Abstract
Pseudoryzomys simplex, the false rice rat, is a monotypic genus of the Oryzomyini tribe (Sigmodontinae) distributed in part of Bolivia, Paraguay, Argentina and Brazil. Its diploid number has been described as 56 acrocentric chromosomes decreasing in size and no karyotype figure has been depicted. Herein, we present karyotypic data on P. simplex, including chromosome banding and molecular fluorescent in situ hybridization using telomeric sequences and the whole X-chromosome of its sister clade Holochilus brasiliensis (HBR) as probes. A case of remarkable autosomal heteromorphism due to the presence of a whole heterochromatic arm leading to the variability of FN is reported, as well as the occurrence of regions of homology between the X and Y chromosomes (pseudoautosomal regions) after chromosome painting with the HBR X probe on P. simplex metaphases.
Keywords: cytogenetics, fluorescent in situ hybridization, heterochromatin, Oryzomyini
Introduction
The subfamily Sigmodontinae (Family Cricetidae), which comprises about 84 genera and is predominantly distributed in South America (D’Elía et al., 2007), is one of the most diverse and complex groups of the Neotropical mammals. Within Sigmodontinae, nine tribes are recognized, from which Oryzomyini is the most specious, with 33 genera and about 140 valid species (D’Elía et al., 2007; Percequillo et al., 2011; Pine et al., 2012). In addition, Oryzomyini displays an exceptional range of diploid and fundamental numbers, ranging from 2n = 16–17 and FN = 25–29 in Nectomys palmipes to 2n = 80 and FN = 140 in Oecomys bicolor, autosomes and sex chromosomes polymorphisms and supernumerary elements (Gardner and Patton, 1976; Almeida and Yonenaga-Yassuda, 1991; Barros et al., 1992; Silva and Yonenaga-Yassuda, 1998; Patton et al., 2000; Weksler, 2006). Pseudoryzomys, the false rice rat, is a poorly known monotypic genus of Oryzomyini, represented by P. simplex. This species has been found from eastern Bolivia, western Paraguay and northeastern Argentina to eastern Brazil, in the states of Mato Grosso,Goiás, Tocantins, Minas Gerais, São Paulo, Bahia and into the northeastern Alagoas and Pernambuco States (Voss and Myers, 1991; Musser and Carleton, 2005; Bonvicino et al., 2008; Brancalion and Percequillo, 2009). Phylogenetic analyses using morphological and molecular data supported a close relationship among Pseudoryzomys, Holochilus and Lundomys, three genera that share several morphological characters, including specializations towards a semiaquatic lifestyle, such as the presence of interdigital membranes (Weksler, 2006).
Cytogenetic data of Pseudoryzomys simplex is restricted to the description of a diploid number (2n) of 56 chromosomes and a fundamental number (FN) of 54, with exclusively acrocentric chromosomes that gradually decrease in size (Voss and Myers, 1991; Bonvicino et al., 2005). No karyotype has ever been presented and cytogenetic data from this genus are still scarce.
In this work, the karyotype of Pseudoryzomys simplex (PSI) was studied using conventional staining, CBG-and GTG-banding, Ag-NOR staining and fluorescent in situ hybridization (FISH) with telomeric sequences and with the whole X chromosome of Holochilus brasiliensis (HBR) as probes. A comparison of the GTG-banding patterns of P. simplex and of its closely related species H. brasiliensis (2n = 56; FN = 56) is also presented.
Material and Methods
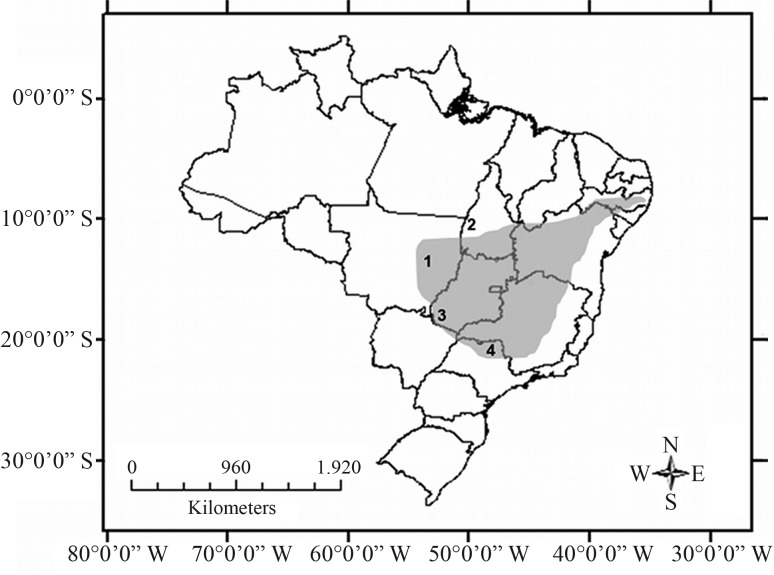
Seven specimens (one male and six females) from four Brazilian states (Goiás, Mato Grosso, São Paulo and Tocantins) were cytogenetically analyzed (Table 1, Figure 1). Chromosome preparations were obtained from bone marrow (Ford and Hamerton, 1956) or from fibroblasts cultured in Dulbecco’s modified Eagle’s medium, supplemented with 20% fetal bovine serum (Freshney, 1986).
Table 1.
Sex, diploid (2n) and fundamental numbers (FN), and collection localities of Pseudoryzomys simplex from Brazil.
| Specimen number | Sex | 2n | FN | Locality | Geographic coordinates | |
|---|---|---|---|---|---|---|
| CIT 603 | F | 56 | 54 | Gaúcha do Norte, MT | 13º11′00″ S | 53º15′23″ W |
| CIT 1152 | M | 56 | 55 | Parque Nacional do Araguaia, TO | 9°50′59″ S | 49°56′59″ W |
| CIT 1159 | F | 56 | 54 | Parque Nacional do Araguaia, TO | 9°50′59″ S | 49°56′59″ W |
| CIT 1168 | F | 56 | 54 | Parque Nacional do Araguaia, TO | 9°50′59″ S | 49°56′59″ W |
| CIT 1219 | F | 56 | 54 | Parque Nacional do Araguaia, TO | 9°50′59″ S | 49°56′59″ W |
| CIT 1333 | F | 56 | 54 | Parque Nacional das Emas, GO | 18°6′23″ S | 52°55′40″ W |
| ROD 286 | F | 56 | 55 | Guará, SP | 20°48′55″ S | 47°48′59″ W |
Brazilian states: MT = Mato Grosso; TO = Tocantins; GO = Goiás, SP = São Paulo.
F: female. M: male.
Figure 1.
Map of Brazil with the collecting localities of Pseudoryzomys simplex. 1. Gaúcha do Norte, Mato Grosso; 2. Parque Nacional do Araguaia, Tocantins; 3. Parque Nacional das Emas, Goiás; and 4. Guará, São Paulo. In gray, the distribution of the genus in Brazil, according to Bonvicino et al. (2008).
Metaphases were analyzed after Giemsa staining, CBG- and GTG-banding and Ag-NOR staining, performed according to routine techniques. For FISH with telomeric probes, the Dako Telomere PNA FISH Kit/FITC (code number 5325) was used following the recommended protocol.
In order to precisely identify the X chromosome, we used a whole X-chromosome painting probe obtained from the Oryzomyini species Holochilus brasiliensis (HBR). The probe was generated from flow-sorted chromosomes from HBR cells at the Cambridge Resource Centre for Comparative Genomics (Department of Veterinary Medicine, University of Cambridge, UK) and amplified by degenerate oligonucleotide-primed PCR (DOP-PCR). Hybridization of the whole HBR X probe was performed according to Yang et al. (1995). The cross-species hybridization was performed for 24 h at 37 °C. Post-hybridization washes included two 5-min incubations in 50% for-mamide/2×SSC at 42 °C followed by two 5 min incubations in 2×SSC and a 4 min in 4×T (100 mL 20×SSC + 400 mL H2O + 250 μL Triton X-100). The biotin-labeled probe was visualized with streptavidin-Cy3.
Results and Discussion
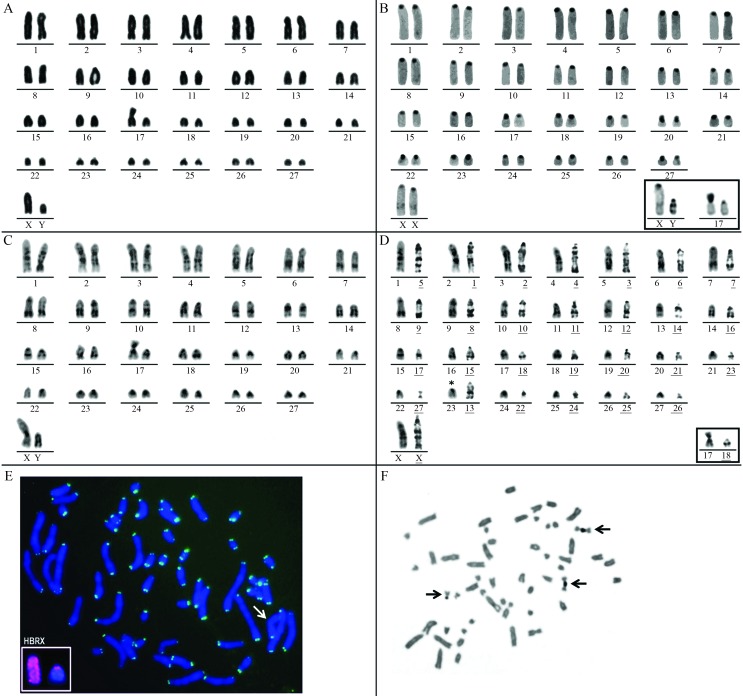
Pseudoryzomys simplex presented 2n = 56 and FN = 54–55. The individuals with FN = 54 presented only acrocentric autosomes decreasing in size. Two specimens (one male from Parque Nacional do Araguaia, TO, and one female from Guará, SP) showed FN = 55 due to the presence of one heteromorphic pair (17) with one metacentric and one acrocentric element (Table 1, Figure 2A). The X chromosome was a large acrocentric and the Y chromosome, a medium acrocentric.
Figure 2.
Karyotype analysis of Pseudoryzomys simplex and comparisons with other species. (A–C) Karyotypes of Pseudoryzomys simplex with 2n = 56. (A) Conventionally stained karyotype of a male with FN = 55; (B) CBG-banded karyotype of a female with FN = 54; inset: sex chromosomes of a male and heteromorphic pair 17; (C) GTG-banded karyotype of a male with FN = 55. (D) Comparison between the GTG-banded chromosomes of P. simplex with 2n = 56 (left) and H. brasiliensis with 2n = 56 (right with underlined numbers). (E–F) Metaphases of P. simplex with 2n = 56. (E) Male metaphase with FN = 55 after telomeric FISH; the arrow shows the biarmed element of the heteromorphic pair 17. Inset: The X and Y chromosomes of P. simplex after chromosome painting with the HBR X probe. * Partial homology detected. (F) After Ag-NOR staining. Five Ag-NORs-bearing chromosomes of PSI are indicated. Note the two chromosome associations.
The CBG-banded metaphases exhibited blocks of constitutive heterochromatin in the pericentromeric region of all autosomes. When pair 17 was heteromorphic, the metacentric element exhibited one entirely heterochromatic arm. The X chromosome presented pericentromeric heterochromatic blocks and an interstitial lightly stained CBG band on its long arm. The Y chromosome presented two conspicuous interstitial heterochromatic bands on its long arm, in addition to the pericentromeric heterochromatin (Figure 2B).
Oryzomyini species usually present easily recognizable sex chromosomes after CBG-banding. Large heterochromatic blocks at Xp or at the pericentromeric regions of acrocentric X chromosomes and almost entirely heterochromatic Y chromosomes have been described for Holochilus brasiliensis, Nectomys squamipes, Euryoryzomys russatus and Oligoryzomys nigripes (Yonenaga-Yassuda et al., 1987; Andrades-Miranda et al., 2000; Paresque et al., 2007). In P. simplex, the X chromosome is recognizable by the tenuous heterochromatic block in its long arm and the Y chromosome by the two interstitial CBG bands in Yq.
GTG-banding allowed the correct identification of all homologues, including the acrocentric and the metacentric elements of the heteromorphic pair 17 and the sex chromosomes (Figure 2C). Comparative analysis of the GTG-banded complements of PSI (2n = 56, FN = 54–55) and HBR (2n = 56, FN = 56) allowed the inference of partial homologies between both karyotypes. A complete homology was verified between 26 autosomes of PSI and HBR, but only partial homology could be inferred between PSI 23 and HBR 13. Besides, the metacentric HBR 27 is similar in size and GTG-banding pattern to the acrocentric PSI 22, suggesting that these chromosomes differ due to a pericentric inversion. Despite the morphological differences of the X chromosomes (subtelocentric in HBR and acrocentric in PSI), their GTG-banding patterns were similar, supporting the idea that the mammalian X chromosome is conserved (Figure 2D). The GTG-banded Y chromosomes could not be compared because they did not present a definite banding pattern.
The high similarity of GTG-banding patterns between the autosomes of P. simplex and H. brasiliensis is in accordance to their close phylogenetic relationship (Weksler, 2006). However, comparative analyses based on GTG-banding may be ineffective because some homologous regions escape detection. For instance, GTG-banding comparisons between Akodon sp. (2n = 10) and A. cursor (2n = 16) (Silva et al., 2006) did not allow the disclosure of complete homology or of the occurrence of the high complex rearrangements between both karyotypes, which could only be detected after cross-species chromosome painting (Ventura et al., 2009).
FISH with telomeric sequences revealed signals exclusively at the ends of all chromosome arms and no interstitial signals were observed (Figure 2E). Interstitial telomeric signals co-localized with regions of constitutive heterochromatin have been described in autosomes and supernumerary chromosomes of some marsupials and rodent species, probably due to amplification of (TTAGGG)n sequences in these regions (Silva and Yonenaga-Yassuda, 1998; Pagnozzi et al., 2002; Ventura et al., 2006). Nevertheless, an amplified non-telomeric heterochromatin (Multani et al., 2001) is found in the heteromorphic pair 17 of P. simplex.
The hybridization of the HBR X painting probe on P. simplex metaphases confirmed the acrocentric form and large size of the X chromosome. The HBR X paint hybridized on the whole PSI X corroborating the conservation of the mammalian X chromosome. The same probe also hybridized to the pericentromeric region of the PSI Y and produced a weak interstitial signal on Yq (Figure 2E, inset), evidencing the pseudoautosomal region (PAR) of the PSI Y. A similar case has been reported for the akodontine genus Oxymycterus, although in that case chromosome painting using an Akodon paranaensis Y chromosome probe allowed the detection of the PAR on both sex chromosomes (whole Xp and Yq) (Ventura et al., 2012). Multiple Ag-NORs, varying from five to eight were localized at the telomeric regions of the short arms of small autosomes. Associations involving two and three chromosomes were frequent (Figure 2F).
Pseudoryzomys simplex has been collected in localities in eastern and central Brazil (Voss and Myers, 1991; Musser and Carleton, 2005; Bonvicino et al., 2008). The specimens collected in Parque Nacional do Araguaia, Tocantins State, extend the northernmost distribution of the species in this state and the specimen from Guará represents the third record of the genus in the State of São Paulo (Brancalion and Percequillo, 2009).
The cytogenetic data presented herein for five specimens of P. simplex with 2n = 56 and FN = 54 is in accordance with previous reports (Voss and Myers, 1991; Bonvicino et al., 2005). In addition, a new karyotype with 2n = 56 and FN = 55 is being described for two individuals, one male from Tocantins and one female from São Paulo. The difference in FN resulted from a heteromorphism due to the presence of a single biarmed element in this karyotype. The GTG-banding patterns allowed the identification of this chromosome as a homologue of pair 17 and the CBG-banding evidenced the addition/amplification of constitutive heterochromatin, as one chromosome arm of the metacentric homologue is entirely heterochromatic. The presence of the heteromorphic pair 17 in two individuals collected in different localities, one in São Paulo and another in Tocantins, may indicate the occurrence of chromosome polymorphisms in Pseudoryzomys simplex.
Among the deer mice Peromyscus spp., heterochromatic whole-arm additions to an otherwise mostly acrocentric chromosomes karyotype has been reported for a few species and for groups of species that exhibit short heterochromatic arms on different chromosomes, which are frequently polymorphic for this character (Greenbaum et al., 1994).
A similar case of chromosome heteromorphism has been recently described by Bezerra et al. (2012) for the spiny rat Clyomys laticeps. The authors described a new karyotype for the species with 2n = 32; FN = 54, as well as the presence of a heteromorphic pair in which only one homologue presented a large heterochromatic block. However, differently from what was observed in P. simplex, the heteromorphic heterochromatic block in Clyomys was reported in an acrocentric pair, causing a difference in size between both homologues, but no variation in FN. A similar case has also been observed in the akodontine Thaptomys (Ventura et al., 2004).
An increase in sampling efforts and the application of molecular cytogenetic techniques as chromosome painting using the microdissected heterochromatic arm of the metacentric element of PSI 17 as probe will help in establishing the complete homology between PSI and its sister clade HBR, to better characterize this unusual heteromorphism and to understand the nature of the amplified heterochromatic region.
Acknowledgments
We thank Drs. Miguel T. Rodrigues, Ana Paula Carmignotto, Flávio H. G. Rodrigues and Ligia Pina for collecting the specimens, Angela Vianna Morgante for providing the facilities for cell culture and Valéria Fagundes for some of the chromosome preparations. Grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 09/54300-0 for KV; 10/03432-0 for CBDN; 05/04557-3 for MJJS) made this work possible.
Footnotes
Associate Editor: Marcelo Guerra
References
- Almeida EJC, Yonenaga-Yassuda Y. Pericentric inversions and sex chromosome heteromorphisms in Oryzomys nigripes (Rodentia, Cricetidae) Caryologia. 1991;44:63–73. [Google Scholar]
- Andrades-Miranda J, Zanchin NIT, Oliveira LFB, Langguth AR, Mattevi MS. Cytogenetic studies in nine taxa of the genus Oryzomys (Rodentia, Sigmodontinae) from Brazil. Mammalia. 2000;65:461–472. [Google Scholar]
- Barros MA, Reig OA, Perez-Zapata A. Cytogenetics and karyosystematics of South American Oryzomyine rodents (Cricetidae, Sigmodontinae) Cytogenet Cell Genet. 1992;59:34–38. doi: 10.1159/000133195. [DOI] [PubMed] [Google Scholar]
- Brancalion BB, Percequillo AR. Pseudoryzomys simplex (Winge, 1887) Rodentia, Cricetidae. In: Bressan PM, Kierulff MCM, Sugieda AM, editors. Fauna Ameaçada de Extinção no Estado de São Paulo - Vertebrados. Fundação Parque Zoológico de São Paulo, Secretaria do Meio Ambiente; 2009. p. 76. [Google Scholar]
- Bonvicino CR, Lemos B, Weksler M. Small mammals of Chapada dos Veadeiros National Park (Cerrado of Central Brazil): Ecologic, karyologic, and taxonomic considerations. Braz J Biol. 2005;65:395–406. doi: 10.1590/s1519-69842005000300004. [DOI] [PubMed] [Google Scholar]
- Bonvicino CR, Oliveira JA, D’Andrea PS. Guia dos Roedores do Brasil: Com chaves para gêneros baseadas em caracteres externos. Centro Pan-Americano de Febre Aftosa-OPAS/OMS; Rio de Janeiro: 2008. p. 49. [Google Scholar]
- Bezerra AMR, Pagnozzi JM, Carmignotto AP, Yonenaga-Yassuda Y, Rodrigues FHG. A new karyotype for the spiny rat Clyomys laticeps (Thomas, 1909) (Rodentia, Echimyidae) from Central Brazil. Comp Cytogenet. 2012;6:153–161. doi: 10.3897/CompCytogen.v6i2.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Elía G, Pardiñas UFJ, Teta P, Patton JL. Definition and diagnosis of a new tribe of sigmodontine rodents (Cricetidae, Sigmodontinae), and a revised classification of the subfamily. Gayana. 2007;71:187–194. [Google Scholar]
- Ford CE, Hamerton JL. A colchicine hypotonic-citrate squash sequence for mammalian chromosome. Stain Technol. 1956;31:247–251. doi: 10.3109/10520295609113814. [DOI] [PubMed] [Google Scholar]
- Freshney RI. Animal Cell Culture - A Practical Approach. IRL Press; Oxford: 1986. p. 247. [Google Scholar]
- Gardner AL, Patton JL. Karyotypic variation in Oryzomyini rodents (Cricetidae) with comments on chromosomal evolution in the Neotropical cricetine complex. Occas Pap Mus Zool Univ Mich. 1976;49:1–48. [Google Scholar]
- Greenbaum IF, Gunn SJ, Smith SA, McAllister BF, Hale DW, Baker RJ, Engstrom MD, Hamilton MJ, Modi WS, Robbins LW, et al. Cytogenetic nomenclature of deer mice, Peromyscus (Rodentia): Revision and review of the standardized karyotype. Cytogenet Cell Genet. 1994;66:181–195. doi: 10.1159/000133696. [DOI] [PubMed] [Google Scholar]
- Multani AS, Ozen M, Furlong CL, Zhao YJ, Hsu TC, Pathak S. Heterochromatin and interstitial telomeric DNA homology. Chromosoma. 2001;110:214–220. doi: 10.1007/s004120100133. [DOI] [PubMed] [Google Scholar]
- Musser GG, Carleton MD. Super Family Muroidea. In: Wilson DE, Redeer DM, editors. Mammal Species of the World: A Taxonomic and Geographic Reference. 3rd edition. Johns Hopkins University Press; Baltimore: 2005. pp. 894–1531. [Google Scholar]
- Pagnozzi JM, Ditchfield AD, Yonenaga-Yassuda Y. Mapping the distribution of the interstitial telomeric (TTAGGG)n sequences in eight species of Brazilian marsupials (Didelphidae) by FISH and the correlation with constitutive heterochromatin. Do ITS represent evidence for fusion events in American marsupials? Cytogenet Genome Res. 2002;98:278–284. doi: 10.1159/000071049. [DOI] [PubMed] [Google Scholar]
- Paresque R, Silva MJJ, Yonenaga-Yassuda Y, Fagundes V. Karyological geographic variation of Oligoryzomys nigripes Olfers, 1818 (Rodentia, Cricetidae) from Brazil. Genet Mol Biol. 2007;30:43–53. [Google Scholar]
- Patton JL, Silva MNF, Malcolm JR. Mammals of the Rio Juruá and the evolutionary and ecological diversification of Amazonia. Bull Am Mus Nat Hist. 2000;244:1–306. [Google Scholar]
- Percequillo AR, Weksler M, Costa LP. A new genus and species of rodent from the Brazilian Atlantic Forest (Rodentia, Cricetidae, Sigmodontinae, Oryzomyini), with comments on oryzomyine biogeography. Zool J Linn Soc. 2011;161:357–390. [Google Scholar]
- Pine RH, Timm RM, Weksler M. A newly recognized clade of trans-Andean Oryzomyini (Rodentia, Cricetidae), with description of a new genus. J Mammal. 2012;93:851–868. [Google Scholar]
- Silva MJJ, Yonenaga-Yassuda Y. Heterogeneity and meiotic behaviour of B and sex chromosomes, banding patterns and localization of (TTAGGG)n sequences by fluorescence in situ hybridization in the neotropical water rat Nectomys (Rodentia, Cricetidae) Chromosome Res. 1998;6:455–462. doi: 10.1023/a:1009248311530. [DOI] [PubMed] [Google Scholar]
- Silva MJJ, Patton JL, Yonenaga-Yassuda Y. Phylogenetic relationships and karyotype evolution in the sigmodontine rodent Akodon (2n = 10 and 2n = 16) from Brazil. Genet Mol Biol. 2006;29:469–474. [Google Scholar]
- Ventura K, Silva MJJ, Fagundes V, Pardini R, Yonenaga-Yassuda Y. An undescribed karyotype for Thaptomys (2n = 50) and the mechanism of differentiation from Thaptomys nigrita (2n = 52) evidenced by FISH and Ag-NORs. Caryologia. 2004;57:89–97. [Google Scholar]
- Ventura K, Silva MJJ, Fagundes V, Christoff AU, Yonenaga-Yassuda Y. Non-telomeric sites as evidence of chromosomal rearrangement and repetitive (TTAGGG)n arrays in heterochromatic and euchromatic regions in four species of Akodon (Rodentia, Muridae) Cytogenet Genome Res. 2006;115:169–175. doi: 10.1159/000095238. [DOI] [PubMed] [Google Scholar]
- Ventura K, O’Brien PCM, Yonenaga-Yassuda Y, Ferguson-Smith MA. Chromosome homologies of the highly rearranged karyotypes of four Akodon species (Rodentia, Cricetidae) resolved by reciprocal chromosome painting: The evolution of the lowest diploid number in rodents. Chromosome Res. 2009;17:1063–1078. doi: 10.1007/s10577-009-9083-5. [DOI] [PubMed] [Google Scholar]
- Ventura K, Yonenaga-Yassuda Y, Ferguson-Smith MA. Variable patterns of Y chromosome homology in Akodontini rodents (Sigmodontinae): A phylogenetic signal revealed by chromosome painting. Chromosome Res. 2012;20:427–433. doi: 10.1007/s10577-012-9286-z. [DOI] [PubMed] [Google Scholar]
- Voss RS, Myers P. Pseudoryzomys simplex (Rodentia, Muridae) and the Significance of Lund’s Colletions from the Caves of Lagoa Santa, Brazil. Bull Am Mus Nat Hist. 1991;206:414–432. [Google Scholar]
- Weksler M. Phylogenetic relationships of oryzomine rodents (Muroidea, Sigmodontinae): Separate and combined analyses of morphological and molecular data. Bull Am Mus Nat Hist. 2006;296:1–149. [Google Scholar]
- Yang F, Carter NP, Shi L, Ferguson-Smith MA. A comparative study of karyotypes of muntjacs by chromosome painting. Chromosoma. 1995;103:642–652. doi: 10.1007/BF00357691. [DOI] [PubMed] [Google Scholar]
- Yonenaga-Yassuda Y, Prado RC, Mello DA. Supernumerary chromosome in Holochilus brasiliensis and comparative cytogenetic analysis with Nectomys squamipes (Cricetidae, Rodentia) Rev Bras Genet. 1987;2:209–220. [Google Scholar]