Abstract
OBJECTIVES
Pulmonary hypertension is a major cause of morbidity and mortality in patients following acute pulmonary embolism. Although thrombolytic therapy decreases pulmonary arterial pressure, compared with anticoagulation alone, it has the propensity for haemorrhagic complications, distal embolization and incomplete recanalization, with the potential risk of late pulmonary hypertension. Surgical embolectomy—once performed solely on critically-ill patients—has now gained favour in a wider range of patients. In this paper we present the outcomes of patients who underwent surgical embolectomy complemented with retrograde technique and follow-up systolic pulmonary arterial pressure (SPAP).
METHODS
From January 2004 to December 2010, 30 consecutive patients with a mean age of 58 ± 15 years underwent pulmonary embolectomy at our centre. The patients were followed for a mean period of 30.5 ± 12 months. Their New York Heart Association (NYHA) classifications were assessed and their SPAPs were measured by echocardiography.
RESULTS
The overall mortality rate was 13.2% (4/30). Of the remaining patients, 19 patients (73.1%) were in NYHA classes I and II, 7 patients (26.9%) in class III and no patient in class IV. The patients' preoperative and postoperative mean SPAPs were 44.9 ± 5.7 and 34.9 ± 7.1 mmHg, respectively, which showed a significant reduction (P < 0.001). The mean SPAP in the follow-up was 29.4 ± 11.5 mmHg, which again showed significant reduction compared with early postoperation values (P < 0.001). No significant correlations were found between the level of SPAP reduction in patients' follow-up with age (P = 0.727) and total days of ICU admission (P = 0.700), but weak correlations with sex (P = 0.016) and total intubation time were noticed (P = 0.035).
CONCLUSIONS
This is the first series reporting the long-term outcome of patients undergoing surgical embolectomy complemented by retrograde embolectomy technique, demonstrating the safety and favourable long-term outcome of this technique. It is also a new element in the growing body of evidence regarding the relevance of surgical embolectomy in patients with acute pulmonary embolism. We concluded that, following surgery, not only does the pulmonary arterial pressure drop immediately, but also the trend toward normalization continues long after operation.
Keywords: Surgical pulmonary embolectomy, Retrograde pulmonary embolectomy, Acute pulmonary embolism, Chronic thrombo-embolic pulmonary hypertension
INTRODUCTION
Chronic thrombo-embolic pulmonary hypertension (CTPH), defined as mean pulmonary arterial pressure above 25 mmHg persisting 6 months after the diagnosis of acute pulmonary embolism, is a relatively rare complication that is estimated to occur in 2–4% of patients after the acute event [1, 2]. This complication causes considerable morbidity and, although late mortality is determined mainly by comorbidities—particularly cancer—it may also cause mortality, mainly due to progressive right ventricular (RV) failure [1–4].
Following acute pulmonary embolism, thrombolytic therapy and catheter embolectomy have been proved to be effective in decreasing pulmonary arterial pressure by restoring patency of vessel, thereby reducing right ventricular pressure and stabilizing patients' haemodynamics [1, 5, 6]. However, these procedures are associated with haemorrhage, distal embolization and failure to completely resolve the intraluminal thrombus, rendering the patient amenable to development of chronic thrombo-embolic pulmonary hypertension [6–8]. Whilst surgical embolectomy has usually been reserved for patients who were critically ill, there has been a resurgence in recent years—driven by improved surgical technique, better postoperative care and, especially, lower mortality rates in recent series—which has resulted in enhanced indications for the use of surgical embolectomy in patients with acute pulmonary embolism [6–18].
Previously, we presented an improved technique for retrograde pulmonary embolectomy, which complemented the current conventional antegrade technique, and published the short-term results on 30 patients; herein we present the long-term follow-up and analysis of the pulmonary arterial pressures of the patients who underwent surgical embolectomy with retrograde technique.
PATIENTS AND METHOD
Patient population
From January 2004 through 30 December 2010, patients with massive and submassive pulmonary embolism underwent pulmonary embolectomy in two university teaching hospitals. The study included 15 men and 15 women with a mean age of 58.2 ± 15.6 years, ranging from 28 to 80 years. The most common risk factors for development of pulmonary embolism were deep vein thrombosis (14.46%), followed by recent surgery (7.23%).
The indications for surgery were severe haemodynamic and/or respiratory compromise in 11 patients (36%) judged to be on the verge of cardiac arrest, with three of them actually experiencing cardiac arrest at some point before operation; lack of response to intravenous thrombolysis in 10 patients (30%); contraindication for thrombolysis in 7 patients (23%) and, in 2 patients (6%), because of concomitant patent foramen ovale.
Surgical technique and hospital course
Surgery involved median sternotomy with bicaval cannulation and total circulatory bypass. Then the main pulmonary artery was opened distal to the pulmonary valve and the incision was extended as needed up to the bifurcation of the artery. The clots were removed by means of atraumatic forceps, bolus saline flush and suction. Thereafter, an atrial septostomy was created and pulmonary vein orifices in the left atrium were identified and cannulated, one by one, with a 5-French to 5.5 French endotracheal tube and irrigated with oxygenated blood with a mean pressure of 30–45 mmHg for 60–120 s for each vein. In this way, any peripherally lodged thrombus fragments were washed out in a retrograde manner. At the end of the operation, the atrial septal defect was closed and the systolic and diastolic pulmonary artery pressure was directly measured before and after cardiopulmonary bypass. The patients were then transferred to the intensive care unit (ICU). All the surviving patients were anticoagulated with unfractioned heparin and then warfarin, and were discharged from hospital for the care of their primary physician.
Study design
For the purposes of this study, the surviving patients were contacted and asked to refer to our outpatient clinic. The study objectives were explained to them and informed consent was obtained. A full physical examination was performed; their New York Heart Association (NYHA) classification was evaluated by a physician and they underwent transthoracic echocardiography to assess their systolic pulmonary arterial pressures (SPAP), carried out by a cardiologist who was unaware of the study goals. The SPAP was estimated from the tricuspid valve regurgitant jet velocity plus the right atrial pressure estimated from the inferior vena cava, and is expressed in mmHg.
Statistical analysis
Repeated measure design was used to compare the SPAPs of patients in the three periods of preoperation, postoperation and long-term follow-up because of variable follow-up in our patient population. We used repeated measure without any covariates that were significant (P < 0.05). Accordingly, we included age as a covariate and sex as a factor and used the full model (sum of squares type III) to construct our model.
In order to analyse correlation of numerical and nominal variables with preoperation and postoperation SPAP values, Spearman's rho- and Mann–Whitney U-tests were used, respectively. P-value <0.05 was considered weakly significant and <0.001 strongly significant.
RESULTS
The mean postoperative follow-up was 30.5 ± 12 months with a range of 6–61 months. The overall mortality rate in our study was 13.2% (4/30). A 60-year-old woman died during operation, due to severe right ventricular dysfunction; a 79-year-old man after 2 months of ICU care, due to heart and renal failure; a 65-year-old man after 6 months, due to renal cell carcinoma related complications and a 75-year-old woman with Cushing's syndrome died after 20 months, due to sepsis. In total, twenty-six patients completed the study; 12 women (46.2%) and 14 men (53.8%) with a mean age of 56.3 ± 15.8 years. The mean intubation time for this group of patients was 52.7 ± 37.1 h and the mean stay in ICU was 7.3 ± 11.7 days. Nineteen patients (73.1%) were in NYHA class I and II—indicative of only slight limitation of activity—and 7 patients (26.9%) were in NYHA class III—showing marked limitation of activity—with no patients in NYHA class IV.
Measured directly in the operating room, the patients' preoperative and postoperative mean SPAPs were 44.9 ± 5.7 and 34.9 ± 7.1, respectively, which showed a significant reduction (P < 0.001). The mean SPAP in the follow-up, measured by transthoracic echocardiography, was 29.4 ± 11.5, which showed significant reduction compared with both preoperation and early postoperation values (P < 0.001), which was independent of age and sex after insertion of these factors (P-values of 0.294 and 0.147, respectively) (Table 1, Fig. 1).
Table 1.
Mean pressures and minimum and maximum ranges of pulmonary systolic pressures measured at three intervals in the study group
| Systolic pressure | Mean ± SD (mmHg) | Minimum (mmHg) | Maximum (mmHg) | P-value |
|---|---|---|---|---|
| Preoperation | 44.9 ± 5.7 | 35.50 | 55.00 | |
| Early postoperation | 34.9 ± 7.1 | 22.50 | 50.00 | 0.001 |
| Follow-up study | 29.4 ± 11.5 | 16.00 | 60.00 |
Figure 1.
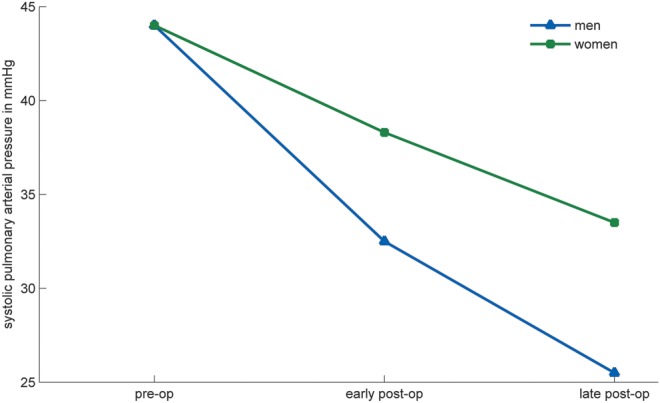
Repeated measure of the systolic pressures in the study group.
We performed a correlation study for baseline SPAP, the immediate fall in SPAP following operation and the further diminution of SPAP in follow-up with different variables (Table 2). There was no correlation between age of patients on admission and total days in ICU with the level of SPAP reduction in patients' follow-up, compared with preoperation values (P = 0.727 and 0.700, respectively) but there was a weak positive correlation with intubation time (P = 0.035) and sex of the patients (P = 0.016). There was no meaningful correlation between age of patients on admission and total days in ICU (r = 0.144, P = 0.483) but there was a weak correlation with total intubation time (r = 0.404, P = 0.041).
Table 2.
Correlation coefficients of age, intubation time and ICU admission days with baseline SPAP and the fall in this value immediately after operation and in the follow-up
| Preop SPAP | Differences in Preop and early postop SPAP | Differences in early and late postop SPAP | |
|---|---|---|---|
| Age | 0.057 | −0.077 | 0.146 |
| Intubation time | 0.039 | 0.085 | 0.442* |
| ICU admission | 0.065 | 0.146 | 0.357 |
SPAP: systolic pulmonary arterial pressure; ICU: intensive care unit.
*P < 0.05.
DISCUSSION
Historically, surgical embolectomy has always been reserved for critically ill patients. Current guidelines still recommend thrombolytic therapy for patients with massive and submassive pulmonary embolism with haemodynamic compromise, and propose surgical embolectomy as a substitute in case thrombolytic therapy is either contraindicated or ineffective [19]. Nevertheless, due to the overwhelmingly encouraging results of surgical embolectomy, many centres now offer this procedure as first-line therapy for such patients and have added patients with right ventricular failure, patent foramen ovale and intracardiac thrombi to the list of patients eligible for surgery [6–18, 20]. A recent paper by Aymard et al. proposed computed tomography scan derived right ventricle/left ventricle ratio (RV/LV CT ratio) as a new index for eligibility of patients for surgery [18].
Thrombolytic agents lyse organized clots, restore flow in the pulmonary artery, improve haemodynamics and have been shown to decrease short-term mortality and the likelihood of development of pulmonary hypertension, compared with anticoagulation alone [1, 2, 5, 6]. Despite these favourable outcomes, thrombolytic therapy is associated with long-term morbidity and is contraindicated in up to 50% of patients [6]. It is associated with the risk of bleeding in as much as 21.9% of patients and intracerebral haemorrhage rate of 0.7–6.4% [5–7, 9, 10]. Another concern that is not addressed in current guidelines is the limited time window available in critically ill patients before thrombolytic agents take effect, since most deaths are reported within the first few hours: what is more distressing is the delay in definitive management in patients with lack of response to thrombolytic therapy, who show higher mortality rates after surgery [6, 9, 11, 18]. Operative mortality increases significantly in patients with preoperative cardiac arrest, with reports as high as 30%, even with preoperative percutaneous cardiopulmonary support, prompting some authors to advocate a strategy of aggressive and early surgery in all patients with impending RV failure [9, 10]. Considering all these shortcomings of thrombolytic therapy, in parallel with emerging reports of favourable outcomes of surgery with comparable and even superior mortality benefit and higher symptomatic relief, many institutes including our centre were encouraged to revise their eligibility criteria for surgical embolectomy [6–18, 20]. The mortality rate in our study was 13.2% (4/30) in a mean follow-up of 30.5 ± 12 months. This is within the range reported by other studies and imitates the trend of decreased mortality rates in recent series [6, 7, 9–18]. The mortality rate was reported to be around 35% before 1985, 20% in the following decade and from 5.3 to 27.2% in recent series, attributed to better surgical techniques, improved perioperative care, early surgery and widened indication for surgery including patients with more stable haemodynamics [9, 11]. In our study, the mortality rate in the subgroup of patients with preoperative cardiac arrest was 33.3% (1/3) again reflecting the need to allocate such patients for earlier operation. It is widely believed that, during the first 30 days after the index event of acute embolism, the main cause of death is RV failure, whereas underlying comorbidity is the primary cause thereafter [14, 21]. In line with this belief, our only mortality in the first 30 days was a woman who died during operation due to RV failure and the other three mortalities were related to underlying problems.
As stated earlier, CTPH is a major cause of late morbidity and even mortality in patients surviving acute pulmonary embolism [2, 3, 8]. Thrombolytic embolectomy lessens the risk of development of CTPH, compared with anticoagulation alone, and surgical embolectomy can also prevent clot organization and thrombo-embolic scarring, resulting in decreased possibility of CTPH [1, 5, 14]. Clot fragmentation with showering to distal arteries is commonly seen with catheter-based embolectomy and incomplete recanalization is always a possibility in patients receiving thrombolytic therapy, rendering the patient amenable to the development of pulmonary hypertension, although no clinical trial has directly compared these two methods in this respect [6]. Retrograde pulmonary embolectomy, which was first performed in 1966 with subsequent modifications, has the advantage of dislodging the remaining clots in distal pulmonary vasculature not approached in antegrade technique [22, 23]. Consequently, compared with the antegrade technique alone, this will help to prevent pulmonary hypertension, without injury to lung parenchyma or the pulmonary arterial wall by forceps or Fogarty catheters [8, 16]. Few studies have directly addressed the issue of patient follow-up based on SPAP. In this study, we have shown that the pulmonary artery pressure falls immediately after operation and the normalization of pulmonary artery pressure continues even in the long-term follow-up. This is in agreement with the findings in other reports demonstrating the dynamic nature of the pulmonary vasculature and its propensity toward higher or lower values, especially in the first two years after the acute event [2]. Potential risk factors for the development of CTPH are multiple episodes of pulmonary embolism, younger age, larger perfusion defect and idiopathic presentation of pulmonary embolism [2]. In our study we analysed age, sex, intubation time and days spent in the ICU and found that higher age is associated with longer intubation time after operation which, in turn, may be associated with higher SPAP in follow-up. Moreover it was demonstrated that female gender may be associated with less favourable response, compared with male gender (Table 2, Fig. 1). These findings, although interesting, were found in correlation studies and need to be confirmed in larger observational studies in order to be relied upon.
NYHA classification is a useful and simple index to evaluate the symptoms of the patient. Both thrombolytic and surgical embolectomy have favourable effects on the symptoms of patients with acute pulmonary embolectomy, with at least one report indicating a lower proportion of patients having subjective complaint of dyspnoea after surgical embolectomy, compared with thrombolytic therapy [20]. In our report 73.1% of patients were in NYHA class I and II, which is near the range of 73.3–86% reported in other studies [7, 20]. Some of these reports excluded patients with left-sided systolic dysfunction and valvular disease while, in our series, five surviving patients had moderate-to-severe left-sided dysfunction (ejection fraction <40%) in the follow-up echocardiography, which can be a cause of dyspnoea. In addition, considering the fact that some patients with CTPH are asymptomatic and some symptomatic patients have causes of dyspnoea not attributable to pulmonary hypertension, and that we did not actively seek for the causes of dyspnoea in our patients, this seems to be an acceptable result [1, 2, 20]. In our study, there was a close association between NYHA classification and SPAP; while 73.1% of patients were in NYHA classes I and II, 76.9% (20/26) had SPAP below 40 mmHg at follow-up. Whether or not NYHA classification is an accurate clinical indicator to follow the patients after pulmonary embolectomy needs to be validated in larger studies, with at least one study reporting acceptable correlation between NYHA classification and SPAP [20].
This is the first series published regarding the long-term outcome of patients undergoing surgical embolectomy complemented by modified retrograde embolectomy technique, demonstrating the safety and encouraging long-term outcome of this technique. It is also a new piece in the growing body of evidence regarding the relevance of surgical embolectomy in patients with pulmonary embolism. Additionally, this is one of the few studies to follow the patients with an objective and reproducible index, i.e. systolic pulmonary arterial pressure. It was also interesting to notice that the pulmonary arterial pressure does not fall only immediately following surgery, but that the pressure normalization continues well into long-term follow-up. Considering the overall safety of pulmonary embolectomy, even in the context of more unstable patients usually selected for this approach, the time has perhaps come to conduct a large, clinical, randomized trial to compare the short-term and long-term outcomes of this approach with thrombolytic embolectomy.
This study has its own limitations: first of all, it is a non-randomized study, subject to bias of patient selection. Additionally, the patients' SPAPs were measured by echocardiography in the follow-up study, while their immediate preoperative and post-operative SPAPs were measured directly in the operating room. Although there is close correlation between echocardiographic and catheter-based measurement of pulmonary arterial pressure, this discrepancy in measurement methods should be considered when analysing the study data [24]. Finally, this is a retrograde study and patients were investigated at different stages of the postoperation period. If patients could be followed prospectively, and echocardiography was performed in the same stage for all patients, more consistent results could be obtained.
Conflict of interest: none declared.
REFERENCES
- 1.Piazza G, Goldhaber SZ. Chronic thrombo-embolic pulmonary hypertension. N Engl J Med. 2011;364:351–60. doi: 10.1056/NEJMra0910203. [DOI] [PubMed] [Google Scholar]
- 2.Pengo V, Lensing AW, Prins MH, Marchiori A, Davidson BL, Tiozzo F, et al. Incidence of chronic thrombo-embolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350:2257–64. doi: 10.1056/NEJMoa032274. [DOI] [PubMed] [Google Scholar]
- 3.Meneveau N, Ider O, Seronde MF, Chopard R, Davani S, Bernard Y, et al. Long-term prognostic value of residual pulmonary vascular obstruction at discharge in patients with intermediate- to high-risk pulmonary embolism. Eur Heart J. 2013;34:693–701. doi: 10.1093/eurheartj/ehs365. [DOI] [PubMed] [Google Scholar]
- 4.Saouti N, Morshuis WJ, Heijmen RH, Snijder RJ. Long-term outcome after pulmonary endarterectomy for chronic thrombo-embolic pulmonary hypertension: a single institution experience. Eur J Cardiothorac Surg. 2009;35:947–52. doi: 10.1016/j.ejcts.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 5.Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M. Moderate pulmonary embolism treated with thrombolysis. Am J Cardiol. 2013;111:273–7. doi: 10.1016/j.amjcard.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 6.Dauphine C, Omari B. Pulmonary embolectomy for acute massive pulmonary embolism. Ann Thorac Surg. 2005;79:1240–44. doi: 10.1016/j.athoracsur.2004.08.081. [DOI] [PubMed] [Google Scholar]
- 7.Vohra HA, Whistance RN, Mattam K, Kaarne M, Haw MP, Barlow CW, et al. Early and late clinical outcomes of pulmonary embolectomy for acute massive pulmonary embolism. Ann Thorac Surg. 2010;90:1747–52. doi: 10.1016/j.athoracsur.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Zarrabi K, Zolghadrasli A, Ostovan MA, Azimifar A. Short-term results of retrograde pulmonary embolectomy in massive and submassive pulmonary embolism: a single-center study of 30 patients. Eur J Cardiothorac Surg. 2011;40:890–93. doi: 10.1016/j.ejcts.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Kilic A, Shah AS, Conte JV, Yuh DD. Nationwide outcomes of surgical embolectomy for acute pulmonary embolism. J Thorac Cardiovasc Surg. 2012 doi: 10.1016/j.jtcvs.2012.01.066. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Taniguchi S, Fukuda W, Fukuda I, Watanabe K, Saito Y, Nakamura M, et al. Outcome of pulmonary embolectomy for acute pulmonary thromboembolism: analysis of 32 patients from a multicentre registry in Japan. Interact CardioVasc Thorac Surg. 2012;14:64–67. doi: 10.1093/icvts/ivr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukuda I, Taniguchi S. Embolectomy for acute pulmonary thromboembolism: from Trendelenburg's procedure to the contemporary surgical approach. Surg Today. 2011;41:1–6. doi: 10.1007/s00595-010-4416-8. [DOI] [PubMed] [Google Scholar]
- 12.Samoukovic G, Malas T, deVarennes B. The role of pulmonary embolectomy in the treatment of acute pulmonary embolism: a literature review from 1968 to 2008. Interact CardioVasc Thorac Surg. 2010;11:265–70. doi: 10.1510/icvts.2009.228361. [DOI] [PubMed] [Google Scholar]
- 13.Fukuda I, Taniguchi S, Fukui K, Minakawa M, Daitoku K, Suzuki Y. Improved outcome of surgical pulmonary embolectomy by aggressive intervention for critically ill patients. Ann Thorac Surg. 2011;91:728–32. doi: 10.1016/j.athoracsur.2010.10.086. [DOI] [PubMed] [Google Scholar]
- 14.Greelish JP, Leacche M, Solenkova NS, Ahmad RM, Byrne JG. Improved midterm outcomes for type A (central) pulmonary emboli treated surgically. J Thorac Cardiovasc Surg. 2011;142:1423–29. doi: 10.1016/j.jtcvs.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 15.Leacche M, Unic D, Goldhaber SZ, Rawn JD, Aranki SF, Couper GS. Modern surgical treatment of massive pulmonary embolism: results in 47 consecutive patients after rapid diagnosis and aggressive surgical approach. J Thorac Cardiovasc Surg. 2005;129:1018–23. doi: 10.1016/j.jtcvs.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 16.Spagnolo S, Grasso MA, Tesler UF. Retrograde pulmonary perfusion improves results in pulmonary embolectomy for massive pulmonary embolism. Tex Heart Inst J. 2006;33:473–476. [PMC free article] [PubMed] [Google Scholar]
- 17.Amirghofran AA, Emami Nia A, Javan R. Surgical embolectomy in acute massive pulmonary embolism. Asian Cardiovasc Thorac Ann. 2007;15:149–53. doi: 10.1177/021849230701500214. [DOI] [PubMed] [Google Scholar]
- 18.Aymard T, Kadner A, Widmer A, Basciani R, Tevaearai H, Weber A, et al. Massive pulmonary embolism: surgical embolectomy versus thrombolytic therapy–should surgical indications be revisited? Eur J Cardiothorac Surg. 2013;43:90–4. doi: 10.1093/ejcts/ezs123. discussion 94. [DOI] [PubMed] [Google Scholar]
- 19.Agnelli G, Becattini C. Acute pulmonary embolism. N Engl J Med. 2010;363:266–74. doi: 10.1056/NEJMra0907731. [DOI] [PubMed] [Google Scholar]
- 20.Habicht JM, Hämmerli R, Perruchoud A, Müller J, Stulz P. Long-term follow-up in pulmonary embolectomy: is NYHA (dyspnoea) classification reliable? Eur J Cardiothorac Surg. 1996;10:32–37. doi: 10.1016/s1010-7940(96)80263-6. [DOI] [PubMed] [Google Scholar]
- 21.Schmitto JD, Doerge H, Post H, Coulibaly M, Sellin C, Popov AF, et al. Progressive right ventricular failure is not explained by myocardial ischemia in a pig model of right ventricular pressure overload. Eur J Cardiothorac Surg. 2009;35:229–34. doi: 10.1016/j.ejcts.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Zarrabi K, Mollazadeh R, Ostovan MA, Abdi Ardekani AR. Retrograde pulmonary embolectomy in 11 patients. Ann Thorac Surg. 2008;85:1471–72. doi: 10.1016/j.athoracsur.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Zarrabi K, Yarmohammadi H, Ostovan MA. Retrograde pulmonary embolectomy in massive pulmonary embolism. Eur J Cardiothorac Surg. 2005;28:897–99. doi: 10.1016/j.ejcts.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 24.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573–619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]


