Abstract
OBJECTIVES
Acute kidney injury (AKI) following cardiac surgery is a common complication associated with serious morbidity and mortality. Activation of inflammatory cascade and vascular endothelial dysfunction plays a vital role during the perioperative period leading to AKI. Statins are known to suppress inflammation and improve endothelial dysfunction over and above the cholesterol lowering efficacy.
METHODS
Observational studies with a defined population in terms of preoperative statin therapy and no preoperative statin therapy undergoing cardiac surgery (CABG, isolated valve surgery or both) and with reported data on the incidence of acute renal failure/injury and/or mortality were identified and analysed for inclusion in the analysis. Outcomes evaluated were occurrence of postoperative acute kidney injury/failure, requirement of any postoperative renal replacement therapy and short-term all-cause mortality rate. A meta-analysis was conducted and a pooled estimate of odds ratio (OR) was calculated using the inverse variance method.
RESULTS
A total of 17 studies with a total population of 24 998 statin users and 22 082 non-statin users were included in the final analysis. PST resulted in a significantly lower incidence of renal replacement therapy in patients undergoing CABG (OR: 0.56 [0.41–0.76]) but not in isolated valve surgery (OR: 1.80 [0.73–4.44]). Also preoperative statin therapy resulted in a significantly lower postoperative mortality (0.72 [0.61–0.84]) irrespective of the type of surgery. There was no effect of preoperative statin therapy on the incidence of AKI in any of the sub-group of the patients.
CONCLUSIONS
Patients undergoing CABG might derive benefit from preoperative statin therapy in terms of reducing the need for postoperative renal replacement therapy and mortality. However, the uncertainty concerning the reno-protective efficacy of preoperative statin therapy in patients undergoing isolated valve surgery needs further investigation.
Keywords: Acute kidney injury, Cardiac surgery; Prevention; Statin
INTRODUCTION
Acute kidney injury (AKI) is a common postoperative complication following cardiac surgery. The common occurrence is attributed to factors like perioperative haemodynamic alterations, comorbid conditions predisposing to renal injury and pharmacological toxins associated with cardiac surgery. It is associated with serious morbidity and mortality. The incidence of AKI following cardiac surgery ranges from 20 to 50% depending upon the criteria used to define renal injury [1]. The rise in serum creatinine, absolute or a percentage increase over a defined time period after surgery, is usually taken to diagnose AKI. The differences in the magnitude of increase and the definition of time period had led to the differences in the incidence observed in various studies. The two commonly used definitions acute kidney injury network (AKIN) criteria (>0.3 mg/dl increase or 50% increase over baseline within 48 h, >100% increase over baseline and >200% increase over baseline or 0.5 mg/dl increase to at least 4.0 mg/dl) and risk injury failure loss and end stage renal disease (RIFLE) criteria (>50% increase over baseline, >100% increase over baseline and >200% increase over baseline or ≥0.5 mg/dl increase to at least 4.0 mg/dl) categorize AKI into three stages. However, irrespective of the definition used, the mortality associated with the occurrence of AKI is more than 60%. Pharmacological preventive treatments are directed towards blocking one or more of the multiple pathological pathways involved in the development of AKI. Improvement of the renal and the splanchnic perfusion, targeting the inflammatory cytokines and oxidative stress have been tried to improve the renal outcomes in high-risk patients undergoing cardiac surgery. Patients undergoing cardiac surgery show increased levels of circulatory cytokines like tumor necrosis factor-α and various interleukins [2]. 3-Hydroxy-3-methyl-glutaryl-CoA reductase (HMG Co-A) reductase inhibitors, commonly termed statins, are known to have many pleiotropic effects in addition to their lipid-lowering properties. These additional properties include the anti-inflammatory, antithrombotic and antioxidant actions and improvement of the endothelial dysfunction. Owing to these properties, the statins are expected to be associated with improved cardiovascular outcomes in patients undergoing cardiac interventional procedures. In a meta-analysis of randomized controlled trials and observational studies involving a large population of patients undergoing cardiac surgery, preoperative statin therapy (PST) was associated with a lower incidence of atrial fibrillation, stroke and all-cause mortality [3]. However, the incidence of renal failure was not significantly different from that in the patients who did not receive preoperative statins.
A retrospective cohort study had demonstrated the benefits of PST on renal outcomes [4], while another study had demonstrated the lack of benefit of PST in the prevention of acute renal injury [5]. The equivocal nature of the published reports on the effect of preventive treatment with statin on renal outcomes following cardiac surgery leaves the question open whether preoperative statins might prevent acute renal injury in patients undergoing cardiac surgery. Few randomized clinical trials had evaluated the effects of PST on renal outcomes. A meta-analysis of four such studies had revealed no statistically significant benefit (odds ratio (OR): 0.41, 95% confidence interval: 0.15–1.12) of PST on renal outcomes [3]. The small sample size of 367 patients in total and the low event rate might have affected the results. Similarly, a small pilot trial showed no benefit of preoperative atorvastatin on the occurrence of AKI using the consensus definition for acute renal injury [6]. However, the study had few limitations, which included significant differences in the baseline characteristics of the study population, small sample size and a short washout period. Conduct of a placebo controlled clinical trial to evaluate the effect of statin in such patient population to generate evidence is limited by the ethical issue of withdrawing statin therapy, as many patients would have been receiving these drugs for the secondary prevention of cardiovascular events. Keeping in view of the published equivocal nature of the data, the present meta-analysis of observational studies evaluating the effect of preoperative statin in patients undergoing cardiac surgery on renal outcomes was undertaken.
METHODS
The observational studies that had reported the effects of PST on the postoperative renal outcomes in adult patients undergoing cardiac surgery were identified and analysed for inclusion. The following inclusion criteria were used for the purposes of selection of the studies: (i) studies with a defined population in terms of preoperative statin users (defined as taking statin prior to surgery irrespective of indication and duration) and non-statin users (defined as patients not taking any statin prior to surgery), (ii) studies with a patient population undergoing cardiac surgery (CABG, valve surgery or both) and (iii) studies with reported data on the incidence of acute renal failure/injury and/or mortality. Two authors independently performed an electronic literature search in PUBMED using MESH terms: ‘HMG Co-A reductase inhibitors’, ‘Cardiac surgery’ and ‘Acute renal injury’. All human studies published in full-text or abstract forms were initially screened for inclusion without applying any language restrictions. Titles and abstracts from the electronic search were reviewed. After initial review of the abstracts, the relevant studies were identified and a detailed evaluation of the full text was done. Additional studies were identified through cross-references from the full-text published article. All data with regard to authorship, year of publication, type of publication study design, study population, type of cardiac surgery and clinical endpoint definition were extracted. Funnel plot analysis was performed for assessment of publication bias. Methodological quality of the included studies was assessed using the Downs and Black score [7].
Outcomes
The primary analysis included the postoperative renal outcome defined as occurrence of postoperative AKI/failure. Secondary outcome was postoperative requirement of any renal replacement therapy (RRT) and short-term all-cause mortality rate.
Statistical analyses
Statistical analyses were performed using RevMan (version 5). For each individual study, the adjusted estimated effects expressed as the OR and its 95% confidence interval (95% CI) were used and summarized by Forest plots. Sub-group analysis was also done to determine the effect of preoperative statin on postoperative renal outcomes depending on the type of cardiac surgery performed (CABG, isolated cardiac valve surgery or any cardiac surgery). Pooled estimate was obtained using the inverse variance method. Q-statistics or I2-statistics was performed to test for the heterogeneity among the included studies. A standard fixed effects model was used in the absence of heterogeneity among the studies. In the presence of heterogeneity, the random effects model was used. A funnel plot was constructed to assess the presence of publication bias and examine the differences between the effects in the large and the small studies. The treatment effects, given as the OR on a logarithmic scale, were plotted against a measure of precision expressed as the inverse standard error.
RESULTS
A total of 17 observational studies were included in the analysis [4, 5, 8–22]. The flow chart of search strategy is provided in Fig. 1. The characteristics and details of the included studies are shown in Table 1. The analysis included a total of 52 770 patients of whom 28 242 were included in the preoperative statin group and 24 528 were included in the no preoperative statin group.
Figure 1:
Flowchart of search.
Table 1:
Characteristics and details of the included studies
| Author | Design | Patient population | AKI definition | Statin users (n) | Non-statin users (n) | AKI |
RRT |
Mortality |
Downs and Black Score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S (n) | NS (n) | Adjusted odds (CI) | S (n) | NS (n) | Adjusted odds (CI) | S (n) | NS (n) | Adjusted odds (CI) | |||||||
| Ali and Buth [8] | RC | C, V, C + V | NR | 3555 | 1914 | NR | NR | NR | NR | NR | NR | 451 | 373 | 0.9 (0.6–1.2) | 20 |
| Allou et al. [9] | RC | C | 50% increase from baseline | 222 | 208 | 22 | 29 | NR | NR | NR | NR | NR | NR | 0.52 (0.25–1.11) | 18 |
| Argalious et al. [5] | RC | C V | RIFLE | 6152 | 4487 | 819 | 467 | 0.97 (0.84–1.12) | 108 | 74 | 0.8 (0.55–1.18) | 105 | 77 | 0.803 (0.56–1.16) | 19 |
| Billings et al. [10] | RC | C | AKIN | 174 | 150 | 39 | 29 | 1.24 (0.66–2.32) | NR | NR | NR | NR | NR | NR | 21 |
| Bolesta et al. [11] | RC | C | AKIN | 356 | 207 | 125 | 54 | 1.36 (0.904–2.05) | NR | NR | NR | NR | NR | NR | 19 |
| Borger et al. [12] | RC | C, V and C + V | Increased Cr or RRT | 4216 | 5538 | NR | NR | NR | 451 | 454 | NR | 316 | 366 | 0.89 (0.75–1.06) | 18 |
| Clark et al. [13] | RC | C, V, C + V | NR | 1044 | 2785 | NR | NR | NR | NR | NR | NR | 26 | 155 | 0.55 (0.32–0.93) | 17 |
| Coleman et al. [14] | RC | C, V, C + V | NR | 1248 | 686 | NR | NR | NR | NR | NR | NR | 53 | 42 | 0.85 (0.51–1.43) | 17 |
| Fedoruk et al. [15] | RC | V | Two times increase from baseline or RRT | 203 | 244 | 8 | 14 | 2.17 (0.824–5.698) | NR | NR | NR | 7 | 13 | 2.7 (0.81–9.05) | 19 |
| Huffmyer et al. [4] | RC | C | RRT or more than 50% decrease in eGFR | 1557 | 1203 | 436 | 331 | 1.012 (0.893–1.145) | 30 | 43 | 0.542 (0.379–0.774) | 31 | 51 | 0.572 (0.42–0.779) | 17 |
| Mithani et al. [16] | RC | C, V, C + V | AKIN | 1434 | 670 | 322 | 173 | 0.79 (0.59–1.06) | 20 | 7 | NR | NR | NR | NR | 21 |
| Powel et al. [17] | RC | C | NR | 2334 | 2405 | NR | NR | NR | NR | NR | NR | 32 | 53 | 0.83 (0.50–1.37) | 17 |
| Subramaniam et al. [18] | RC | C | New onset renal failure requiring dialysis | 1835 | 652 | 8 | 8 | 1 (0.37–2.68) | 8 | 8 | 1 (0.37–2.68) | 5 | 8 | 0.62 (0.20–1.91) | 17 |
| Tabata et al. [19] | RC | C | RRT, two times increase in S Cr and S Cr of >2 mg/dl | 1039 | 763 | NR | NR | 0.54 (0.3–0.99) | NR | NR | NR | NR | NR | NR | 18 |
| Virani et al. [20] | RC | V | NR | 255 | 570 | NR | NR | NR | NR | NR | NR | 9 | 31 | 0.89 (0.38–2.03) | 18 |
| Virani et al. [21] | RC | C, V, C + V | Any increase Sr Cr, ATN, AIN or Anuria | 1675 | 13 26 | 42 | 46 | 0.6 (0.38–0.95) | NR | NR | 0.71 (0.41–1.24) | NR | NR | NR | 17 |
| Pan et al. [22] | RC | C | RRT, at least 2 mg/dl, 0.7 mg/dl rise or RF on autopsy | 943 | 720 | 59 | 39 | NR | NR | NR | NR | 17 | 27 | 0.53 (0.28–0.99) | 18 |
AKIN: acute kidney injury network; ATN: acute tubular necrosis; AIN: acute interstitial nephritis; RC: retrospective cohort; C: CABG; V: isolated valve surgery; Cr: creatinine; C + V: any cardiac surgery (including CABG, valve or combined); RRT: renal replacement therapy; RIFLE: risk injury failure loss and end stage renal disease; NR: not reported; S: preoperative statin treatment group; NS: no preoperative statin group; eGFR: estimated glomerular filtration rate; Sr: serum.
Primary outcome
Occurrence of postoperative acute kidney injury/failure
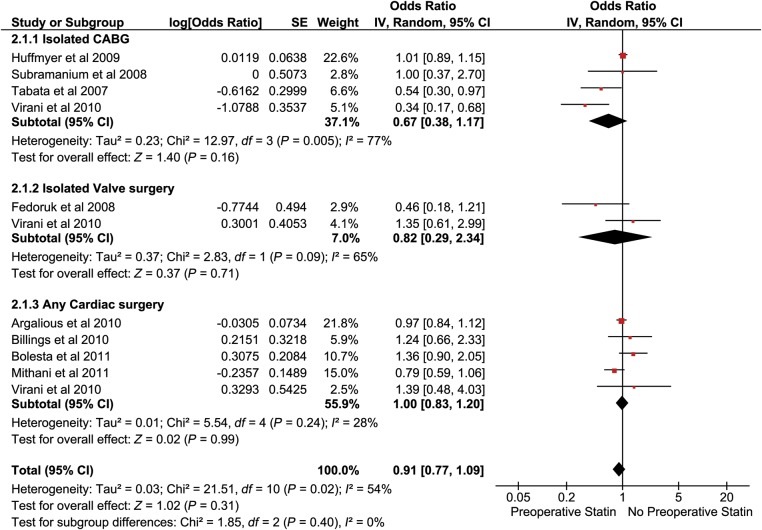
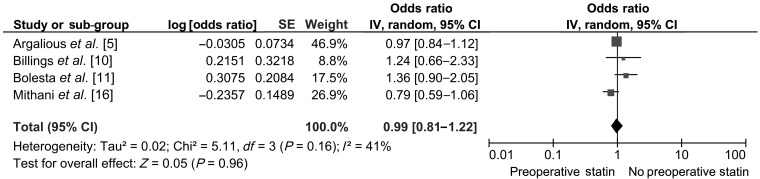
Out of the 17 studies identified for the analysis, 11 studies reported postoperative renal outcomes. Nine studies with a total population of 24 127 (14 425 statin users and 9702 no statin users) had reported adjusted OR for postoperative acute kidney/renal injury were included for the analysis of AKI. The studies that were included had differing definitions for AKI (Table 1). Four studies had used the AKIN/RIFLE criteria for defining the postoperative kidney injury in the patients undergoing cardiac surgery. A separate analysis was done including only those studies that had used the AKIN/RIFLE criteria. The pooled OR (95% CI) for primary outcomes from the nine studies was 0.91 [0.77–1.09] (Fig. 2). Sub-group analysis had revealed an odds of AKI following CABG to be 0.67 [0.38–1.17], following isolated valve surgery (IVS) to be 0.82 [0.29–2.34] and following any cardiac surgery to be 1.00 [0.83–1.20] (Fig. 2). A sensitivity analysis was done by including the four studies which had a common definition of AKI. It had revealed no significant change in the results (an OR of 0.99 [0.81–1.22]) (Fig. 3).
Figure 2:
Pooled analysis of AKI.
Figure 3:
Sensitivity analysis of AKI (including studies using AKIN or RIFLE criteria).
Secondary outcomes
Postoperative requirement of renal replacement therapy
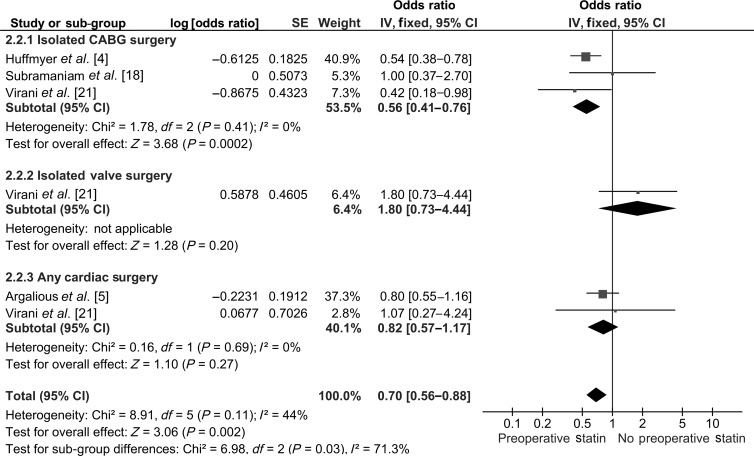
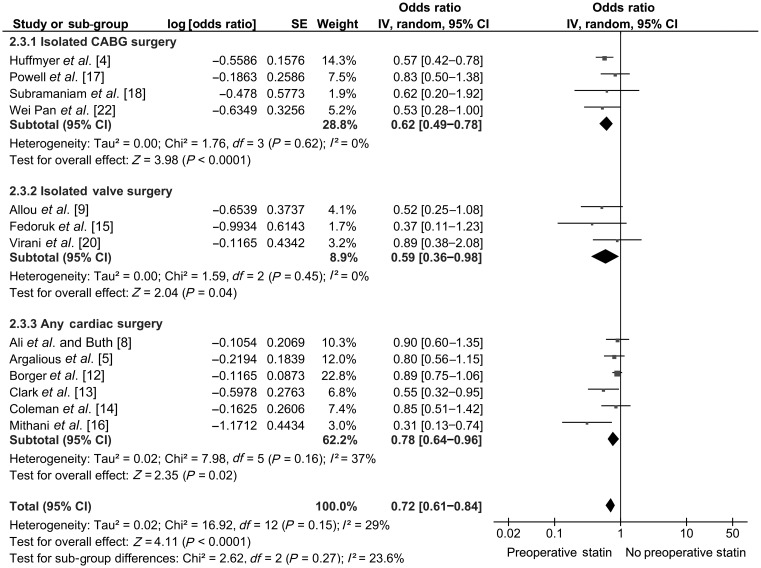
Four studies with a total population of 18 887 (11 219 statin users and 7668 non-statin users) were included for pooled analysis of postoperative RRT. The pooled OR was 0.70 [0.56–0.88] favouring PST. Sub-group analysis revealed that preoperative statin administration was associated with significantly less postoperative RRT (OR: 0.56 [0.41–0.76]; P = 0.0002) in patients with isolated CABG surgery but not in patients who underwent IVS (OR: 1.80 [0.73–4.44]; P = 0.20) and any cardiac surgery (OR: 0.82 [0.57–1.17]; P = 0.27) (Fig. 4).
Figure 4:
Pooled analysis of RRT.
Mortality
Thirteen studies with a total population of 47 080 (24 998 statin users and 22 082 non-statin users) had reported adjusted OR and were included in the pooled analysis. The pooled OR was 0.72 [0.61–0.84]; P < 0.0001. PST was associated with a lower short-term mortality in the patients undergoing cardiac surgery. The reduction in mortality with PST was seen across all three sub-groups (Fig. 5).
Figure 5:
Pooled analysis of mortality.
Assessment of publication bias
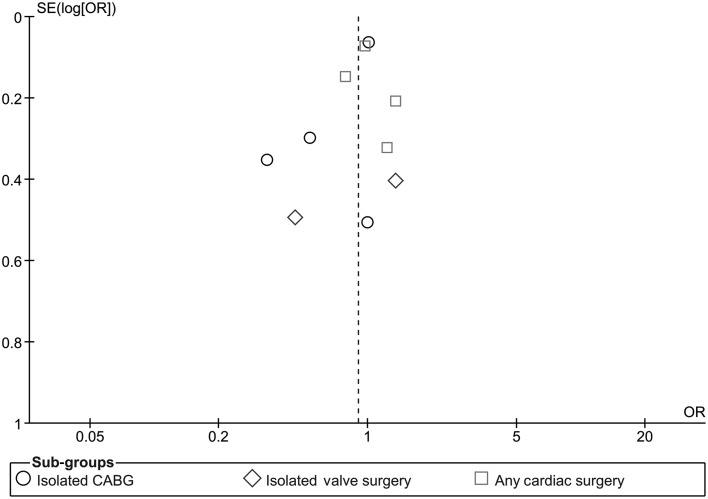
Funnel plot analysis using data of studies included for primary outcome showed no significant publication bias (Fig. 6).
Figure 6:
Funnel plot for assessment of publication bias.
DISCUSSION
Renal dysfunction occurring postoperatively in patients undergoing cardiac surgery is associated with significant morbidity and mortality. The results of the present study were consistent with the results of the earlier studies that had reported the mortality-reducing effect of PST in patients undergoing cardiac surgery. Furthermore, statin therapy in the preoperative period was associated with a reduction in postoperative myocardial infarction, stroke and atrial fibrillation [23]. Nevertheless, the protective effects of PST on the occurrence of postoperative renal dysfunction have not been established unequivocally. Few randomized controlled trials had been conducted with an objective to evaluate the reno-protective effects of preoperative statin treatment. Further, the published observational studies had conflicting results. The results of the present observational study had suggested that the patients undergoing CABG derived benefit from preoperative administration of statin in terms of lesser need for postoperative RRT, an effect that was not seen in patients undergoing IVS. The beneficial effect of PST was also observed on the incidence of postoperative RRT in the overall population included in the analysis. Interestingly, there was a trend towards lower incidence of postoperative AKI with PST in patients undergoing CABG (OR: 0.67 [0.38–1.17]; P = 0.16). The results suggested that PST might possibly lower the incidence of postoperative AKI though not statistically significantly. However, a sensitivity analysis did not reveal any significant difference in the AKI outcomes following PST using the RIFLE/AKIN criteria.
However, the differential effects of statin with respect to the type of surgery might be explained on the basis of distinct pathophysiology of the two conditions. Patients who undergo CABG have a generalized atherosclerotic component involving the vasculature compared with patients undergoing isolated cardiac valve surgery. However, the pathogenesis of postoperative AKI after cardiac surgery involves reduction in renal perfusion pressure, activation of proinflammatory cytokines and direct nephrotoxicity. Cardiopulmonary bypass leads to development of systemic inflammatory response syndrome due to contact of blood components with artificial surface of the bypass circuit. Statins are known to have pleiotropic effects, which include anti-inflammatory, immunomodulatory and antioxidant properties [24]. Moreover, treatment with statin improves endothelial dysfunction by promoting the synthesis of nitric oxide synthase enzyme and has an inhibitory effect on transendothelial neutrophil migration [25]. Animal studies have shown the protective effect of statins in preventing development of ischaemic renal failure. Keeping in view of the mechanisms mentioned above, it might be possible to explain the observed reno-protective effect of statins in patients undergoing cardiac surgery. However, the reno-protective effect of preoperative statin in isolated cardiac valve surgery needs to be explored further. Importantly, the reno-protective efficacy of preoperative statin in patients undergoing isolated cardiac valve surgery could not be ruled out completely as it was based upon the analysis of only one study and as well due to the efficacy of preoperative statin in improving systemic endothelial dysfunction in patients undergoing non-coronary artery surgery. Moreover, the reno-protective effect of preoperative statin on RRT persisted in the overall population included in the analysis. More research in the patients undergoing IVS is needed to evaluate the beneficial effects of statin as reno-protective agents.
It is known that patients requiring RRT postoperatively are at a higher risk of complications like multiorgan dysfunction and systemic infections thereby associated with increased mortality rate. In the present analysis, it was observed that there was a 33% reduction in the risk of RRT in patients who had undergone CABG. This effect would additionally contribute to the beneficial effect of PST on postoperative mortality. Also the effect could translate into improvement in morbidity by decreasing the risk of complications associated with renal failure requiring specific treatment.
The present evidence was generated primarily on the basis of an analysis including retrospective studies that have various limitations of their own. The recent clinical trial that had compared preoperative atorvastatin with placebo [6] did not find a significant difference in renal outcomes in patients post-cardiac surgery. However, the limitations of the study included a statistical power of 60% only which might have resulted in the non-significant outcome. Additionally, the studies included in the analysis did not evaluate the dose–response relationship, duration of therapy prior to surgery and the effect of individual statins. However, the evidence generated from the present observation regarding the reno-protective effects of PST needs to be confirmed through a large-scale prospective clinical study.
Conclusion
The present meta-analysis strengthens the evidence for the recommendations for preoperatively statin therapy in patients undergoing CABG, based on the efficacy of preoperative statin in reducing the need for postoperative RRT and mortality. The uncertainty concerning the reno-protective efficacy of PST in patients undergoing cardiac valve surgery needs further investigation.
Conflict of interest: none declared.
REFERENCES
- 1.Kumar AB, Suneja M. Cardiopulmonary bypass-associated acute kidney injury. Anesthesiology. 2011;114:964–70. doi: 10.1097/ALN.0b013e318210f86a. [DOI] [PubMed] [Google Scholar]
- 2.Halter J, Steinberg J, Fink G, Lutz C, Picone A, Maybury R, et al. Evidence of systemic cytokine release in patients undergoing cardiopulmonary bypass. J Extra Corpor Technol. 2005;37:272–7. [PMC free article] [PubMed] [Google Scholar]
- 3.Liakopoulos OJ, Kuhn EW, Slottosch I, Wassmer G, Wahlers T. Preoperative statin therapy for patients undergoing cardiac surgery. Cochrane Database Syst Rev. 2012;4:CD008493. doi: 10.1002/14651858.CD008493.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Huffmyer JL, Mauermann WJ, Thiele RH, Ma JZ, Nemergut EC. Preoperative statin administration is associated with lower mortality and decreased need for postoperative hemodialysis in patients undergoing coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2009;23:468–73. doi: 10.1053/j.jvca.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Argalious M, Xu M, Sun Z, Smedira N, Koch CG. Preoperative statin therapy is not associated with a reduced incidence of postoperative acute kidney injury after cardiac surgery. Anesth Analg. 2010;111:324–30. doi: 10.1213/ANE.0b013e3181d8a078. [DOI] [PubMed] [Google Scholar]
- 6.Prowle JR, Calzavacca P, Licari E, Ligabo EV, Echeverri JE, Haase M, et al. Pilot double-blind, randomized controlled trial of short-term atorvastatin for prevention of acute kidney injury after cardiac surgery. Nephrology (Carlton) 2012;17:215–24. doi: 10.1111/j.1440-1797.2011.01546.x. [DOI] [PubMed] [Google Scholar]
- 7.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–84. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali IS, Buth KJ. Preoperative statin use and outcomes following cardiac surgery. Int J Cardiol. 2005;103:12–8. doi: 10.1016/j.ijcard.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Allou N, Augustin P, Dufour G, Tini L, Ibrahim H, Dilly MP, et al. Preoperative statin treatment is associated with reduced postoperative mortality after isolated cardiac valve surgery in high-risk patients. J Cardiothorac Vasc Anesth. 2010;24:921–6. doi: 10.1053/j.jvca.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Billings FT, Pretorius M, Siew ED, Yu C, Brown NJ. Early postoperative statin therapy is associated with a lower incidence of acute kidney injury after cardiac surgery. J Cardiothorac Vasc Anesth. 2010;24:913–20. doi: 10.1053/j.jvca.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolesta S, Uhrin LM, Guzek JR. Preoperative statins and acute kidney injury after cardiac surgery: utilization of a consensus definition of acute kidney injury. Ann Pharmacother. 2011;45:23–30. doi: 10.1345/aph.1P384. [DOI] [PubMed] [Google Scholar]
- 12.Borger MA, Seeburger J, Walther T, Borger F, Rastan A, Doenst T, et al. Effect of preoperative statin therapy on patients undergoing isolated and combined valvular heart surgery. Ann Thorac Surg. 2010;89:773–9. doi: 10.1016/j.athoracsur.2009.12.001. discussion 79–80. [DOI] [PubMed] [Google Scholar]
- 13.Clark LL, Ikonomidis JS, Crawford FA, Jr, Crumbley A, 3rd, Kratz JM, Stroud MR, et al. Preoperative statin treatment is associated with reduced postoperative mortality and morbidity in patients undergoing cardiac surgery: an 8-year retrospective cohort study. J Thorac CardioVasc Surg. 2006;131:679–85. doi: 10.1016/j.jtcvs.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Coleman CI, Lucek DM, Hammond J, White CM. Preoperative statins and infectious complications following cardiac surgery. Curr Med Res Opin. 2007;23:1783–90. doi: 10.1185/030079907X210570. [DOI] [PubMed] [Google Scholar]
- 15.Fedoruk LM, Wang H, Conaway MR, Kron IL, Johnston KC. Statin therapy improves outcomes after valvular heart surgery. Ann Thorac Surg. 2008;85:1521–5. doi: 10.1016/j.athoracsur.2008.01.078. discussion 25–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mithani S, Kuskowski M, Slinin Y, Ishani A, McFalls E, Adabag S. Dose-dependent effect of statins on the incidence of acute kidney injury after cardiac surgery. Ann Thorac Surg. 2011;91:520–5. doi: 10.1016/j.athoracsur.2010.10.061. [DOI] [PubMed] [Google Scholar]
- 17.Powell BD, Bybee KA, Valeti U, Thomas RJ, Kopecky SL, Mullany CJ, et al. Influence of preoperative lipid-lowering therapy on postoperative outcome in patients undergoing coronary artery bypass grafting. Am J Cardiol. 2007;99:785–9. doi: 10.1016/j.amjcard.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 18.Subramaniam K, Koch CG, Bashour A, O'Connor M, Xu M, Gillinov AM, et al. Preoperative statin intake and morbid events after isolated coronary artery bypass grafting. J Clin Anesth. 2008;20:4–11. doi: 10.1016/j.jclinane.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Tabata M, Khalpey Z, Pirundini PA, Byrne ML, Cohn LH, Rawn JD. Renoprotective effect of preoperative statins in coronary artery bypass grafting. Am J Cardiol. 2007;100:442–4. doi: 10.1016/j.amjcard.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 20.Virani SS, Nambi V, Lee VV, Elayda M, Reul RM, Wilson JM, et al. Does preoperative statin therapy improve outcomes in patients undergoing isolated cardiac valve surgery? Am J Cardiol. 2008;102:1235–9. doi: 10.1016/j.amjcard.2008.06.055. [DOI] [PubMed] [Google Scholar]
- 21.Virani SS, Nambi V, Polsani VR, Lee VV, Elayda M, Kohsaka S, et al. Preoperative statin therapy decreases risk of postoperative renal insufficiency. Cardiovasc Ther. 2010;28:80–6. doi: 10.1111/j.1755-5922.2009.00124.x. [DOI] [PubMed] [Google Scholar]
- 22.Pan W, Pintar T, Anton J, Lee VV, Vaughn WK, Collard CD. Statins are associated with a reduced incidence of perioperative mortality after coronary artery bypass graft surgery. Circulation. 2004;110:II45–9. doi: 10.1161/01.CIR.0000138316.24048.08. [DOI] [PubMed] [Google Scholar]
- 23.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 24.Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinsella A, Raza A, Kennedy S, Fan Y, Wood AE, Watson RW. The impact of high-dose statin therapy on transendothelial neutrophil migration and serum cholesterol levels in healthy male volunteers. Eur J Clin Pharmacol. 2011;67:1103–8. doi: 10.1007/s00228-011-1062-z. [DOI] [PubMed] [Google Scholar]