Abstract
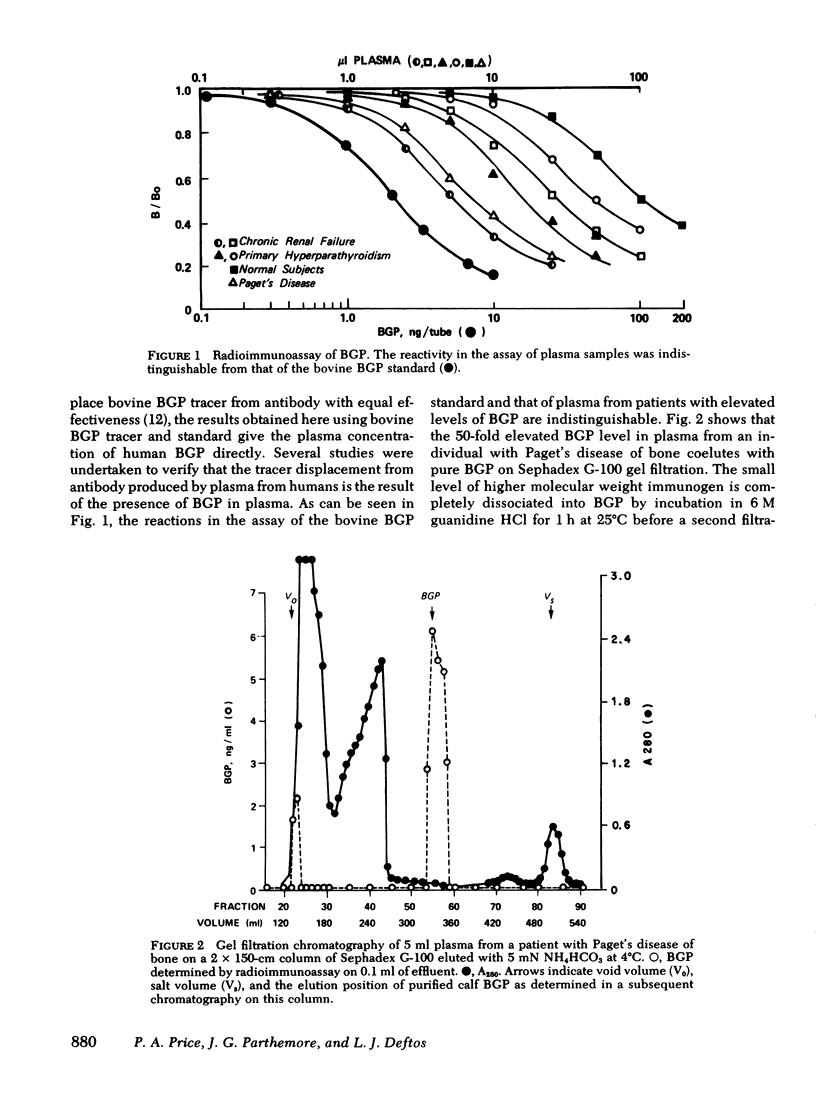
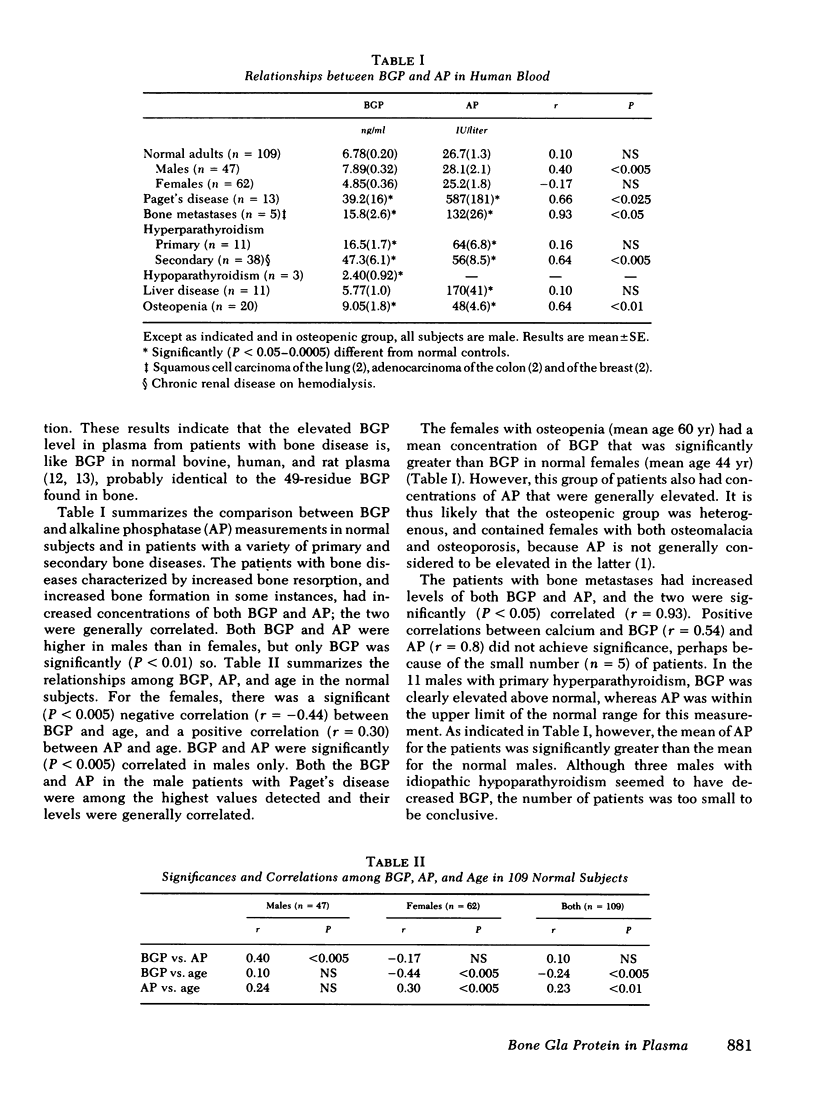
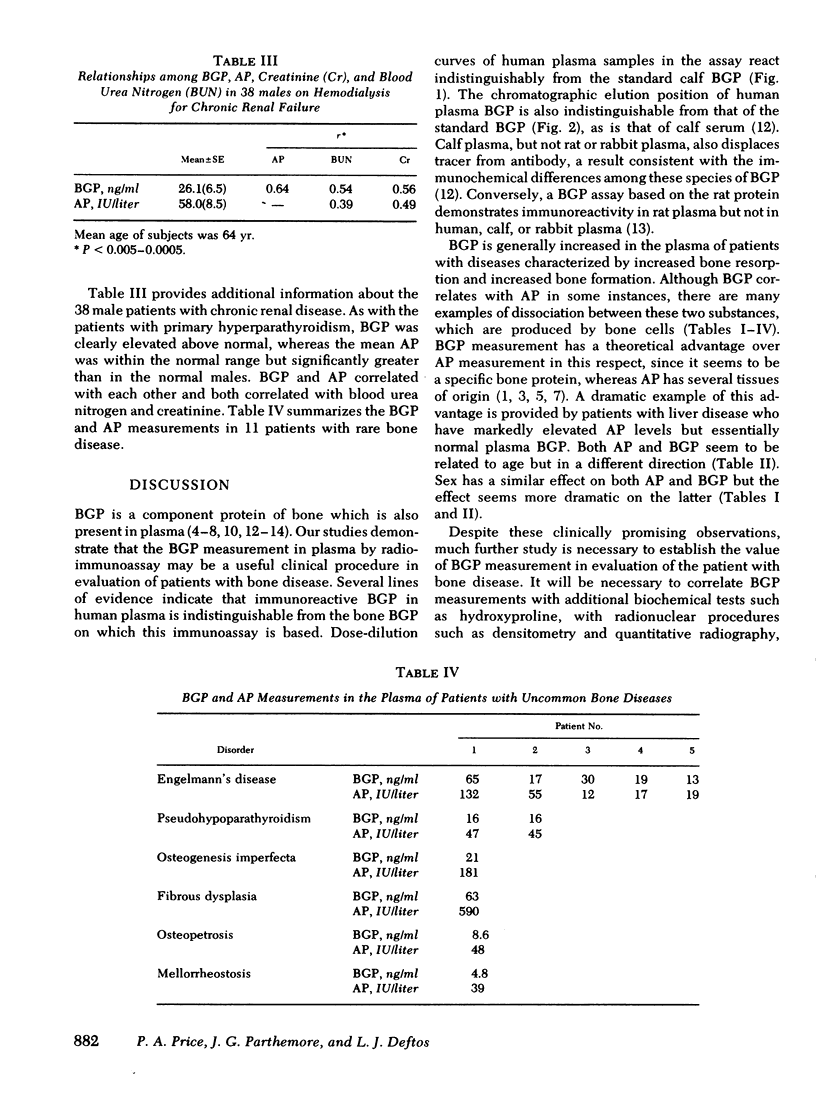
gamma-Carboxyglutamic acid-containing protein of bone (BGP) is an abundant noncollagenous protein of mammalian bone. BGP has a molecular weight of 5,800 and contains three residues of the vitamin K-dependent amino acid, gamma-carboxyglutamic acid. We have applied a radioimmunoassay based on calf BGP for the measurement of the protein in the plasma of 109 normal humans and 112 patients with various bone diseases. BGP in human plasma was demonstrated to be indistinguishable from calf BGP by assay dilution studies and gel permeation chromatography. The mean (+/- SE) concentration of BGP in normal subjects was 6.78 (+/- 0.20) ng/ml, 7.89 (+/- 0.32) for males and 4.85 (+/- 0.35) for females. Plasma BGP was increased in patients with Paget's disease of bone, bone metastases, primary hyperparathyroidism, renal osteodystrophy, and osteopenia. Plasma BGP did correlate with plasma alkaline phosphatase (AP) in some instances, but there were dissociations between the two. It was additionally observed that patients with liver disease had normal plasma BGP despite increased plasma AP, a reflection of the lack of specificity of AP measurements for bone disease. Our studies indicate that the radioimmunoassay of plasma BGP can be a useful and specific procedure for evaluating the patient with bone disease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Deftos L. J., Roos B. A., Parthemore J. G. Calcium and skeletal metabolism. West J Med. 1975 Dec;123(6):447–458. [PMC free article] [PubMed] [Google Scholar]
- Hauschka P. V., Lian J. B., Gallop P. M. Direct identification of the calcium-binding amino acid, gamma-carboxyglutamate, in mineralized tissue. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3925–3929. doi: 10.1073/pnas.72.10.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luben R. A., Wong G. L., Cohn D. V. Biochemical characterization with parathormone and calcitonin of isolated bone cells: provisional identification of osteoclasts and osteoblasts. Endocrinology. 1976 Aug;99(2):526–534. doi: 10.1210/endo-99-2-526. [DOI] [PubMed] [Google Scholar]
- Nishimoto S. K., Price P. A. Proof that the gamma-carboxyglutamic acid-containing bone protein is synthesized in calf bone. Comparative synthesis rate and effect of coumadin on synthesis. J Biol Chem. 1979 Jan 25;254(2):437–441. [PubMed] [Google Scholar]
- Nishimoto S. K., Price P. A. Secretion of the vitamin K-dependent protein of bone by rat osteosarcoma cells. Evidence for an intracellular precursor. J Biol Chem. 1980 Jul 25;255(14):6579–6583. [PubMed] [Google Scholar]
- Poser J. W., Price P. A. A method for decarboxylation of gamma-carboxyglutamic acid in proteins. Properties of the decarboxylated gamma-carboxyglutamic acid protein from calf bone. J Biol Chem. 1979 Jan 25;254(2):431–436. [PubMed] [Google Scholar]
- Price P. A., Lothringer J. W., Nishimoto S. K. Absence of the vitamin K-dependent bone protein in fetal rat mineral. Evidence for another gamma-carboxyglutamic acid-containing component in bone. J Biol Chem. 1980 Apr 10;255(7):2938–2942. [PubMed] [Google Scholar]
- Price P. A., Nishimoto S. K. Radioimmunoassay for the vitamin K-dependent protein of bone and its discovery in plasma. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2234–2238. doi: 10.1073/pnas.77.4.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price P. A., Otsuka A. A., Poser J. W., Kristaponis J., Raman N. Characterization of a gamma-carboxyglutamic acid-containing protein from bone. Proc Natl Acad Sci U S A. 1976 May;73(5):1447–1451. doi: 10.1073/pnas.73.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price P. A., Poser J. W., Raman N. Primary structure of the gamma-carboxyglutamic acid-containing protein from bovine bone. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3374–3375. doi: 10.1073/pnas.73.10.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I., Tuderman L., Guzman N. A. The biosynthesis of collagen and its disorders (first of two parts). N Engl J Med. 1979 Jul 5;301(1):13–23. doi: 10.1056/NEJM197907053010104. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Kemmler W., Tager H. S., Peterson J. D. Proteolytic processing in the biosynthesis of insulin and other proteins. Fed Proc. 1974 Oct;33(10):2105–2115. [PubMed] [Google Scholar]
- Tzehoval E., Segal S., Stabinsky Y., Fridkin M., Spirer Z., Feldman M. Tuftsin (an Ig-associated tetrapeptide) triggers the immunogenic function of macrophages: implications for activation of programmed cells. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3400–3404. doi: 10.1073/pnas.75.7.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]


