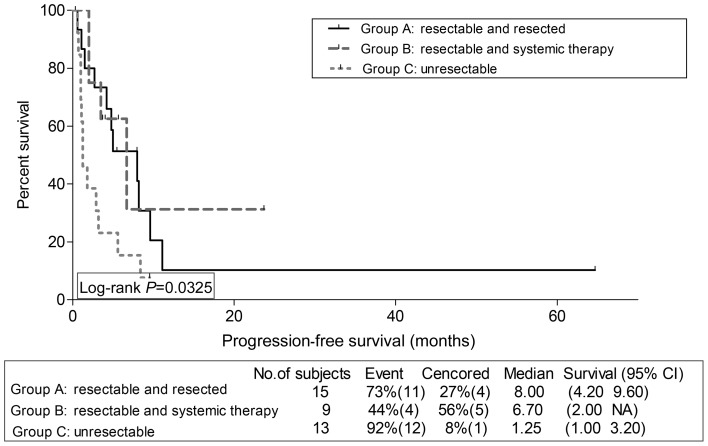
We assessed survival outcomes per group, for osteosarcoma patients, on one hand, and for non-osteosarcoma patients, on the other hand. Interestingly, PFS and OS were significantly superior for Group A compared with B for osteosarcoma patients only; PFS 21.6 and 3.65 months, respectively, P = 0.011 (Fig. 2), OS 34.00 and 12.39 months, respectively, P = 0.0044 (Fig. 3). For non-osteosarcoma patients, there were no such statistically significant differences in PFS (Fig. 4), nor in OS (Fig. 5) between Groups A and B.
Figure 2:
Kaplan–Meier progression-free survival estimation for osteosarcoma patients per group.
Figure 3:
Kaplan–Meier overall survival estimation for osteosarcoma patients per group.
Figure 4:
Kaplan–Meier progression-free survival estimation for non-osteosarcoma patients per group.
Figure 5:
Kaplan–Meier overall survival estimation for non-osteosarcoma patients per group.
Finally, a subanalysis for osteosarcoma patients with multiple (≥3 nodules) and bilateral lung metastases (n = 13) showed a trend towards improved OS for Group A compared with B, OS 32.00 and 12.39 months, respectively, P = 0.094.
DISCUSSION
Sarcomas are rare and heterogeneous group of neoplasms which have the propensity for haematogenous spread to lungs. Around 20% of patients with sarcomas of the extremities will develop lung metastases at some point during the course of their disease [15, 16]. Compared with extremity sarcomas, retroperitoneal and visceral sarcomas are less likely to develop lung metastases and have more propensities for local recurrences [17]. Although studies had shown that osteosarcoma, synovial sarcoma, liposarcoma and malignant fibrous histiocytoma are the most commonly encountered metastatic histologies [8, 18], sarcomas of any histological subtype or primary site can metastasize to lungs [18, 19].
Many studies have shown that surgical resection of lung metastases in osteosarcoma and soft-tissue sarcoma patients is associated with a favourable prognosis [5–9]. The encouraging survival rates in these series could be explained, in part, by the limited nature of the metastases. In fact, the historical comparisons with patients treated with chemotherapy are not sufficient to make a firm conclusion that PM improves survival because the patients who are treated with non-operative therapy have such an advanced disease that is beyond resectability. For that reason, we elected to categorize the metastases into resectable and unresectable categories as a first step, before we retrospectively identified the therapeutic strategies for every patient.
Our findings suggest that PM is associated with improvement in survival of osteosarcoma patients with resectable metastases. In fact, osteosarcoma patients with resectable metastases in our study, who were managed without PM, were more than five times at risk of progression and more than four times at risk of death compared with osteosarcoma patients with resectable metastases who were managed with PM. We emphasize the importance of complete resection (R0 resection) of the metastases because it is the most consistent prognostic factor among studies [7, 8, 20–22]. On the other hand, non-osteosarcoma patients with resectable lung metastases in our study, who were managed with PM, had PFS and OS rates that were not different from those treated with systemic therapy alone.
These findings suggest that the influence of PM on survival in sarcoma is variable and is dependent in part on the histological subtype. We think that future studies should address specific sarcoma histologies.
We acknowledge the limitation of our retrospective design, and the recognized differences in two important disease-related variables within patients in the resectable group: laterality and number of lung nodules. More patients within Group B when compared with Group A had bilateral metastases; additionally, the number of metastatic nodules was higher in Group B patients. Apart from one study [5], laterality of metastases was never found to influence survival in the numerous studies of PM [6–8, 23]. Data from the literature are conflicting regarding the prognostic significance of the number of metastatic nodules; while some studies have demonstrated that the number has prognostic significance [5, 23–25]; other studies have shown that whenever all nodules are completely resected, the number becomes not important as a prognostic factor [1, 4, 8, 19, 25]. We recognize the differences in these factors between the two groups; however, that limitation was unavoidable given the retrospective design of our study.
To limit the influence of laterality and number of metastases on our results, we performed the subanalysis for osteosarcoma patients with resectable multiple (≥3 nodules) and bilateral metastases and found a trend towards improved survival with PM compared with chemotherapy alone for that group. This finding suggests that even those patients with multiple metastases can benefit from a strategy of complete resection of all metastatic nodules. The difference in survival was clinically meaningful (32.0 vs 12.4 months). However, it is possible that the small number of patients was the reason why the difference did not reach statistical significance (P = 0.09).
An important limitation of our study is that pulmonary function tests were not available for the patients with resectable metastases who were treated with non-operative therapy; however, these patients were free of documented pulmonary or cardiac diseases and were of young age (mean age 27.6 years). Furthermore, they had adequate performance status, which minimize the possible influence of comorbidities, advanced age and poor performance status on the demonstrated inferior outcomes.
We acknowledge our limitation in assessing the quality of life for the three groups. Quality-of-life assessment is applicable to prospective studies and not to retrospective studies as the case of our study. We assessed the combined outcome of reporting shortness of breath and any requirements for home oxygen therapy in an attempt to have an idea about the respiratory status of the patients. It is worth noting that no previous studies addressed the respiratory status for sarcoma patients who are treated with PM as Treasure et al. [12] demonstrated in a recent systematic review. Furthermore, we compared the hospitalizations in order to have an idea about the level of morbidity following therapy of the metastases. In our series, patients who had undergone metastasectomy had significantly lower hospitalizations when compared with resectable patients who were treated with chemotherapy alone. Furthermore, we demonstrated that patients who had undergone metastasectomy, a significant proportion of whom had undergone repeated resections, are not at significant risk of increased requirement of home oxygen, and a lung parenchyma saving approach is both effective and safe. This approach will preserve lung tissue to allow future repeated resection if needed.
Patients with unresectable metastases in our study had an extremely poor prognosis and spent a significant proportion of their remaining life (25% of their follow-up time) admitted to hospital. The poor survival with chemotherapy suggests that alternative therapeutic strategies including evaluation of novel targeted therapies are urgently needed. These patients should be managed with a palliative intent because their disease is considered non-curable with currently available therapies.
Conclusions
PM is associated with improvement in PFS and OS for osteosarcoma patients with resectable lung metastases. In contrary, non-osteosarcoma patients did not derive a survival advantage from PM in our study. Patients with unresectable lung metastasis have extremely poor prognosis.
ACKNOWLEDGEMENTS
We thank Dalia Al-Rimawi and Ayat Taqash, Statistical Programmers at King Hussein cancer center, for helping with the statistics.
Conflict of interest: none declared.
REFERENCES
- 1.Rehders A, Hosch SB, Scheunemann P, Stoecklein NH, Knoefel WT, Peiper M. Benefit of surgical treatment of lung metastasis in soft tissue sarcoma. Arch Surg. 2007;142:70–5. doi: 10.1001/archsurg.142.1.70. discussion 76. [DOI] [PubMed] [Google Scholar]
- 2.García Franco CE, Algarra SM, Ezcurra AT, Guillén-Grima F, San-Julián M, Mindán JP, et al. Long-term results after resection for soft tissue sarcoma pulmonary metastases. Interact CardioVasc Thorac Surg. 2009;9:223–6. doi: 10.1510/icvts.2009.204818. [DOI] [PubMed] [Google Scholar]
- 3.Predina JD, Puc MM, Bergey MR, Sonnad SS, Kucharczuk JC, Staddon A, et al. Improved survival after pulmonary metastasectomy for soft tissue sarcoma. J Thorac Oncol. 2011;6:913–9. doi: 10.1097/JTO.0b013e3182106f5c. [DOI] [PubMed] [Google Scholar]
- 4.Robinson MH, Sheppard M, Moskovic E, Fischer C. Lung metastasectomy in patients with soft tissue sarcoma. Br J Radiol. 1994;67:129–35. doi: 10.1259/0007-1285-67-794-129. [DOI] [PubMed] [Google Scholar]
- 5.Casson AG, Putnam JB, Natarajan G, Johnston DA, Mountain C, McMurtrey M, et al. Five-year survival after pulmonary metastasectomy for adult soft tissue sarcoma. Cancer. 1992;69:662–8. doi: 10.1002/1097-0142(19920201)69:3<662::aid-cncr2820690311>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 6.The International Registry of Lung Metastases. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg. 1997;113:37–49. doi: 10.1016/s0022-5223(97)70397-0. [DOI] [PubMed] [Google Scholar]
- 7.Smith R, Pak Y, Kraybill W, Kane Iii JM. Factors associated with actual long-term survival following soft tissue sarcoma pulmonary metastasectomy. Eur J Surg Oncol. 2009;35:356–61. doi: 10.1016/j.ejso.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Billingsley KG, Burt ME, Jara E, Ginsberg RJ, Woodruff JM, Leung DH, et al. Pulmonary metastases from soft tissue sarcoma: analysis of patterns of diseases and postmetastasis survival. Ann Surg. 1999;229:602–10. doi: 10.1097/00000658-199905000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mentzer SJ, Antman KH, Attinger C, Shemin R, Corson JM, Sugarbaker DJ. Selected benefits of thoracotomy and chemotherapy for sarcoma metastatic to the lung. J Surg Oncol. 1993;53:54–9. doi: 10.1002/jso.2930530114. [DOI] [PubMed] [Google Scholar]
- 10.Frost DB. Pulmonary metastasectomy for soft tissue sarcoma; is it justified? J Surg Oncol. 1995;59:110–5. doi: 10.1002/jso.2930590208. [DOI] [PubMed] [Google Scholar]
- 11.Fey MF, Rauch D. Metastasectomy—a direct therapeutic effect or an illusion due to patient selection? Ther Umsch. 2001;58:726–31. doi: 10.1024/0040-5930.58.12.726. [DOI] [PubMed] [Google Scholar]
- 12.Treasure T, Fiorentino F, Scarci M, Møller H, Utley M. Pulmonary metastasectomy for sarcoma: a systematic review of reported outcomes in the context of Thames Cancer Registry data. BMJ Open. 2012;2:pii. doi: 10.1136/bmjopen-2012-001736. e001736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aberg T, Malmberg KA, Nilsson B, Nöu E. The effect of metastasectomy: fact or fiction? Ann Thorac Surg. 1980;30:378–84. doi: 10.1016/s0003-4975(10)61278-7. [DOI] [PubMed] [Google Scholar]
- 14.Thomford NR, Woolner L, Clagett O. The surgical treatment of metastatic tumours in the lung. J Thorac Cardiovasc Surg. 1965;49:357–63. [PubMed] [Google Scholar]
- 15.Gadd MA, Casper ES, Woodruff JM, McCormack PM, Brennan MF. Development and treatment of pulmonary metastases in adult patients with extremity soft tissue sarcoma. Ann Surg. 1993;218:705–12. doi: 10.1097/00000658-199312000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Songür N, Dinç M, Ozdilekcan C, Eke S, Ok U, Oz M. Analysis of lung metastases in patients with primary extremity sarcoma. Sarcoma. 2003;7:63–7. doi: 10.1080/13577140310001607284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brennan MF. Management of extremity soft-tissue sarcoma. Am J Surg. 1989;158:71–8. doi: 10.1016/0002-9610(89)90319-x. [DOI] [PubMed] [Google Scholar]
- 18.Krishnamoorthy N, Desai SS, Rekhi B, Jambhekar NA. A clinico-morphological study of 95 cases of sarcomas with metastases to the lungs. Indian J Cancer. 2011;48:335–8. doi: 10.4103/0019-509X.84942. [DOI] [PubMed] [Google Scholar]
- 19.Lewis JJ, Brennan MF. Soft tissue sarcomas. Curr Probl Surg. 1996;33:817–72. [PubMed] [Google Scholar]
- 20.Sardenberg RA, Figueiredo LP, Haddad FJ, Gross JL, Younes RN. Pulmonary metastasectomy from soft tissue sarcomas. Clinics (Sao Paulo) 2010;65:871–6. doi: 10.1590/S1807-59322010000900010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfannschmidt J, Klode J, Muley T, Dienemann H, Hoffmann H. Pulmonary metastasectomy in patients with soft tissue sarcomas: experiences in 50 patients. Thorac Cardiovasc Surg. 2006;54:489–92. doi: 10.1055/s-2006-924248. [DOI] [PubMed] [Google Scholar]
- 22.Pfannschmidt J, Klode J, Muley T, Hoffmann H, Dienemann H. Pulmonary resection for metastatic osteosarcomas: a retrospective analysis of 21 patients. Thorac Cardiovasc Surg. 2006;54:120–3. doi: 10.1055/s-2005-872855. [DOI] [PubMed] [Google Scholar]
- 23.Putnam JB, Jr, Roth JA, Wesley MN, Johnston MR, Rosenberg SA. Analysis of prognostic factors in patients undergoing resection of pulmonary metastases from soft tissue sarcomas. J Thorac Cardiovasc Surg. 1984;87:260–8. [PubMed] [Google Scholar]
- 24.Choong PF, Pritchard DJ, Rock MG, Sim FH, Frassica FJ. Survival after pulmonary metastasectomy in soft tissue sarcoma: prognostic factors in 214 patients. Acta Orthop Scand. 1995;66:561–8. doi: 10.3109/17453679509002316. [DOI] [PubMed] [Google Scholar]
- 25.Temeck BK, Wexler LH, Steinberg SM, McClure LL, Horowitz M, Pass HI. Metastasectomy for sarcomatous pediatric histologies: results and prognostic factors. Ann Thorac Surg. 1995;59:1385–9. doi: 10.1016/0003-4975(95)00233-b. discussion 90. [DOI] [PubMed] [Google Scholar]