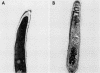
Abstract
In previous studies, we noted that Candida hyphae and pseudohyphae could be damaged and probably killed by neutrophils, primarily by oxygen-dependent nonphagocytic mechanisms. In extending these studies, amount of damage to hyphae again was measured by inhibition of [14C]cytosine uptake. Neutrophils from only one of four patients with chronic granulomatous disease damaged hyphae at all, and neutrophils from this single patient damaged hyphae far less efficiently than simultaneously tested neutrophils from normal control subjects. Neutrophils from neither of two subjects with hereditary myeloperoxidase deficiency damaged the hyphae. This confirmed the importance of oxidative mechanisms in general and myeloperoxidase-mediated systems in particular in damaging Candida hyphae.
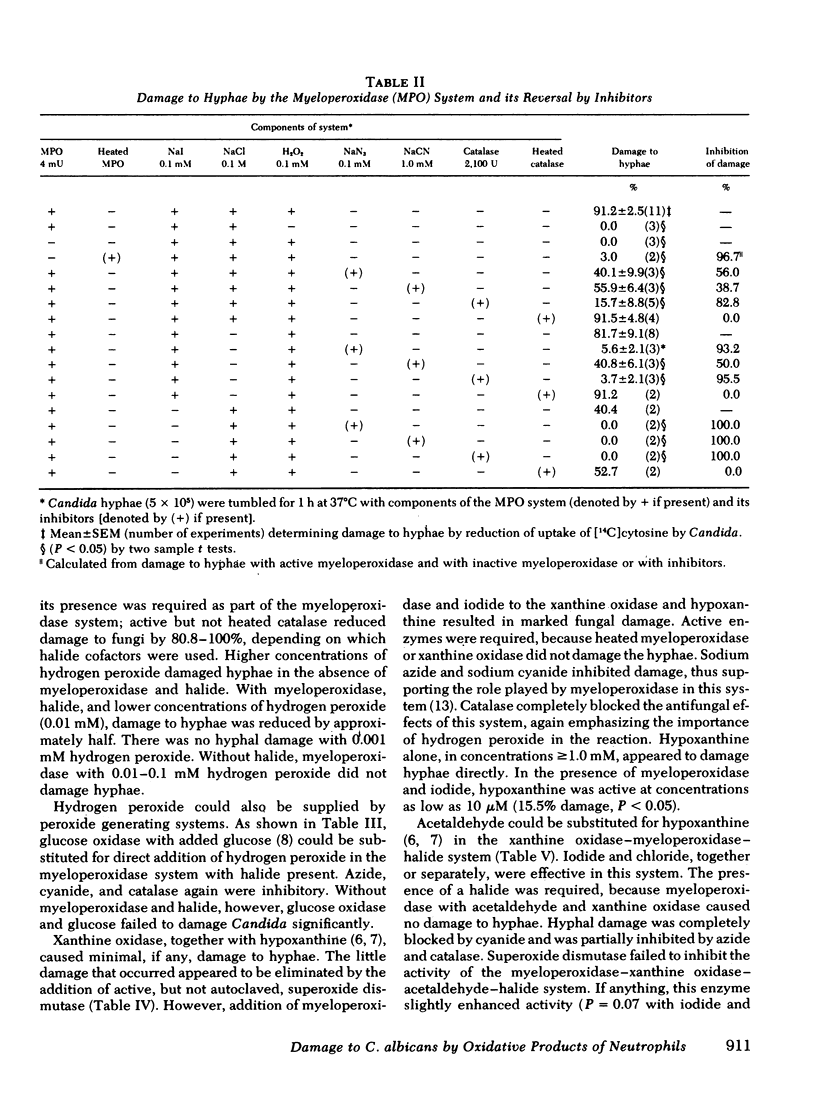
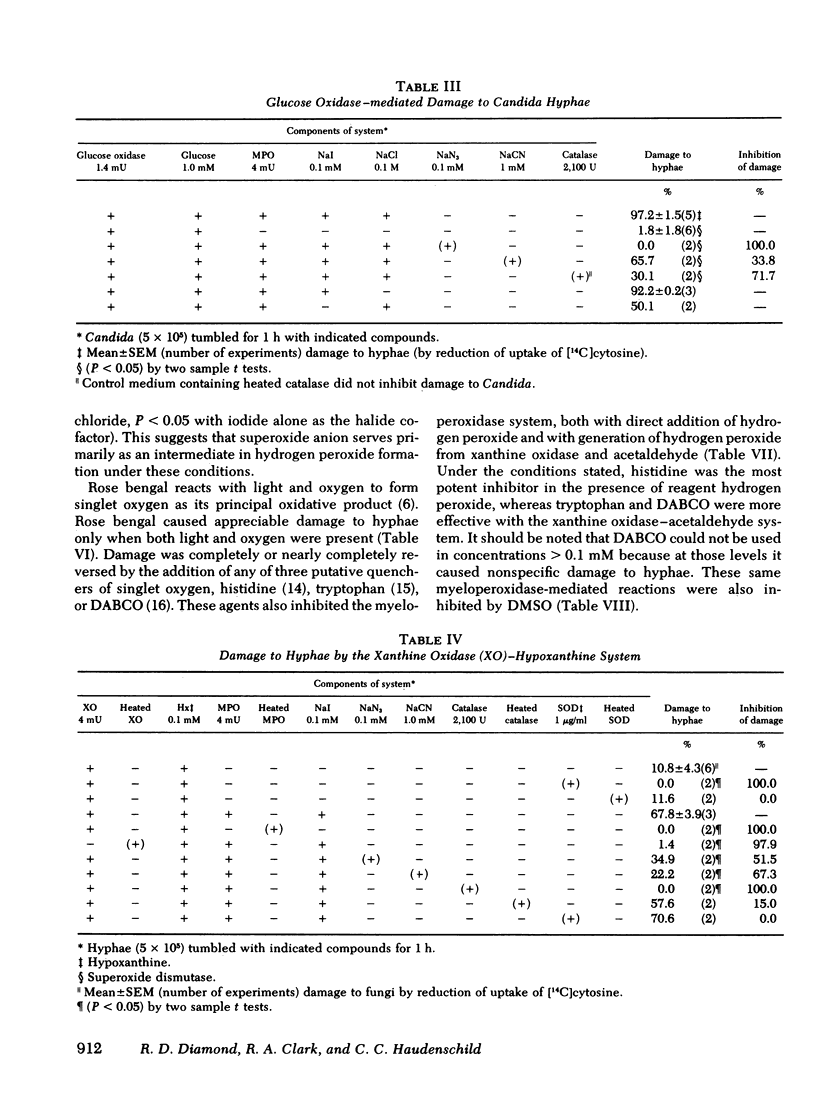
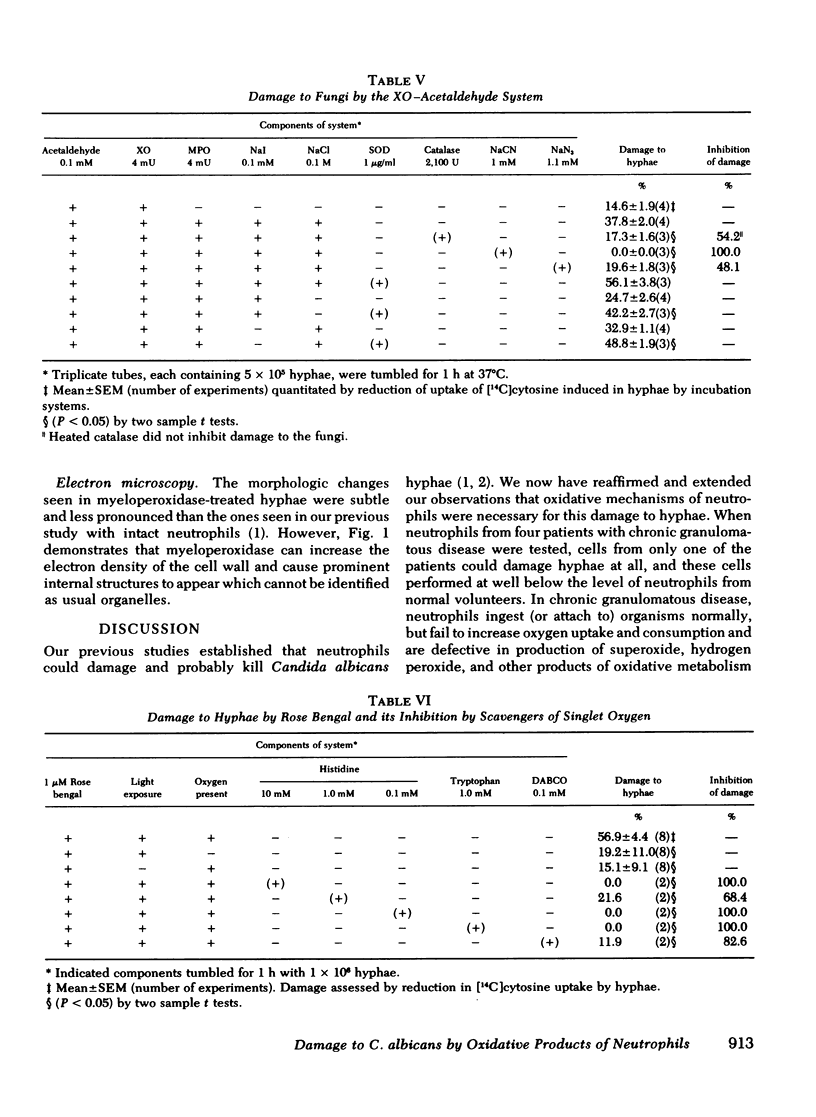
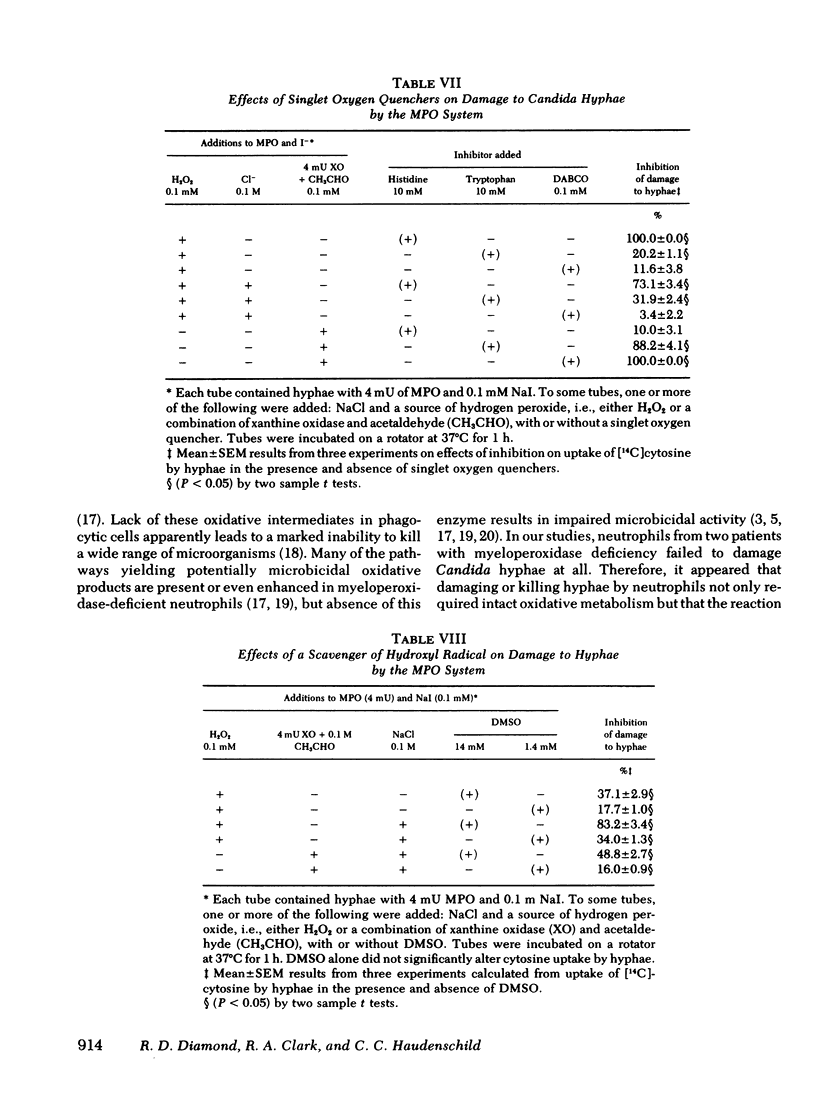
Several potentially fungicidal oxidative intermediates are produced by metabolic pathways of normal neutrophils, but their relative toxicity for Candida hyphae was previously unknown. To help determine this, cell-free in vitro systems were used to generate these potentially microbicidal products. Myeloperoxidase with hydrogen peroxide, iodide, and chloride resulted in 91.2% damage to hyphal inocula in 11 experiments. There was less damage when either chloride or iodide was omitted, and no damage when myeloperoxidase was omitted or inactivated by heating. Azide, cyanide, and catalase (but not heated catalase) inhibited the damage. Systems for generation of hydrogen peroxide could replace reagent hydrogen peroxide in the myeloperoxidase system. These included glucose oxidase, in the presence of glucose, and xanthine oxidase, in the presence of either hypoxanthine or acetaldehyde. In the presence of myeloperoxidase and a halide, the toxicity of the xanthine oxidase system was not inhibited by superoxide dismutase and, under some conditions, was marginally increased by this enzyme. This suggested that superoxide radical did not damage hyphae directly but served primarily as an intermediate in the production of hydrogen peroxide. The possible damage to hyphae by singlet oxygen was examined using photoactivation of rose bengal. This dye damaged hyphae in the presence of light and oxygen. The effect was almost completely inhibited by putative quenchers of singlet oxygen: histidine, tryptophan, and 1,4-diazobicyclo[2.2.2]octane. These agents also inhibited damage to hyphae by myeloperoxidase, halide, and either hydrogen peroxide or a peroxide source (xanthine oxidase plus acetaldehyde). Myeloperoxidase-mediated damage to hyphae was also inhibited by dimethyl sulfoxide, an antioxidant and scavenger of the hydroxyl radical.
These data support the involvement of oxidative mechanisms and the myeloperoxidase-H2O2-halide system, in particular in damaging hyphae in vitro and perhaps in vivo as well.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978 Mar 30;298(13):721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Myeloperoxidase--H2O2--halide system: cytotoxic effect on human blood leukocytes. Blood. 1977 Jul;50(1):65–70. [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Neutrophil-platelet interaction mediated by myeloperoxidase and hydrogen peroxide. J Immunol. 1980 Jan;124(1):399–405. [PubMed] [Google Scholar]
- Daimond R. D., Krzesicki R. Mechanisms of attachment of neutrophils to Candida albicans pseudohyphae in the absence of serum, and of subsequent damage to pseudohyphae by microbicidal processes of neutrophils in vitro. J Clin Invest. 1978 Feb;61(2):360–369. doi: 10.1172/JCI108946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Krzesicki R., Jao W. Damage to pseudohyphal forms of Candida albicans by neutrophils in the absence of serum in vitro. J Clin Invest. 1978 Feb;61(2):349–359. doi: 10.1172/JCI108945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J. E., Watson B. D., Schultz J. Myeloperoxidase and singlet oxygen: a reappraisal. FEBS Lett. 1978 Aug 15;92(2):327–332. doi: 10.1016/0014-5793(78)80780-7. [DOI] [PubMed] [Google Scholar]
- Held A. M., Hurst J. K. Ambiguity associated with use of singlet oxygen trapping agents in myeloperoxidase-catalyzed oxidations. Biochem Biophys Res Commun. 1978 Apr 14;81(3):878–885. doi: 10.1016/0006-291x(78)91433-x. [DOI] [PubMed] [Google Scholar]
- Hodgson E. K., Fridovich I. The production of superoxide radical during the decomposition of potassium peroxochromate(V). Biochemistry. 1974 Aug 27;13(18):3811–3815. doi: 10.1021/bi00715a030. [DOI] [PubMed] [Google Scholar]
- Kim M. H., Rodey G. E., Good R. A., Chilgren R. A., Quie P. G. Defective candidacidal capacity of polymorphonuclear leukocytes in chronic granulomatous disease of childhood. J Pediatr. 1969 Aug;75(2):300–303. doi: 10.1016/s0022-3476(69)80403-8. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Clark R. A. Hemolysis and iodination of erythrocyte components by a myeloperoxidase-mediated system. Blood. 1975 May;45(5):699–707. [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase: contribution to the microbicidal activity of intact leukocytes. Science. 1970 Sep 11;169(3950):1095–1097. doi: 10.1126/science.169.3950.1095. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Rosen H. Ethylene formation by polymorphonuclear leukocytes. Role of myeloperoxidase. J Exp Med. 1978 Aug 1;148(2):490–506. doi: 10.1084/jem.148.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. Antifungal effects of peroxidase systems. J Bacteriol. 1969 Aug;99(2):361–365. doi: 10.1128/jb.99.2.361-365.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969 Aug;48(8):1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. Measurement of candidacidal activity of specific leukocyte types in mixed cell populations I. Normal, myeloperoxidase-deficient, and chronic granulomatous disease neutrophils. Infect Immun. 1970 Jul;2(1):42–47. doi: 10.1128/iai.2.1.42-47.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. The fungicidal mechanisms of human monocytes. I. Evidence for myeloperoxidase-linked and myeloperoxidase-independent candidacidal mechanisms. J Clin Invest. 1975 Feb;55(2):338–346. doi: 10.1172/JCI107937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K., Ishiwara K., Noguchi Y., Takahashi T., Tadokoro I. New type of antibody-enzyme conjugate which specifically kills Candida albicans. Infect Immun. 1980 Feb;27(2):690–692. doi: 10.1128/iai.27.2.690-692.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., Eaton J. W., Anders M. W., Hoidal J. R., Fox R. B. Generation of hydroxyl radical by enzymes, chemicals, and human phagocytes in vitro. Detection with the anti-inflammatory agent, dimethyl sulfoxide. J Clin Invest. 1979 Dec;64(6):1642–1651. doi: 10.1172/JCI109626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Bactericidal activity of a superoxide anion-generating system. A model for the polymorphonuclear leukocyte. J Exp Med. 1979 Jan 1;149(1):27–39. doi: 10.1084/jem.149.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Formation of singlet oxygen by the myeloperoxidase-mediated antimicrobial system. J Biol Chem. 1977 Jul 25;252(14):4803–4810. [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Hydroxyl radical generation by polymorphonuclear leukocytes measured by electron spin resonance spectroscopy. J Clin Invest. 1979 Dec;64(6):1725–1729. doi: 10.1172/JCI109637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Vadasz J. A. Singlet oxygen: a major reactive species in the furocoumarin photosensitized inactivation of E. coli ribosomes. Photochem Photobiol. 1978 Oct-Nov;28(4-5):539–545. doi: 10.1111/j.1751-1097.1978.tb06966.x. [DOI] [PubMed] [Google Scholar]
- Tauber A. I., Babior B. M. Evidence for hydroxyl radical production by human neutrophils. J Clin Invest. 1977 Aug;60(2):374–379. doi: 10.1172/JCI108786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Rustagi P. K., LoBuglio A. F. Human granulocyte generation of hydroxyl radical. J Exp Med. 1978 Feb 1;147(2):316–323. doi: 10.1084/jem.147.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]