Abstract
OBJECTIVES
Thoracoscopic thymectomy has gradually replaced conventional sternotomy for resection of thymoma; however, a thoracoscopic approach for thymoma remains controversial. We evaluated the oncological outcomes of thoracoscopic thymectomy for the treatment of stages I–III thymomas.
METHODS
Sixty-two patients who underwent thoracoscopic thymectomy for the treatment of thymoma were retrospectively reviewed between July 2005 and September 2011 at Jikei University Hospital. Surgical outcomes and pathological results between stages I + II and stage III were compared.
RESULTS
Twenty-nine patients had Masaoka stage I, 28 had stage II and 5 had stage III. Three stage III patients needed conversions to open surgery. Masaoka stage III comprised pathological type B3 in 3 patients and thymic carcinoma in 2. For all patients, the 5-year overall survival rate was 100%. Three recurrences, diagnosed as thymic carcinoma, were observed in the Masaoka stage II or III patients. The 5-year disease-free survival rate was 94.2% for all patients, 100% for Masaoka stage I, 96.1% for stage II and 37.5% (55 months) for stage III (P = 0.002). The 5-year disease-free survival rate was 100% for the World Health Organization classification types A, AB and B1–3 and 0% for thymic carcinoma (P < 0.0001). Significant differences were found in the 5-year disease-free survival stratified by the Masaoka stage or WHO classification, but not by surgical procedures.
CONCLUSIONS
Thoracoscopic thymectomy for Masaoka stages I and II thymomas presented acceptable oncological outcomes. Further investigation in a large series with longer follow-up is required. Masaoka stage III thymoma requires careful consideration of the approaches, including median sternotomy.
Keywords: Thymoma, Thoracoscopic, Thymectomy
INTRODUCTION
Thymoma is primarily approached through surgical resection comprising thymectomy, which has typically been performed via standard median sternotomy [1]; however, because of development in instrumentation and improvement in visualization, a minimally invasive approach using thoracoscopic thymectomy for thymoma has been developed [2–4]. Recently, thoracoscopic thymectomy has been performed more frequently for resection of early-stage thymoma. However, several controversies exist with regard to the surgical treatment of thymoma, including the criteria for operative indication for thoracoscopic thymectomy, and the extent of resection. Furthermore, there are no oncological principles that accept the feasibility of thoracoscopic thymectomy for treatment of not only early-stage but also advanced-stage thymoma. The purpose of this study was to evaluate the oncological outcomes of thoracoscopic thymectomy for the treatment of stages I–III thymomas. We also present our thoracoscopic procedure for the treatment of thymoma.
MATERIALS AND METHODS
We retrospectively reviewed patients who underwent thoracoscopic thymectomy for the treatment of primary thymoma and thymic carcinoma with a curative intent between July 2005 and September 2011 at Jikei University Hospital. The institutional review board at the Jikei University School of Medicine approved this study, and informed consent was obtained from all study participants after explaining the advantages and disadvantages of thoracoscopic surgery. In each case, the anterior mediastinal tumour demonstrated features highly suggestive of thymoma as demonstrated by computed tomography (CT) and magnetic resonance imaging. Regardless of the greatest diameter of the tumour, the operative indication for thoracoscopic thymectomy for the treatment of thymoma and thymic carcinoma was no invasion to surrounding organs such as the vascular structures, especially the brachiocephalic vein, lungs and chest wall.
We reviewed the duration of surgery and postoperative hospital stay, the amount of intraoperative blood loss and postoperative complications. The recurrence of thymoma was examined by chest CT at 6 and 12 months after surgery and once yearly thereafter. The diagnoses of all resected thymomas, surgical margins and Masaoka stages were confirmed by histopathological examination. Tumours were classified histologically according to the World Health Organization (WHO) classification into types A, AB, B1, B2, B3 and thymic carcinoma. Data are reported as median (range) values. JMP® 9 (SAS Institute, Inc., Cary, NC, USA) was used to perform statistical analysis comparing stages I + II and III. Patient characteristics between the two groups were compared using the Pearson χ2 test for categorical variables and the Wilcoxon test for continuous variables. The Kaplan–Meier method was used to plot the curves, and the log-rank test was used to analyse the overall and disease-free survival curves of the patients. Survival time was defined as the time from surgery to death or the last follow-up or recurrence as recorded in the charts. Differences were considered statistically significant when P < 0.05.
OPERATIVE PROCEDURES
Our thoracoscopic procedures were extended thymectomy (ET), total thymectomy (TT), sub-TT (STT) and hemithymectomy (HT). In ET, the thymus and surrounding fat tissues were resected together. ET of the bilateral thoracic cavity was indicated for thymoma complicated by myasthenia gravis. TT and STT of the unilateral thoracic cavity were our standard procedures for thymoma. The bilateral approach was indicated because of technical necessity. In STT, complete resection of the superior and inferior poles in a contralateral thymus is not required. The left or right lobe of the thymus is resected in HT. Recently, the majority of the resections for thymoma have been attempted by thoracoscopic procedures. However, if, after starting by the thoracoscopic approach, the tumour shows invasion to surrounding vascular structures, the patient is converted to an open approach.
The technique of thoracoscopic thymectomy has been previously described [5] and can be performed with a unilateral or bilateral thoracic approach. In brief, for thoracoscopic thymectomy, a double-lumen tube for split-lung ventilation was used to anaesthetize the patients who were placed in the lateral position. The diseased side of the thoracic cavity was approached via a four- or five-port technique. A thoracoscope at an oblique angle of 30° was introduced through the port in the fourth intercostal space at the posterior axillary line and fixed with an exclusive arm. The working ports were placed in the third, fourth and sixth lateral intercostal spaces at the anterior axillary line conforming to the mammary crease, and another working port could be placed in the sixth intercostal space at the posterior axillary line. The mediastinal pleura was incised just anterior to the phrenic nerve, and the inferior portion of the thymus was mobilized. Dissection was started on the side opposite of the tumour in the thymus to avoid any breaches in the capsule. The dissection plane was extended cranially, preserving the integrity of the phrenic nerve. The base of the thymic lobe was mobilized and retracted cranially. All thymic lobes and perithymic fatty tissues were mobilized en bloc with the tumour, allowing exposure of the underlying pericardium and the aorta. The thymus was then carefully dissected along the left brachiocephalic vein, and the thymic veins were divided with an ultrasonically activated device. After the diseased side of the thymus was dissected from the inferior pole to the superior pole, the dissection was continued to the contralateral thymic lobe. The cervical horns were mobilized and swept inferiorly with the thymus. The contralateral portion of the thymus was mobilized free from the pericardium overlying the ascending aorta and pulmonary artery after the left brachiocephalic vein was exposed. The specimen was removed in a plastic retrieval bag.
RESULTS
We performed 72 consecutive surgical treatments for Masaoka stages I–III thymomas during this period. Ten patients were excluded because 5 of them with stage III thymoma underwent thymectomy via median sternotomy, 2 underwent thoracoscopic biopsy and 3 underwent tumour excision for recurrent thymoma.
We reviewed the surgical and oncological outcomes of the 62 patients who underwent thoracoscopic thymectomy for Masaoka stages I–III. Patient characteristics are shown in Table 1. Twelve patients had myasthenia gravis, and all patients had preoperative clinical Masaoka stage I or II tumour. Pathological examination confirmed complete resection of the tumour in all patients.
Table 1:
Characteristics of patients with thymoma and thymic carcinoma
| Characteristics | Thoracoscopic surgery | Open sternotomy | Biopsy | Recurrence following surgery | P-value |
|---|---|---|---|---|---|
| No. of patients | 62 | 5 | 2 | 3 | |
| Male/female | 35/27 | 3/2 | 2/0 | 2/1 | |
| Age years (range) | 57 (21–80) | 55 (29–71) | 63 (55–71) | 53 (44–61) | 0.7514 |
| Clinical Masaoka stage | |||||
| I + II | 62 | 0 | 0 | 0 | |
| III | 0 | 5 | 2 | 0 | |
| IVa | 0 | 0 | 0 | 3 | |
| Tumour size mm (range) | 43.5 (14–95) | 80 (38–130) | 70 (60–80) | 15 (12–18) | 0.0157 |
| Myasthenia gravis | 12 | 0 | 0 | 0 | |
The surgical outcomes of the patients are shown in Table 2. The approaches and procedures were not significantly different between the pathological stages I + II group and the stage III group (P = 0.2788 and 0.5359, respectively). The duration of surgery between the Masaoka stages I + II group (225 min) and stage III group (410 min) differed significantly (P = 0.0025) as did the intraoperative amount of blood loss (100 and 400 ml, respectively; P = 0.0003). There were no significant differences in the tumour size (P = 0.0589) and the hospital stay (P = 0.4891).
Table 2:
Surgical outcomes and Masaoka stages of patients with thymoma and thymic carcinoma
| Characteristics | Pathological Masaoka stage |
Total | P-value | |||
|---|---|---|---|---|---|---|
| I | II | I + II | III | |||
| Number of patients | 29 | 28 | 57 | 5 | 62 | |
| Approach | ||||||
| Unilateral | 24 | 22 | 46 | 5 | 51 | 0.2788 |
| Bilateral | 5 | 6 | 11 | 0 | 11 | |
| Procedure | ||||||
| ET | 5 | 6 | 11 | 2 | 13 | |
| TT | 3 | 3 | 6 | 0 | 6 | |
| STT | 14 | 18 | 32 | 3 | 35 | |
| HT | 7 | 1 | 8 | 0 | 8 | 0.5359 |
| Tumour size (mm) | 30 (14–95) | 45 (19–91) | 40 (14–95) | 60 (45–90) | 0.0589 | |
| Duration of surgery (min) | 186 (90–477) | 237 (100–488) | 225 (90–488) | 410 (315–537) | 0.0025 | |
| Blood loss (ml) | 100 (5–260) | 130 (10–400) | 100 (5–400) | 400 (300–750) | 0.0003 | |
| POHS (days) | 4 (2–37) | 4 (3–10) | 4 (2–37) | 5 (3–8) | 0.4891 | |
| Blood transfusion | 0 | 0 | 0 | 0 | 0 | |
| Conversion | 0 | 0 | 0 | 3 | 3 | <0.0001 |
| Postoperative complications | 3 | 3 | 6 | 1 | 7 | 0.5210 |
| PTMG | 1 | 1 | 2 | 1 | 3 | |
| WHO classification | ||||||
| A | 6 | 3 | 9 | 0 | 9 | 0.0001 |
| AB | 10 | 5 | 15 | 0 | 15 | |
| B1 | 6 | 10 | 16 | 0 | 16 | |
| B2 | 6 | 5 | 11 | 0 | 11 | |
| B3 | 1 | 3 | 4 | 3 | 7 | |
| Thymic carcinoma | 0 | 2 | 2 | 2 | 4 | |
Conversion: converted to open surgery; HT: left or right lobe HT; POHS: postoperative hospital stay; PTMG: post-thymectomy myasthenia gravis; A, AB, B1, B2, B3: thymoma by WHO classification; WHO: World Health Organization.
Although no patient in the pathological stages I + II group underwent conversion to an open surgery, 3 patients in the stage III group needed conversions to an open surgery because of difficulties in removal of the tumours from the pericardium, superior vena cava or brachiocephalic vein. No surgical complications, such as massive bleeding, were observed in this series. Seven patients experienced postoperative complications. Phrenic nerve palsy was observed in 3 patients of which 2 had atelectasis and 1 had pleural effusion. Another patient experienced prolonged air leakage because of lung injury. There were no perioperative mortalities. Of the 5 patients with Masaoka stage III, 3 had pathological type B3 and 2 had thymic carcinoma. Seven patients in the stages I + II group and 4 in the stage III group underwent postoperative radiotherapy.
Three cases of post-thymectomy myasthenia gravis appeared between 3 and 6 months after surgery. We performed completion thymectomy through the contralateral thoracic cavity in 1 patient who underwent STT.
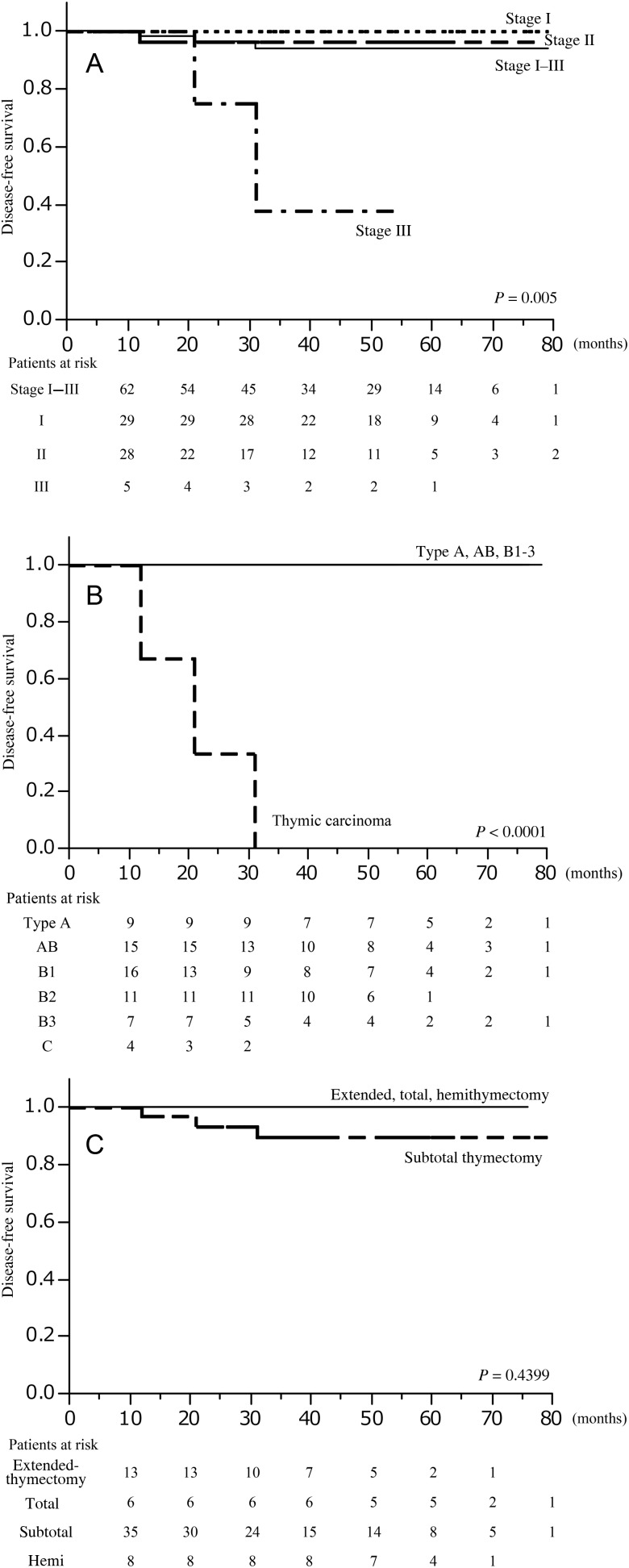
During the postoperative follow-up period of 43 (range: 10–79) months, no patient died, and the 5-year overall survival rate for all patients was 100%. The 5-year disease-free survival rate for all patients was 94.2% (95% confidence interval: 87–97%), 100% for the Masaoka stage I, 96.1% (95% confidence interval: 77–99%) for Masaoka stage II and 37.5% (55 months) for Masaoka stage III (P = 0.002) (Fig. 1A). The 5-year disease-free survival rate was 100% for the WHO classification types A, AB and B1–3 and 0% for thymic carcinoma (P < 0.0001) (Fig. 1B). Significant differences were found in the 5-year disease-free survival stratified by Masaoka stage or WHO classification. There were no significant differences stratified by surgical procedures (Fig. 1C). Three recurrences were observed during the median postoperative follow-up period of 43 (range: 10–79) months (Table 3). Of these 3 patients, all had thymic carcinoma, 2 experienced recurrences in the lung, and the other had local recurrence in the anterior mediastinum. These patients had Masaoka stage II or III tumours. One patient underwent wedge lung resection for lung metastasis, and 1 received systemic chemotherapy.
Figure 1:
(A) Kaplan–Meier analysis for disease-free survival for thoracoscopic thymectomy in thymoma stratified by Masaoka stage. (B) Kaplan–Meier analysis for disease-free survival for thoracoscopic thymectomy in thymoma stratified by the WHO classification. (C) Kaplan–Meier analysis for disease-free survival for thoracoscopic thymectomy in thymoma stratified by the procedures. The P-value was calculated by two-sided log-rank test. Time, in months, from thymectomy plotted on the X-axis.
Table 3:
Recurrent cases of thymoma or thymic carcinoma after thoracoscopic thymectomy
| Patient | Tumour (mm) | Primary procedure | Masaoka stage | WHO classification | Adjuvant therapya | Type of recurrence | Period to recurrence (months) | Treatment |
|---|---|---|---|---|---|---|---|---|
| 56 F | 60 | STT | III | Thymic carcinoma | Radiation | Multiple lung metastases | 21 | Chemotherapy |
| 68 M | 67 | STT | III | Thymic carcinoma | Radiation | Lung metastasis | 31 | Lung resection |
| 44 F | 45 | STT | II | Thymic carcinoma | None | Local recurrence | 12 | None |
M: male; F: female.
aAfter primary procedure.
DISCUSSION
Quite a few authors believe that thoracoscopic surgery would become the procedure of choice not only for benign mediastinal tumours but also for thymomas [6, 7]. Thoracoscopic thymectomy for thymoma achieved results equal to those for conventional open thymectomy. Furthermore, thoracoscopic thymectomy with the da Vinci robotic system has developed [8]. However, we found only a few major series that had previously reported the surgical outcomes of thoracoscopic thymectomy for thymoma [9–13]. We previously reported an original method [5] that enables satisfactory visualization of the field and safe surgery for thymoma.
The objective of this study was to evaluate the surgical and oncological outcomes of our thoracoscopic thymectomy in patients with thymoma. We evaluated 62 patients who underwent thoracoscopic thymectomy for Masaoka stages I–III thymomas and thymic carcinoma. To the best of our knowledge, this is the largest series of the published studies to evaluate thoracoscopic surgery not only for early-stage but also advanced-stage thymoma.
The mid-term surgical outcomes in our series demonstrated the safety and feasibility of thoracoscopic thymectomy for Masaoka stages I and II thymomas, as evidenced by the lack of severe surgical complications such as injury to the thoracic great vessels and lack of conversion to open surgery. In addition, there were no serious postoperative complications. In Masaoka stage III, further examination was required to estimate the feasibility of thoracoscopic thymectomy because there were three conversions to open surgery and two recurrences. Conversion to open surgery seemed mainly dependent on tumour invasion to the great vessels, regardless of the tumour size.
Several authors [14, 15] have reported that complete tumour resection is always the treatment of choice in patients with thymoma. However, no definitive guidelines have yet been established regarding the surgical procedure or the resection extent of the thymus for thymoma. Sakamaki et al. [16] reported that unnecessary wide resection of the thymus for a smaller lesion may increase the potential risk of surgical complications in some cases. Our hypothesis is that TT, including excision of the superior and inferior poles of the contralateral lobe for a smaller lesion of Masaoka stage I or II, may be more invasive and increases the potential risk of surgical complications, whereas thoracoscopic STT might be less invasive. In our series, the disease-free survival rate did not significantly differ when stratified by the resected extent of the thymus. In this study, STT was performed for 35 cases, and ET and TT were performed for 13 and 6 cases, respectively. STT was performed most frequently in our department and might have contributed to lowering of the surgical risk of an oncological radical cure. Concern about intrathymic relapse or local recurrence may limit the indications of the procedures except for those of TT for thymoma because the appropriate resected extent of the thymus for thymoma is unclear. In addition, there is no consensus on the appropriate size of thymoma for thoracoscopic thymectomy. Some studies have reported the incidence of multicentric tumour development to be ∼2% of thymomas [17], whereas intrathymic relapse of the tumour has hardly represented a major problem in clinical practice even after partial thymectomy for non-invasive thymoma. As we have not experienced a local recurrence, we believe that thoracoscopic STT for Masaoka stages I and II is permissible as long as the microscopically intact resection margin can be obtained.
A recent report of surgical treatment for thymoma demonstrated that the overall 5-year survival rate of patients with stages I and II thymomas ranged from 89 to 100% and 71 to 95%, respectively [18]. Very few data presenting the oncological outcomes of thoracoscopic thymectomy for thymoma are available. In one of the few studies that explored thoracoscopic treatment for thymoma, Pennathur et al. [19] demonstrated oncological outcomes of 18 thoracoscopic resections for stages I and II thymomas. Both the 5-year overall and 5-year recurrence-free survival rates were 100% during the follow-up period. Cheng et al. [9] showed no significant differences in either recurrence or the survival rate between thoracoscopic resection of stage II thymoma in 12 patients and open resection in 10. In our series, the 5-year overall and 5-year recurrence-free survival rates were 100 and 92.9%, respectively. There was no recurrence of thymoma in pathological Masaoka stages I and II. In contrast, 2 of 5 patients with Masaoka stage III had recurrences. Of the 4 patients with thymic carcinoma, 3 had recurrences. There was no recurrence diagnosed other than thymic carcinoma. Several authors [20, 21] have reported the overall survival rate for thymic carcinoma to be 38–65% at 5 years and 28–35% at 10 years. Lee et al. [22] reported that complete resection is the most important factor for disease control and long-term survival of patients with thymic carcinoma of early Masaoka stage. Kondo and Monden [23] reported that postoperative adjuvant therapy after complete resection had not been shown to have a significant effect on thymoma and thymic carcinoma. The role of multimodality treatment in completely resected thymic carcinoma has not yet been established. Histological grade of aggressiveness is also an important factor for recurrence or prognosis. Multimodality treatment is more frequently recommended for patients with thymic carcinoma than for patients with other WHO classifications. Our series suggested that adjuvant systemic chemotherapy should be performed for thymic carcinoma. However, further study is required for establishment of adjuvant therapy for thymic carcinoma.
Post-thymectomy myasthenia gravis is known to occur in ∼1–3% of non-myasthenia gravis patients after extirpation of thymoma but has appeared even after ET [24], which suggests that ET has no extra benefits compared with partial thymectomy in terms of avoiding the incidence of post-thymectomy myasthenia gravis. Removing the remnant thymus (completion thymectomy) resolved this extremely rare complication in selected cases [25]. In our experience the mediastinum after thoracoscopic thymectomy usually allows completion thymectomy to be performed more safely than after sternotomy.
CONCLUSIONS
Thoracoscopic thymectomy for Masaoka stages I and II thymomas presented acceptable oncological outcomes. Nevertheless, this procedure requires further investigation in a large series with a longer follow-up. It is necessary to carefully consider the approaches, including median sternotomy, for Masaoka stage III thymoma.
Conflict of interest: none declared.
REFERENCES
- 1.Masaoka A, Monden Y, Nakahara K, Tanioka T. Follow-up study of thymomas with special reference to their clinical stages. Cancer. 1981;48:2485–92. doi: 10.1002/1097-0142(19811201)48:11<2485::aid-cncr2820481123>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 2.Augustin F, Schmid T, Sieb M, Lucciarini P, Bodner J. Video-assisted thoracoscopic surgery versus robotic-assisted thoracoscopic surgery thymectomy. Ann Thorac Surg. 2008;85:S768–71. doi: 10.1016/j.athoracsur.2007.11.079. [DOI] [PubMed] [Google Scholar]
- 3.Mussi A, Fanucchi O, Davini F, Lucchi M, Picchi A, Ambrogi MC, et al. Robotic extended thymectomy for early-stage thymomas. Eur J Cardiothorac Surg. 2012;41:43–7. doi: 10.1093/ejcts/ezr322. [DOI] [PubMed] [Google Scholar]
- 4.Toker A, Sonett J, Zielinski M, Rea F, Tomulescu V, Detterbeck FC. Standard terms, definitions, and policies for minimally invasive resection of thymoma. J Thorac Oncol. 2011;6(7 Suppl 3):S1739–42. doi: 10.1097/JTO.0b013e31821ea553. doi:10.1097/JTO.0b013e31821ea553. [DOI] [PubMed] [Google Scholar]
- 5.Odaka M, Akiba T, Yabe M, Hiramatsu M, Matsudaira H, Morikawa T, et al. Unilateral thoracoscopic subtotal thymectomy for the treatment of stage I and II. Eur J Cardiothorac Surg. 2010;37:824–6. doi: 10.1016/j.ejcts.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Sugarbaker DJ. Thoracoscopy in the management of anterior mediastinal masses. Ann Thorac Surg. 1993;56:653–6. doi: 10.1016/0003-4975(93)90942-b. [DOI] [PubMed] [Google Scholar]
- 7.Gossot D, Izquierdo RR, Girard P, Stern JB, Magdeleinat P. Thoracoscopic resection of bulky intrathoracic benign lesions. Eur J Cardiothorac Surg. 2007;32:848–51. doi: 10.1016/j.ejcts.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Rückert JC, Ismail M, Swierzy M, Sobel H, Rogalla P, Meisel A, et al. Thoracoscopic thymectomy with the da Vinci robotic system for myasthenia gravis. Ann N Y Acad Sci. 2008;1132:329–35. doi: 10.1196/annals.1405.013. [DOI] [PubMed] [Google Scholar]
- 9.Cheng YJ, Kao EL, Chou SH. Videothoracoscopic resection of stage II thymoma: prospective comparison of the results between thoracoscopy and open methods. Chest. 2005;128:3010–12. doi: 10.1378/chest.128.4.3010. [DOI] [PubMed] [Google Scholar]
- 10.Cheng YJ, Hsu JS, Kao EL. Characteristics of thymoma successfully resected by video-thoracoscopic surgery. Surg Today. 2007;37:192–6. doi: 10.1007/s00595-006-3383-6. [DOI] [PubMed] [Google Scholar]
- 11.Takeo S, Tsukamoto S, Kawano D, Katsura M. Outcome of an original video-assisted thoracoscopic extended thymectomy for thymoma. Ann Thorac Surg. 2011;92:2000–5. doi: 10.1016/j.athoracsur.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 12.Jurado J, Javidfar J, Newmark A, Lavelle M, Bacchetta M, Gorenstein L, et al. Minimally invasive thymectomy and open thymectomy: outcome analysis of 263 patients. Ann Thorac Surg. 2012;94:974–82. doi: 10.1016/j.athoracsur.2012.04.097. [DOI] [PubMed] [Google Scholar]
- 13.Detterbeck FC, Nicholson AG, Kondo K, Van Schil P, Moran C. The Masaoka-Koga stage classification for thymic malignancies: clarification and definition of terms. J Thorac Oncol. 2011;6(7 Suppl 3):S1710–6. doi: 10.1097/JTO.0b013e31821e8cff. doi:10.1097/JTO.0b013e31821e8cff. [DOI] [PubMed] [Google Scholar]
- 14.Blumberg D, Port JL, Weksler B, Delgado R, Rosai J, Bains MS, et al. Thymoma: a multivariate analysis of factors predicting survival. Ann Thorac Surg. 1995;60:908–14. doi: 10.1016/0003-4975(95)00669-c. [DOI] [PubMed] [Google Scholar]
- 15.Singhal S, Shrager JB, Rosenthal DI, LiVolsi VA, Kaiser LR. Comparison of stages I-II thymoma treated by complete resection with or without adjuvant radiation. Ann Thorac Surg. 2003;76:1635–41. doi: 10.1016/s0003-4975(03)00819-1. discussion 1641–1642. [DOI] [PubMed] [Google Scholar]
- 16.Sakamaki Y, Kido T, Yasukawa M. Alternative choices of total and partial thymectomy in video-assisted resection of noninvasive thymomas. Surg Endosc. 2008;22:1272–7. doi: 10.1007/s00464-007-9606-0. [DOI] [PubMed] [Google Scholar]
- 17.Bernatz PE, Harrison EG, Clagett OT. Thymoma: a clinicopathologic study. J Thorac Cardiovasc Surg. 1961;42:424–44. [PubMed] [Google Scholar]
- 18.Davenport E, Malthaner RA. The role of surgery in the management of thymoma: a systematic review. Ann Thorac Surg. 2008;86:673–84. doi: 10.1016/j.athoracsur.2008.03.055. [DOI] [PubMed] [Google Scholar]
- 19.Pennathur A, Qureshi I, Schuchert MJ, Dhupar R, Ferson PF, Gooding WE, et al. Comparison of surgical techniques for early-stage thymoma: feasibility of minimally invasive thymectomy and comparison with open resection. J Thorac Cardiovasc Surg. 2011;141:694–701. doi: 10.1016/j.jtcvs.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Ogawa K, Toita T, Uno T, Fuwa N, Kakinohana Y, Kamata M, et al. Treatment and prognosis of thymic carcinoma: a retrospective analysis of 40 cases. Cancer. 2002;94:3115–9. doi: 10.1002/cncr.10588. [DOI] [PubMed] [Google Scholar]
- 21.Blumberg D, Burt ME, Bains MS, Downey RJ, Martini N, Rusch V, et al. Thymic carcinoma: current staging does not predict prognosis. J Thorac Cardiovasc Surg. 1998;115:303–8. doi: 10.1016/S0022-5223(98)70273-9. discussion 308–309. [DOI] [PubMed] [Google Scholar]
- 22.Lee CY, Bae MK, Park IK, Kim DJ, Lee JG, Chung KY. Early Masaoka stage and complete resection is important for prognosis of thymic carcinoma: a 20-year experience at a single institution. Eur J Cardiothorac Surg. 2009;36:159–62. doi: 10.1016/j.ejcts.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 23.Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg. 2003;76:878–84. doi: 10.1016/s0003-4975(03)00555-1. [DOI] [PubMed] [Google Scholar]
- 24.Kondo K, Monden Y. Myasthenia gravis appearing after thymectomy for thymoma. Eur J Cardiothorac Surg. 2005;28:22–5. doi: 10.1016/j.ejcts.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 25.Pompeo E, Nofroni I, Iavicoli N, Mineo TC. Thoracoscopic completion thymectomy in refractory nonthymomatous myasthenia. Ann Thorac Surg. 2000;70:918–23. doi: 10.1016/s0003-4975(00)01566-6. [DOI] [PubMed] [Google Scholar]