Abstract
OBJECTIVES
Left ventricular (LV) diastolic dysfunction after aortic valve replacement (AVR) carries a substantial risk of development of heart failure and reduced survival. In addition to echocardiography, B-type natriuretic peptide (BNP) provides a powerful incremental assessment of diastolic function. This study evaluates BNP as a marker of LV diastolic dysfunction in a cohort of patients with preserved LV ejection fraction who underwent AVR for pure aortic stenosis and the relationship between BNP values and the grade of LV diastolic dysfunction.
METHODS
A total of 113 patients were included in the study. Echocardiographic evaluation was performed preoperatively, 5 days postoperatively and at 12-month follow-up, to assess LV dimensional and functional parameters. Diastolic function was labelled as normal, mild, moderate or severe dysfunction. Concomitantly, BNP levels were evaluated.
RESULTS
Mild to severe diastolic dysfunction occurred preoperatively in all patients. At 12-month follow-up, 65 (62.5%) patients had mild and 25 (24.1%) moderate to severe diastolic dysfunction. BNP values, categorized for quartile distribution, correlated with diastolic dysfunction grade (P < 0.001 for each comparison). At receiver operating characteristic analysis, the BNP level of 120 pg/ml was 91% sensitive and 85% specific for diastolic disease, while 300 pg/ml was 80% sensitive and 91% specific for moderate or severe diastolic dysfunction. Twelve months after AVR, BNP values were strongly correlated with the significant echocardiographic parameters suggestive of diastolic dysfunction (P ≤ 0.006 in all cases).
CONCLUSIONS
The BNP level following AVR is related to diastolic disease severity and may complement echocardiographic evaluation when symptoms are unclear and LV function is difficult to interpret.
Keywords: Aortic valve replacement, Aortic valve stenosis, Diastolic function, Natriuretic peptides, Echocardiography
INTRODUCTION
Aortic stenosis (AS) is associated with left ventricular (LV) hypertrophy, which involves diastolic and systolic disturbances. Aortic valve replacement (AVR) leads to early postoperative haemodynamic and metabolic recovery, contributing to the improvement in LV function and geometry. A successful AVR often results in persistent LV hypertrophy and/or in LV outflow tract obstruction, which jeopardizes systolic and diastolic function recovery [1–4]. Persistent LV diastolic dysfunction after AVR, although largely underestimated in the past, has recently been shown to be an independent predictor of hospitalization and mortality [5, 6] and to carry a substantial risk of the subsequent development of heart failure also in asymptomatic patients with preserved left ventricular ejection fraction (LVEF) [7]. When present, diastolic dysfunction is graded on three levels that reflect the severity and the progression of the disease. The more advanced the stage is, the higher the filling pressures and the worse the outcomes are [7, 8]. Therefore, the early identification of LV diastolic dysfunction in patients without overt symptoms may provide an opportunity to manage the underlying cause and to prevent progression to congestive heart failure.
Echocardiographic assessment is the gold standard for diastolic function monitoring; however, it is usually complex and time-demanding and, sometimes, difficult to interpret. In addition to echocardiography, B-type natriuretic peptide (BNP) has been used as a non-invasive marker of LV dysfunction [8, 9].
BNP is a cardiac neurohormone secreted in the cardiac ventricles as a response to wall stress due to ventricular volume expansion and pressure overload. The BNP level reflects the severity of heart failure, is associated with LV structure and function in severe AS as well as is an independent predictor of complication and outcome after AVR [10–15]. Moreover, it is strongly related to the development of symptom onset and increases progressively with worsening postoperative New York Heart Association (NYHA) functional class [11]. In the current literature, however, there are limited data on the relationship between the BNP level and diastolic dysfunction—or its degree—in patients who underwent AVR for pure AS. To evaluate the postoperative BNP level as a reliable biomarker to facilitate the diagnosis of subclinical diastolic dysfunction following AVR, we analysed whether BNP levels rise as a reflection of LV diastolic dysfunction in a large homogeneous series of patients.
MATERIALS AND METHODS
This prospective study was conducted at a single cardiac surgery centre. Informed consent was obtained from each patient before enrolment in the study. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in a priori approval by the institution's human research committee.
Patient selection
Between January 2006 and January 2009, 113 consecutive patients undergoing AVR for pure AS who fulfilled the inclusion criteria were prospectively selected for this study. Demographic variables and preoperative, operative and postoperative clinical data were collected for each patient. The extent of dyspnoea was classified according to the NYHA. Clinical and investigation data were used to define surgical risk using the European System for Cardiac Operative Risk Evaluation (EuroSCORE) model [16].
Indications for surgery were mean gradient >50 mmHg and/or jet velocity >4 m/s and/or aortic valve area <1.0 cm2. Coronary angiograms were evaluated in all patients before surgery. To obtain an homogeneous population and avoid any confounding interference on BNP results, exclusion criteria were as follows: age over 75 years, evidence of coronary lesions, previous myocardial infarction, associated valve disease (except trace mitral regurgitation), associated aortic diseases, poor cardiac function as indicated by an LV ejection fraction (LVEF) of ≤45%, chronic atrial fibrillation, history of previous heart failure and contraindications to valve replacement with bioprostheses. Further exclusion criteria were all significant causes of elevated or reduced BNP levels other than LV dysfunction as indicated in the current literature: serum creatinine concentration >150 μmol/l, severe pulmonary disease (acute respiratory distress syndrome, lung disease with right heart failure, primary pulmonary hypertension and other causes of pulmonary hypertension) and obesity defined as body mass index (BMI) >30. Concurrent patients complying with the inclusion criteria and receiving mechanical valve substitutes were not enrolled to obtain homogeneity of patient age and postoperative haemodynamic performance (that could influence BNP levels) [3–4, 17]. Nevertheless, bioprostheses are not routinely treated with warfarin postoperatively in our department, and thus, a potential effect of warfarin on plasma BNP was avoided.
All study patients underwent physical examination, BNP analysis and echocardiographic assessment preoperatively, 5 days after surgery when the patient was presumed to be euvolemic, and at 12 months postoperatively thereafter. Follow-up was fixed at 12 months mainly because prosthetic gradients of bioprostheses usually change during the first postoperative year, with significant impacts on the haemodynamic performance [18].
The AVR was undertaken through a median sternotomy, with standard cardiopulmonary bypass under mild hypothermia (32°C), with cold crystalloid cardioplegia. All valves were implanted with 2-0 polyester non-pledget-supported, interrupted, non-everting mattress sutures.
Operative mortality was defined as death within 30 days after the operation. Preoperative and surgical variables are summarized in Table 1.
Table 1:
Preoperative and surgical variables
| Variable | Patients (N = 113) |
|---|---|
| Preoperative variables | |
| Age (years) | 67.2 ± 8.6 |
| Male sex | 77 (68.1%) |
| Body mass index | 24.6 ± 3.1 |
| New York Heart Association Class | |
| I–II | 87 (76.9%) |
| III | 26 (23.1%) |
| Logistic EuroSCORE | 5.1 ± 2.4 |
| Surgical variables | |
| Bicuspid aortic valve | 15 (13.3%) |
| Pump time (min) | 89.8 ± 14.4 |
| Cross-clamp time (min) | 65.7 ± 14.5 |
| Prosthetic valve (mm) | |
| 19 | 41 (36.3%) |
| 21 | 61 (53.9%) |
| 23 | 11 (9.8%) |
| Medtronic Mosaic Ultra porcine valve | 88 (77.8%) |
| Carpentier-Edwards Perimount Magna pericardial valve | 25 (22.2%) |
Values expressed as mean ± SD or n (%).
Echocardiographic measurements and calculations
A comprehensive transthoracic examination was performed according to the guidelines of the American Society of Echocardiography (ASE) [19]. When the transthoracic exam was assessed poor or inadequate, the transoesophageal approach was employed. LV systolic function was evaluated by the Simpson's rule. Left atrial volume was measured by the Simpson's method in an apical four- and two-chamber views and indexed for the body surface area. Timing for measurement was defined at the maximal expansion of the left atrium. LV mass was calculated from LV linear dimensions by the ASE-recommended formula based on modelling the LV as a prolate ellipse of revolution [20]. LV hypertrophy was defined as an indexed LV mass (ILVM) of ≥120 g/m2 in men and ≥100 g/m2 in women [20]. Calculation of relative wall thickness permitted categorization of an increase in LV mass as either concentric (≥0.42) or eccentric (≤0.42) hypertrophy and allowed the identification of concentric remodelling (normal LV mass with increased relative wall thickness). The peak and mean prosthetic gradients were calculated from continuous wave Doppler measurements using the modified Bernoulli equation. The continuity equation was used to calculate the effective orifice area indexed to the patient's body surface area.
For diastolic function evaluation, mitral flow was recorded at the tip of mitral leaflets in the four-chamber view. Colour flow imaging was used for optimal alignment of the Doppler beam. Spectral mitral velocity recordings were initially obtained at sweep speeds of 25–50 mm/s for the evaluation of respiratory variation of flow velocities, as seen in patients with pulmonary disease. If variations were not present, the sweep speed was increased to 100 mm/s, at end-expiration, and averaged over three consecutive cardiac cycles. From the mitral velocity tracings, early flow velocity (E) and peak velocity during atrial systole (A) were measured. The E/A ratio was calculated. Pulmonary venous flow velocities, obtained using the apical four- and two-chamber views, was evaluated whenever possible because of their significant relationship with diastolic function. Peak velocities during systole (S) and diastole (D) were measured. The S/D ratio was calculated. Doppler tissue imaging was taken from the septal mitral annulus to evaluate systolic velocities (S′) and early (E′) and late (A′) diastolic velocities. The mitral E/E′ ratio was subsequently calculated.
Diastolic function was evaluated by integrating mitral flow and pulmonary venous information according to the recommendations for the evaluation of left ventricular diastolic function by echocardiography [21]. In addition to E/A, E/E′ and S/D ratios, the deceleration time of early filling velocity and the isovolumetric relaxation time were also measured to distinguish between normal diastolic function and mild diastolic dysfunction. Mid-diastolic flow was considered as an important signal to recognize: low velocities can occur in normal subjects, but when increased (>20 cm/s), they often represent markedly delayed LV relaxation and elevated filling pressures [22].
Diastolic dysfunction was graded as mild (Grade 1, impaired relaxation), moderate (Grade 2, pseudonormal) or severe (Grade 3, restrictive; Table 2).
Table 2:
Echocardiographic definition of diastolic dysfunction
| Mild dysfunction | Moderate dysfunction | Severe dysfunction | |
|---|---|---|---|
| E/A ratio | <0.8 | 0.8–1.0 | >2 |
| Deceleration time (ms) | >200 | 150–200 | <160 |
| Isovolumetric relaxation time (ms) | >100 | 60–95 | <60 |
| S/D ratio | S > D | S < D | S < D |
| MVAdur/PVAdur | MVAdur > PVAdur | MVAdur < PVAdur | MVAdur < PVAdur |
| Annular E′ (cm/s) | <8.0 | <8.0 | |
| E/E′ ratio | <8.0 | 9–14 | >14 |
A: peak velocity during atrial systole; D: peak velocity during diastole; E: early flow velocity; E′: early diastolic velocity; MVAdur: mitral valve A flow duration; PVAdur: atrial reversal velocity duration; S: peak velocity during systole.
Echocardiographic results were interpreted by two experienced cardiologist blinded to laboratory results.
B-type natriuretic peptide analysis
Rapid quantitative test for BNP assay was performed by means of the immunofluorometric triage method (Triage, Biosite Diagnostics, San Diego, CA, USA). Whole venous blood sample (5 ml) was taken after 20 min of rest in the supine position and collected on an ethylenediaminetetraacetic acid (EDTA) tube, and the BNP level was analysed immediately, following the manufacturer's instructions. The assay is linear throughout the measurable range of the test. The measurable range of the assay is 5.0–1300 pg/ml, and the intra-assay variations range is between 9.5 and 13.9% for BNP levels of 25 and 1200 pg/ml, respectively. The average 95% confidence limit of the analytical sensitivity of the test is <5 pg/ml [95% confidence interval (95% CI) 0.2–4.8 pg/ml].
Statistical analysis
Continuous data are presented as mean and standard deviation (SD), and categorical data as proportion. Data were analysed by SPSS version 15 for Windows (SPSS, Inc., Chicago, IL, USA). Stata 6.0 statistical software package was used for receiver operating characteristic (ROC) analysis. Comparison between continuous variables was done by the Student's t-test for normally distributed features. The Mann–Whitney U-test was used for variables not normally distributed. Categorical variables were analysed by the χ2 test. Patients' echocardiographic characteristics were compared across the quartiles of BNP distribution either by means of the Kruskall–Wallis test or by the χ2 test for continuous and categorical variables, respectively. Pearson's correlation coefficient was used to assess the association between BNP levels and echocardiographic data. In addition, pairwise correlations were determined by using linear regression analysis. Multiway analysis of variance with correction for serial measurements was performed for echocardiographic data and BPN values. Statistical significance was assumed for P-value <0.05. The ROC curve was determined to evaluate the reliability of BNP to predict diastolic dysfunction. The optimal cut-off points for BNP levels were chosen when the sensitivity and specificity were maximized, and areas under the curve indicated.
RESULTS
Overall operative mortality was 3.5% (4 of 113). Non-fatal complications occurred in 9 (7.9%) patients (1 pleural effusion, 3 transient low output status, 3 ventilatory dependence >36 h and 2 bleeding requiring surgical revision).
Twelve-month follow-up was 100% complete for mortality and clinical findings, and 97.2% for BNP and echocardiographic evaluations. Within a follow-up duration of 12 months, 2 (2.6%) patients died: one suddenly and one of endocarditis. Three more patients (2.8%) were hospitalized for congestive heart failure. At follow-up, 55% of patients were receiving b-blockers and 15% angiotensin-converting enzyme inhibitors; 30%, b-blockers and angiotensin-converting enzyme inhibitor therapy started after surgery. Twelve percent of patients were taking furosemide.
Echocardiographic evaluation
The echocardiographic parameters at baseline and follow-up are presented in Table 3. The overall interobserver agreement for diastolic dysfunction classification was 94%. The intraobserver reproducibility for diastolic dysfunction classification was 95%.
Table 3:
Echocardiographic and functional parameters at baseline and follow-up
| Baseline (N = 113) | 5 days after surgery (N = 110) | 12 months after surgery (N = 104) | P-value | |
|---|---|---|---|---|
| Systolic blood pressure (mmHg) | 135 ± 12 | 125 ± 15 | 138 ± 14 | 0.07 |
| Diastolic blood pressure (mmHg) | 85 ± 8 | 75 ± 5 | 83 ± 5 | 0.009 |
| Left ventricular ejection fraction (%) | 52.8 ± 5.7 | 54.1 ± 4.6 | 54.2 ± 5.1 | 0.05 |
| Indexed left ventricular mass (g/m2) | 175.9 ± 45.3 | 174 ± 45.7 | 159.2 ± 42.9 | 0.009 |
| Peak gradient (mmHg) | 95.6 ± 31.5 | 30.6 ± 6.3 | 25.1 ± 4.4 | <0.001 |
| Mean gradient (mmHg) | 55.7 ± 13.4 | 15.1 ± 4.3 | 13.1 ± 3.7 | <0.001 |
| Left ventricular diastolic diameter (mm) | 46.2 ± 5.7 | 45.8 ± 5.9 | 44.9 ± 5.8 | 0.06 |
| Left ventricular systolic diameter (mm) | 29.6 ± 5.8 | 30.2 ± 6.1 | 31.1 ± 5.9 | 0.06 |
| Indexed left atrial volume (ml/m2) | 35.2 ± 9.5 | 36.4 ± 10.9 | 29.1 ± 6.3 | <0.001 |
| Diastolic function | ||||
| Normal | – | – | 14 (13.4%) | <0.001 |
| Mild (impaired relaxation) | 28 (24.7%) | – | 65 (62.5%) | <0.001 |
| Moderate (pseudonormal) | 71 (62.8%) | – | 17 (16.4%) | <0.001 |
| Severe (restrictive) | 14 (12.5%) | – | 8 (7.7%) | 0.3 |
| B-type natriuretic peptide (pg/ml) | 295 ± 125 | 430 ± 242 | 245 ± 168 | 0.01a |
| Indexed effective orifice area cm2/m2 (mean) | – | 0.75 ± 0.07 | 0.76 ± 0.06 | <0.001 |
Values expressed as mean ± SD or n (%).
aBaseline vs 12 months after surgery.
At 12-month follow-up, 104 patients were available for echocardiographic evaluations.
Mild to severe diastolic dysfunction occurred preoperatively in all patients. Twelve months after AVR, a significant change in the distribution of estimated diastolic type was registered when compared with preoperative assessment. LV systolic function was slightly better (P = 0.06) early after surgery compared with preoperative examinations, but no further significant improvement was registered 12 months later. Preoperative cardiac hypertrophy occurred in 91.1% (103 of 113). At 12-month follow-up, cardiac hypertrophy persisted in 76.9% of patients available for echocardiographic evaluation (80 of 104). Notably, although 1 year after surgery, an overall reduction in indexed left ventricular mass values had been registered compared with preoperative values (P = 0.009), the average value of indexed left ventricular mass remained persistently above the normal limit. Postoperative indexed effective orifice area. ≤0.75 cm2/m2 occurred with a significant prevalence in patients who had a Medtronic Mosaic Ultra valve implanted (P < 0.001). Thirteen (11.5%) patients had an indexed effective orifice area of ≤0.65 cm2/m2.
B-type natriuretic peptide measurement relationships with echocardiographic findings
Preoperatively, the mean plasma BNP was 295 ± 125 pg/ml. There was a dramatic increase immediately postoperatively to 430 ± 342 pg/ml, whereas BNP level sampled on the 12th postoperative month decreased to 215 ± 148 pg/ml (median 180, interquartile range 150–250 pg/ml; P = 0.01).
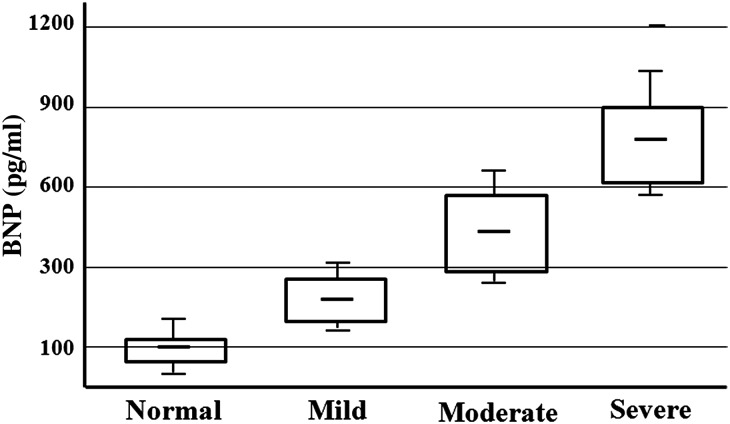
Twelve months after surgery, the mean BNP level was significantly higher in patients with LV diastolic dysfunction (282.5 ± 179.6 pg/ml) than in those with normal diastolic function (84.3 ± 10.5 pg/ml; P < 0.001). ROC analysis showed that the optimal cut-off value of the BNP level to detect diastolic dysfunction 12 months after AVR was 120 pg/ml, with a sensitivity of 91% (95% CI: 83.23–96.07%) and specificity of 85% (95% CI: 57.16–97.80%), with an area under the curve of 0.86. Similarly, BNP values >300 pg/ml were strongly correlated to echocardiographic findings suggestive of moderate or severe diastolic dysfunction with a sensitivity of 80% (95% CI: 59.29–93.09%) and specificity of 91% (95% CI: 83.57–96.57%), area under the curve of 0.83. When patients were categorized according to quartiles of postoperative BNP values sampled on the 12th postoperative month [90 (60–135), 160 (140–170), 210 (175–270) and 500 (275–1100) pg/ml], a fair correlation was found between the value of BNP and the diastolic dysfunction grade: the lower quartile of BNP distribution was related to normal diastolic function, the second and third quartiles to mild diastolic dysfunction and the upper quartile to moderate or severe diastolic dysfunction (P < 0.001 for all comparison; Fig. 1). The relationships between BNP values and echocardiographic parameters, evaluated by means of Pearson's correlation, are reported in Table 4.
Figure 1:
Comparisons between plasma BNP level and grade of LV diastolic function related to 12 months postoperatively. Box defines the interquartile range, with the mean indicated by the crossbar. The error bars indicate the SD.
Table 4:
Relationships between BNP values and echocardiographic parameters
| r-value | P-value | |
|---|---|---|
| BNP | ||
| Left ventricular ejection fraction (%) | −0.2 | 0.09 |
| Indexed effective orifice area (cm2/cm2) | −0.68 | 0.001 |
| Peak gradient | 0.21 | 0.3 |
| Mean gradient | 0.15 | 0.52 |
| Indexed left ventricular mass (g/m2) | 0.31 | 0.04 |
| Indexed left atrial volume | 0.52 | 0.006 |
| E/A | 0.81 | <0.001 |
| E/E′ | 0.83 | <0.001 |
| S/D | −0.69 | <0.001 |
| Isovolumetric relaxation time (ms) | −0.72 | <0.001 |
| Deceleration time (m/s) | −0.65 | 0.003 |
Relationship evaluated by Pearson's correlation.
E/A: early flow velocity (E) to peak atrial velocity (A) ratio; E/E′: transmitral early diastolic velocity (E) to myocardial early diastolic velocity (E′) of the lateral mitral annulus by tissue Doppler; S/D: peak systolic velocity (S) to diastolic velocity (D) ratio.
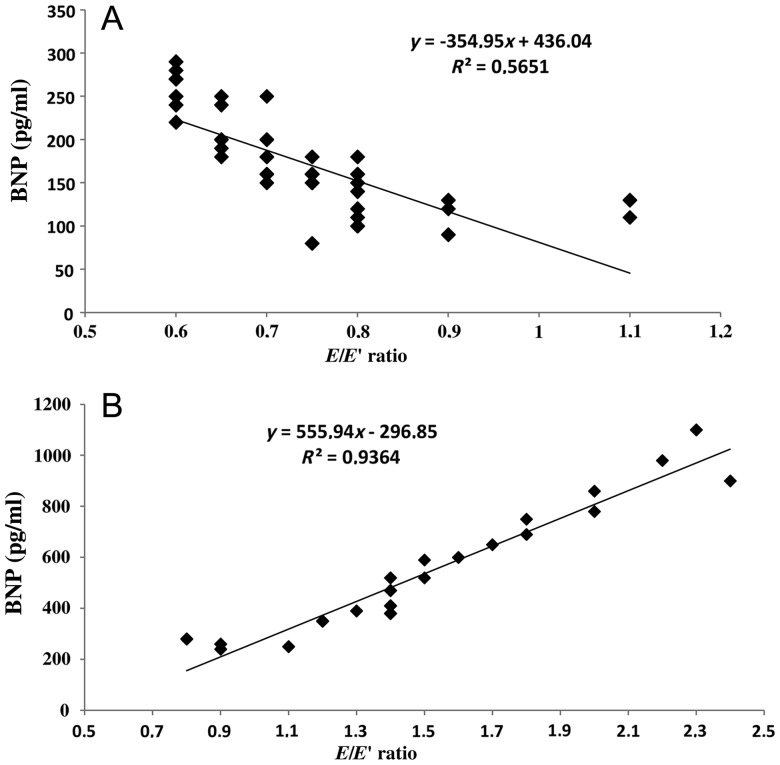
In addition, for each patient identified as having mild or moderate to severe diastolic dysfunction 12 months after surgery, the E/E′ ratio values were forced in the linear regression model for correlation to BNP values. The grade of diastolic dysfunction was strictly associated with BNP values, as reflected by significant correlations between BNP and E/E′ ratio ≤0.8 (R2 = 0.56; y = −354.9x + 436, P < 0.001) and BNP and E/E′ ratio from 0.8 to 2.4 (R2 = 0.93; y = 555.9x − 296.8, P < 0.001; Fig. 2A and B). Finally, there was no significant correlation between the NYHA class and BNP levels, probably as a reasonable consequence of preserved systolic function and good gradient at 12-month follow-up. This was, moreover, not surprising taking into account that more than two-third of our patients were in NYHA Class I or II preoperatively, and thus, there was little room for NYHA class improvement in these patients, even though plasma BNP decreased significantly.
Figure 2:
Diastolic function parameter (E/E′ ratio) 12 month after AVR analysed for correlation to BNP: (A) patients with mid-diastolic dysfunction and (B) patients with moderate or severe diastolic dysfunction.
DISCUSSION
In the present study, we tried non-invasively to identify isolated LV diastolic dysfunction 1 year after successful AVR and to demonstrate the potential of plasma BNP measurement for the sensitive detection of this condition. The results can be summarized as follows: (i) BNP levels were higher before operation in patients undergoing AVR for pure AS despite relatively preserved LVEF; (ii) early after operation, there was a dramatic transient increase in BNP levels; (iii) at 12-month follow-up, BNP levels decreased significantly compared with the preoperative levels, although normal values were reached in only few patients within 12 months; (iv) BNP levels reflected LV diastolic function and its severity also in asymptomatic patients; (v) BNP values >120 pg/ml 12 months after AVR were strongly correlated to echocardiographic findings suggestive of diastolic dysfunction; (vi) BNP values >300 pg/ml were strongly correlated to echocardiographic findings suggestive of moderate or severe diastolic dysfunction.
Following AVR, the afterload of the left ventricle is immediately reduced, and this leads to a recovery of wall stress, LV ejection time, LV volume, coronary perfusion and the load of the left atrium [4, 17, 23]. Conversely, persistence of LV hypertrophy is common after AVR and its regression is a slow process that takes years after correction of the primary haemodynamic abnormality and may continue for decades after surgery [23]. LV hypertrophy, stiffness (or reduced compliance) and subsequently impaired myocardial relaxation are considered the main determinant of postoperative diastolic dysfunction, which is worldwide considered predictive of the long-term adverse postoperative outcome [2, 5, 6, 23].
Echocardiography is the method of choice for diastolic function evaluation; however, it is usually complex and time-demanding and carries potential errors, particularly in distinguishing normal and pseudonormal stages. Tissue Doppler imaging, used in addition to traditional echocardiographic variables, minimizes these potential errors, but, in general, the definition of diastolic dysfunction (and the assessment of the stage) is difficult, and sometimes the measurements are less reproducible. In our experience, we found non-negligible intra- and interobserver variabilities of 5 and 6%, respectively, which is in line with those known from the literature.
In addition to echocardiography, BNP assessment has been validated as a highly sensitive and accurate method for the detection of LV systolic and diastolic dysfunction [24] and has emerged as a key biomarker for the diagnosis, evaluation and management of congestive heart failure [25]. In surgical patients with AS undergoing AVR, the BNP level has been identified as a strong predictor of early and long-term postoperative outcomes [9–14].
In the setting of LV diastolic function, we found significant correlations between the BNP level and echocardiographic evidence of postoperative diastolic dysfunction. As a further result, this study demonstrated that BNP values are directly related to several transmitral flow parameters and tissue Doppler findings indicative of impaired LV diastolic function. These results were substantiated by the significant linear correlation between E/E′ ratio and BNP levels in patients with mild or moderate to severe diastolic dysfunction. Using ROC analysis, we found that a BNP level of >120 pg/ml was a discriminative threshold for normal or impaired diastolic function after AVR. Similarly, a BNP level of >300 pg/ml was a relevant cut-off level, leading to higher sensitivity and specificity in terms of identifying moderate or severe diastolic dysfunction. As a consequence, BNP values, coupled with LV diastolic status, may bring more specific evaluation of isolated LV diastolic dysfunction and could be used as a simple, not operator-dependent, relatively inexpensive and reproducible tool for assessing LV function after AVR and for monitoring the evolution of diastolic dysfunction after AVR.
The clinical relevance and the prognostic value of BNP have been well elucidated by Berger et al. who found that an elevated hormone level was associated with a significantly higher number of any-cause death in symptomatic patients who underwent AVR for AS [10]. More recently, Pedrazzini et al. [12] demonstrated that higher preoperative BNP values (>312 pg/ml) were strong predictors of hospital mortality and, more importantly, long-term survival and were more accurate in predicting postoperative adverse outcome compared with logistic EuroSCORE or echocardiographic evaluation. These findings have been recently substantiated by Iwahashi et al. [11] who confirmed that BNP >312 pg/ml was accurate enough for predicting perioperative complications and was associated with the risk of major cardiac and cerebrovascular events after AVR.
These results, taken together, provide the necessary clinical support to our study, considering that BNP appears to reflect the actual haemodynamic state and could predict non-invasively the progression of diastolic dysfunction from one class to the next and, consequently, the transition from compensated to decompensated heart status. Thus, BNP assessment may represent an important adjunct for the early and adequate recognition of LV dysfunction and may contribute to medical treatment optimization before the development of symptoms that are non-specific and subtle at onset and often difficult to evaluate clinically or by echocardiography.
The study has several limitations: (i) there was always a potential error in interpreting diastolic function echocardiographically, particularly in distinguishing normal and pseudonormal; however, a number echocardiographic findings were evaluated in the present study in order to minimize possible bias; (ii) we were not able, with few events, to draw any meaningful conclusions on the relationship between BNP values and postoperative deaths or need for hospitalization; (iii) patient follow-up was performed at 12 months after surgery, so the results need a further long-term validation.
The present study indicates the relationship between BNP and LV diastolic function after AVR for AS. The serial measurements of BNP may complement echocardiographic and clinical evaluation, particularly when symptoms are unclear or when LV function is difficult to interpret. However, further large prospective studies are needed to determine whether serial measurements of BNP levels can be used to monitor disease progression in the late stages and whether it may be useful as an additional tool for planning an effective pharmacological strategy in preventing LV deterioration.
Conflict of interest: none declared..
ACKNOWLEDGEMENTS
The authors are deeply grateful to GiovanBattista Mannacio from the Department of Mathematics–Statistics Section of Imperial College of London for assistance with the statistical analysis.
REFERENCES
- 1.Yarbrough WM, Mukherjee R, Ikonomidis JS, Zile MR, Spinale FG. Myocardial remodeling with aortic stenosis and after aortic valve replacement: mechanisms and future prognostic implications. J Thorac Cardiovasc Surg. 2012;143:656–64. doi: 10.1016/j.jtcvs.2011.04.044. doi:10.1016/j.jtcvs.2011.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bové T, Van Belleghem Y, François K, Caes F, Van Overbeke H, Van Nooten G. Stentless and stented aortic valve replacement in elderly patients: factors affecting midterm clinical and hemodynamical outcome. Eur J Cardiothorac Surg. 2006;30:706–13. doi: 10.1016/j.ejcts.2006.07.017. doi:10.1016/j.ejcts.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Mannacio VA, De Amicis V, Di Tommaso L, Iorio F, Vosa C. Influence of prosthesis-patient mismatch on exercise-induced arrhythmias: a further aspect after aortic valve replacement. J Thorac Cardiovasc Surg. 2009;138:632–8. doi: 10.1016/j.jtcvs.2009.01.009. doi:10.1016/j.jtcvs.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Mannacio V, Di Tommaso L, De Amicis V, Stassano P, Vosa C. Coronary perfusion: impact of flow dynamics and geometric design of 2 different aortic prostheses of similar size. J Thorac Cardiovasc Surg. 2012;143:1030–5. doi: 10.1016/j.jtcvs.2011.06.011. doi:10.1016/j.jtcvs.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Gjertsson P, Caidahl K, Bech-Hanssen O. Left ventricular diastolic dysfunction late after aortic valve replacement in patients with aortic stenosis. Am J Cardiol. 2005;96:722–7. doi: 10.1016/j.amjcard.2005.04.052. doi:10.1016/j.amjcard.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 6.Licker M, Cikirikcioglu M, Inan C, Cartier V, Kalangos A, Theologou T, et al. Preoperative diastolic function predicts the onset of left ventricular dysfunction following aortic valve replacement in high-risk patients with aortic stenosis. Crit Care. 2010;14:R101. doi: 10.1186/cc9040. doi:10.1186/cc9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure: abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–9. doi: 10.1056/NEJMoa032566. doi:10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 8.Fox AA, Shernan SK, Collard CD, Liu KY, Aranki SF, DeSantis SM, et al. Preoperative B-type natriuretic peptide is as independent predictor of ventricular dysfunction and mortality after primary coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2008;136:452–61. doi: 10.1016/j.jtcvs.2007.12.036. doi:10.1016/j.jtcvs.2007.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50:2357–68. doi: 10.1016/j.jacc.2007.09.021. doi:10.1016/j.jacc.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 10.Berger R, Huelsman M, Strecker K, Bojic A, Moser P, Stanek B, et al. B-type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation. 2002;105:2392–7. doi: 10.1161/01.cir.0000016642.15031.34. doi:10.1161/01.CIR.0000016642.15031.34. [DOI] [PubMed] [Google Scholar]
- 11.Iwahashi N, Nakatani S, Umemura S, Bojic A, Petra Moser P, Stanek B, et al. Usefulness of plasma B-type natriuretic peptide in the assessment of disease severity and prediction of outcome after aortic valve replacement in patients with severe aortic stenosis. J Am Soc Echocardiogr. 2011;24:984–91. doi: 10.1016/j.echo.2011.03.012. doi:10.1016/j.echo.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Pedrazzini GB, Masson S, Latini R, Klersy C, Rossi MG, Pasotti E, et al. Comparison of brain natriuretic peptide plasma levels versus logistic EuroSCORE in predicting in-hospital and late postoperative mortality in patients undergoing aortic valve replacement for symptomatic aortic stenosis. Am J Cardiol. 2008;102:749–54. doi: 10.1016/j.amjcard.2008.04.055. doi:10.1016/j.amjcard.2008.04.055. [DOI] [PubMed] [Google Scholar]
- 13.Patel DN, Bailey SR. Role of BNP in patients with severe asymptomatic aortic stenosis. Eur Heart J. 2004;25:1972–3. doi: 10.1016/j.ehj.2004.09.001. doi:10.1016/j.ehj.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Neverdal NO, Knudsen CW, Husebye T, Vengen OA, Pepper J, Lie M, et al. The effect of aortic valve replacement on plasma B-type natriuretic peptide in patients with severe aortic stenosis: one year follow-up. Eur J Heart Fail. 2006;8:257–62. doi: 10.1016/j.ejheart.2005.08.004. doi:10.1016/j.ejheart.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Qi W, Mathisen P, Kjekshus J, Simonsen S, Endresen K, Bjørnerheim R, et al. The effect of aortic valve replacement on N-terminal natriuretic propeptides in patients with aortic stenosis. Clin Cardiol. 2002;25:174–80. doi: 10.1002/clc.4960250408. doi:10.1002/clc.4960250408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michel P, Roques F, Nashef SA. Logistic or additive EuroSCORE for high risk patients. Eur J Cardiothorac Surg. 2003;23:684–7. doi: 10.1016/s1010-7940(03)00074-5. doi:10.1016/S1010-7940(03)00074-5. [DOI] [PubMed] [Google Scholar]
- 17.Mannacio V, Di Tommaso L, Stassano P, De Amicis V, Vosa C. Myocardial metabolism and diastolic function after aortic valve replacement for aortic stenosis: influence of patient–prosthesis mismatch. Eur J Cardiothorac Surg. 2012;41:316–21. doi: 10.1016/j.ejcts.2011.05.039. doi:10.1016/j.ejcts.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto K. Patient-prosthesis mismatch: the Japanese experience. Ann Thorac Cardiovasc Surg. 2006;12:159–65. [PubMed] [Google Scholar]
- 19.Zoghbi WA, Chambers JB, Dumesnil JG, Foster E, Gottdiener JS, Grayburn PA, et al. American Society of Echocardiography's Guidelines and Standards Committee. Recommendations for evaluation of prosthetic valves with echocardiography and Doppler ultrasound. J Am Soc Echocardiogr. 2009;22:975–1014. doi: 10.1016/j.echo.2009.07.013. doi:10.1016/j.echo.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Lang RM, Bierig M, Devereux RB, Foster E, Flachskampf FA, Foster E, et al. Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. doi:10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–33. doi: 10.1016/j.echo.2008.11.023. doi:10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 22.Ha JW, Oh JK, Redfield MM, Ujino K, Seward JB, Tajik AJ. Triphasic mitral inflow velocity with middiastolic filling: clinical implications and associate echocardiographic findings. J Am Soc Echocardiogr. 2004;17:428–31. doi: 10.1016/j.echo.2004.02.007. doi:10.1016/j.echo.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Villari B, Vassalli G, Monrad ES, Monrad ES, Chiariello M, Turina M, et al. Normalization of diastolic dysfunction in aortic stenosis late after valve replacement. Circulation. 1995;91:2353–8. doi: 10.1161/01.cir.91.9.2353. doi:10.1161/01.CIR.91.9.2353. [DOI] [PubMed] [Google Scholar]
- 24.Guarracino F, De Lorenzo B, Vullo C, Pasquini C, Boldrini A. Plasma BNP and diastolic dysfunction in patients with preserved systolic function. Eur J Anaesthesiol. 2007;24:53–4. doi:10.1097/00003643-200706001-00197. [Google Scholar]
- 25.Jourdain P, Jondeau G, Funck F, Gueffet P, Le Helloco A, Donal E, et al. Plasma brain natriuretic peptide-guided therapy to improve outcome in heart failure: the STARS-BNP Multicenter Study. J Am Coll Cardiol. 2007;49:1733–9. doi: 10.1016/j.jacc.2006.10.081. doi:10.1016/j.jacc.2006.10.081. [DOI] [PubMed] [Google Scholar]