Abstract
The primary symptom of fibromyalgia (FM) is chronic, widespread pain; however, patients report additional symptoms including decreased concentration and memory. Performance based deficits are seen mainly in tests of working memory and executive function. Neural correlates of executive function were investigated in 18 FM patients and 14 age-matched HCs during a simple go/no-go task (response inhibition) while they underwent functional magnetic resonance imaging (fMRI). Performance was not different between FM and HC, in either reaction time or accuracy. However, fMRI revealed that FM patients had lower activation in the right pre-motor cortex, supplementary motor area (SMA), mid cingulate cortex (MCC), putamen and, after controlling for anxiety, in the right insular cortex (IC) and right inferior frontal gyrus (IFG). A hyper-activation in FM patients was seen in the right inferior temporal gyrus/fusiform gyrus. Despite the same RTs and accuracy, FM patients show less brain activation in cortical structures in the inhibition network (specifically in areas involved in response selection/motor preparation) and the attention network along with increased activation in brain areas not normally part of the inhibition network. We hypothesize that response -inhibition and pain perception may rely on partially overlapping networks, and that in chronic pain patients resources taken up by pain processing may not be available for executive functioning tasks such as response inhibition. Compensatory cortical plasticity may be required to achieve performance on par with control groups.
Keywords: pain, fibromyalgia, fMRI, executive function, response inhibition
Introduction
The primary symptom of fibromyalgia (FM) is chronic, widespread pain accompanied by diffuse tenderness.48, 49 Individuals with FM, however, often report additional complaints and symptoms including perturbed mood, fatigue, disturbed sleep, reduced functional status, stiffness, and problems with concentration and memory.18, 19, 29 This latter complaint, problems with memory and concentration is termed “fibrofog” in patient parlance and “dyscognition” in the medical literature. Performance based tests of dyscognition in FM find deficits in working memory, executive control and attention.12, 13, 18, 26, 32 These deficits are most often found in tasks with high demands on attention (e.g., when distraction is present). Performance differences are not always observed in less demanding tasks.
Executive control is an umbrella term for many cognitive processes, including maintenance of long-term goals, planning, the ability to ignore distracting information, and to suppress inappropriate responses. As such, executive control is an important part of the working memory system, responsible for maintaining relevant items in the short-term memory store, removing items no longer needed, and ignoring items that are not relevant to the task at hand. Since individuals with FM rarely perform poorly on tests of short-term memory storage,23, 26 it is likely that memory problems in FM are attributable to deficits in executive functions. Likewise, executive deficits in the ability to inhibit pre-potent or habitual responses may contribute to the poor performance observed in FM on attention control tests.12, 13, 18, 26 Furthermore, failures in executive function leading to a susceptibility to distraction could make cognitive problems very troubling for FM patients in real-world settings where distractions are ubiquitous.12, 13 Thus, neuroimaging studies of brain function during executive function tasks could be very informative about the nature of cognitive dysfunction in these patients.
Several neuroimaging studies have documented altered brain activity in FM compared to healthy controls (HC) during painful stimulation; (i.e. individuals with FM showed augmented activity in cortical areas of the brain associated with pain perception).3, 9, 20 Specifically, the magnitude of the neuronal activation in these cortical regions involved in pain processing was greater than seen in controls given the same intensity stimulus. It is important to note that many cortical regions involved in the processing of pain are also involved in other sensory and cognitive tasks (i.e. prefrontal cortex, anterior and mid cingulate cortex (ACC and MCC), supplementary motor area (SMA), and insula cortex (IC)).1 Along these lines it has been suggested that individuals with FM experience cognitive problems when these overlapping brain systems are preferentially engaged in processing painful stimuli.36 This idea has been supported by neuroimaging findings from studies conducted in chronic fatigue syndrome (CFS), a condition similar to FM that also includes cognitive problems, fatigue and often wide-spread pain. In particular, these studies show a pattern of increased activity in multiple brain areas during effortful working memory tasks in patients that exceed that of HCs.5, 10, 24 Thus, the patients demonstrated a pattern of recruiting additional or atypical cortical areas compared to HC in order to perform the same task. A similar finding would be expected in FM where the influence of pain would be expected to be more profound. In addition, behavioural tests of cognitive function in FM frequently show a negative correlation with reported level of pain, although it should be noted that this relationship is not always observed and some studies also find negative correlations with depression, anxiety and fatigue.
In order to investigate neural correlates of executive functioning in individuals with FM during a cognitive task, we administered the Go/NoGo task (a test of response inhibition)21, to a group of individuals with FM and a group of HCs while undergoing functional magnetic resonance imaging. This simple and easy task was chosen so that FM patients could perform as well as the controls, albeit requiring different levels of effort/neural processing. In contrast, a more difficult task where the patients would not be able to perform as well as controls would limit our inferences regarding mechanisms. Go/NoGo tasks involve speeded reactions, usually key presses, in response to stimuli (the Go trials). On some trials, a stimulus is presented that indicates participants should withhold their response (the NoGo trials). Since the Go trials are more numerous than the NoGo trials, participants build up a prepotency to respond, and thus have to actively inhibit a response on the NoGo trials. Prior research using such tasks has revealed a pattern of neural activation and de-activation in healthy participants during trials where the reaction is withheld. The brain areas commonly involved in this response-inhibition network include the right inferior frontal cortex (IFC), the SMA, the pre-SMA, the ACC, and structures of the basal ganglia (caudate and subthalamic nucleus).8, 15, 35 Although there is some debate about the specific roles that each of these brain areas fills during response inhibition, it appears that the IFC responds to the NoGo stimulus and facilitates the inhibitory processes carried out via the pre-SMA, SMA, and basal ganglia structures. Medial frontal areas such as the ACC appear to be involved in error detection and resolving competing response options. Given that the pain system and the response inhibition network share common resources, such as structures in the medial frontal wall, (i.e. the SMA, and the ACC)1 we hypothesized that the pattern of neural activity in FM patients would differ from controls during the NoGo trials. Specifically, individuals with FM may be actively engaging this network for both NoGo response inhibition and for pain processing/inhibition. If this is the case, the subtraction of Go trials activation from NoGo trials activation will result in lower activation levels in these areas among the FM patients.
To summarize, although competition for this network’s resources could result in increased errors on the NoGo trials, on this simple taskwe expect to find comparable performance withutilization of expanded cortical resources not common to HC. Thus we expected to find that FM patients would show an activation pattern with less activation in the response-inhibition network, but increased activation in other cortical areas. Furthermore, we investigated correlations between neural activation in FM patients during response inhibition and several symptom categories (fatigue, depression and anxiety). These factors are often negatively correlated with performance on cognitive tasks. We did not have a priori hypotheses about the direction of relationship between various brain areas and these symptom categories. As a final step, we conducted post-hoc analyses of functional connectivity in cortical areas where FM patients showed greater activation than HC. This allows further exploration of possible compensating mechanisms in FM that can be investigated in future studies.
Methods
Subjects
We investigated 18 individuals with FM and 14 age-matched HCs. Initially 20 individuals with FM and 16 HCs were consented for participation. One individual with FM was excluded due to a misunderstanding of the Go/NoGo instructions. The other three participants (1 FM, 2 HC) were excluded due to mechanical failures in collecting the Go/NoGo data in the context of the fMRI scanner. All participants were taking part in a larger randomized controlled clinical treatment trial for FM in which a subset of participants underwent baseline neuroimaging. This study was conducted within the Chronic Pain and Fatigue Research Center (CPFRC) at the University of Michigan. The study sample was drawn from a registry of individuals diagnosed with FM who had previously indicated interest in participating in clinical research within the Center. The study was conducted in accordance with the Institutional Review Boards of the University of Michigan, and the Department of Defense (co-sponsor). All participants were females.
In order to participate in the study, individuals with FM needed to (1) fulfill the American College of Rheumatology (ACR) research classification criteria for FM,49 (2) be 18 years of age, and (3) be under the standard medical care of a physician for FM. Participants were ineligible if they had any of the following: (1) a severe physical impairment (e.g., complete blindness, or deafness, paraplegia) or co-existing physical injury (e.g. sprained ankle, neck injury etc.), (2) co-morbid medical illnesses (e.g. morbid obesity, autoimmune diseases, cardiopulmonary disorders, uncontrolled endocrine or allergic disorders or malignancy within 2 years, (3) any present psychiatric disorder involving a history of psychosis, current suicide risk or attempt within 2 years of the study, or substance abuse within 2 years, and (4) a pending status associated with disability or the receipt of disability compensation for less than two years. HCs needed to meet the same inclusion/exclusion criteria except for meeting ACR criteria for FM.
Demographic and clinical data
The following clinical features were assessed: (1) demographics (i.e., age, education), (2) pain (i.e., average weekly pain intensity and percentage bodily pain distribution), (3) mood (depression and anxiety), and (4) co-occuring symptoms (i.e. medical symptoms, fatigue, somnolence, and perceived cognitive difficulties).
Demographics – a standardized demographics form was used to record age, education, and sex (i.e., all female in this study) as well medical status.
Clinical Pain – pain was assessed using a patient experience diary (PED), a Palm Pilot-like device programmed to collect pain ratings 6–8 times per day on a real-time random basis. This approach to clinical pain assessment is considered to be superior methodologically to a single weekly recall of pain intensity.44 Data was then aggregated to determine the weekly average pain rating ranging between “0” for “no pain” and “100” for “extreme pain”. Participants were also asked to complete a body map from the Brief Pain Inventory (BPI)7 which was segmented into 70 body regions that could be shaded to indicate the presence of pain. The percentage of the body regions (%BR) that were shaded indicated the extent to which pain was localized or wide-spread, an approach similar to that being used for the clinical diagnosis of fibromyalgia using the new American College of Rheumatology criteria.48
Mood – State depression was assessed using the Center for Epidemiological Studies-Depression Scale ((CES-D).34 The CES-D is a 20-item self-report instrument that was developed by the National Institute of Mental Health to detect major or clinical depression in adolescents and adults in both clinical (medical) or normal populations. The questions are easily interpreted and address most of the areas included in the diagnostic criteria for depression. Most commonly a single summary score is obtained with a cut-off of 16 used to indicate a high degree of depressive symptoms. The CES-D has been used in urban and rural populations, and in cross-cultural studies of depression. Studies using the CES-D indicate that it has very good internal consistency, acceptable test-retest stability, and construct validity (Hertzog, Van Alstine, Usala, Hultsch, & Dixon, 1990).
State anxiety was assessed using the State-Trait Personality Inventory (STPI), a refinement of the State-Trait Anxiety Inventory.40 The STPI is an 80-item self-report questionnaire with eight 10-item scales for measuring state and trait anxiety, anger, depression, and curiosity. For purposes of this study, a subset of 20-items were used to assess state anxiety. The STPI retains the strong psychometric properties for the assessment of anxiety found in the State-Trait Anxiety Inventory.41
Co-occuring symptoms – Medical symptoms that commonly co-occur with FM were assessed with the Complex Medical Symptom Inventory (CMSI),47 a 48-item checklist. based upon the diagnostic criteria for commonly co-morbid conditions such as chronic fatigue syndrome, irritable bowel syndrome, headache, etc. The checklist can be summed to reveal a metric of symptom burden.
Mental fatigue was assessed using the Multidimensional Fatigue Inventory (MFI).39 The MFI contains 20-items and has 5 factor analytically confirmed subscales assessing general fatigue, physical fatigue, reduced activity, reduced motivation, and mental fatigue. The MFI differs from other multidimensional fatigue measures by purposely retaining a relatively short list of items, and by eliminating somatic items. The mental fatigue scale is one of the five independent fatigue dimensions assessed by this instrument. Support exists for the valid use of this instrument in the assessment of fatigue in individuals with FM.16, 27
Somnolence (i.e., daytime sleepiness), was assessed by the corresponding scale from MOS Sleep Scale.42 The Medical Outcomes Study Sleep Scale (MOS Sleep Scale) was originally developed as part of the Medical Outcomes Study (MOS) which was a four-year observational study of health outcomes for chronically ill patients. The MOS Sleep Scale represents the portion of this larger assessment protocol that specifically focused upon sleep.43 The MOS Sleep Scale is a non-disease specific measure of multiple aspects of sleep problems. The instrument contains 12 items that can be scored to reveal 6 subscales, one of which is somnolence. Support for valid use of the MOS Sleep Scale comes from numerous clinical studies including those specific to FM.4
Perceived cognitive difficulties were assessed using the Multiple Abilities Self-Report Questionnaire (MASQ).37 The MASQ was purposely designed to assess the self-perception of cognitive difficulties in contrast to the more traditional “objective” neuropsychological assessment by a clinician.37 The MASQ contains items about perceived cognitive difficulties in 5 domains of clinical neuropsychological evaluation (i.e., Language, Visual-Perceptual ability (VP), Verbal Memory, Visual-Spatial Memory (VSM), and Attention/Concentration (AC). A total MASQ score can be derived that combines each of these areas of perceived cognitive problems. Support for the psychometric integrity of the MASQ is found in the original publication.37
2.3 Data acquisition and analysis
2.3.1 fMRI Task
A Go/NoGo task was used to probe response inhibition. Participants were instructed to respond to target letters (i.e letters other than X) by pressing a button (Go trials) but make no response to infrequent non-target stimuli (i.e., X; NoGo trials). Stimulus duration was 500 msec, followed by a 3500 msec screen with a fixation cross. There were five runs of 49 trials, each lasting 3 min 24 sec and containing 11, 12, or 13 NoGo trials (a total of 60 NoGo trials out of 245 total trials). Before scanning, all participants had a practice session of 49 trials on a desktop computer. False-alarm rate (i.e. responding to the NoGo signal) and reaction times (RTs) for correct responses were calculated as performance measures.
2.3.2 MRI Data acquisition
Whole-brain BOLD functional images were acquired on a 3.0 Tesla GE Signa scanner (Milwaukee, Wisconsin) using T2* weighted single-shot combined spiral in-out sequence with the following parameters: TR = 2000 msec, TE = 30 msec, FA = 90°, FOV = 200 mm, matrix size = 64 × 64; in plane resolution = 3.12 mx 3.12 mm, and slice thickness = 4 mm. The entire volume of 29 axial slices was acquired every 2 sec. Participants’ motion was minimized using foam pads placed around the head along with a forehead strap.
2.3.3 Pre-processing and statistical analyses
Data were quality checked, pre-processed and analyzed using SPM (Statistical Parametric Mapping) software packages, version 5 (Functional Imaging Laboratories, London, UK), running under Matlab 7.5b (Mathworks, Sherborn, MA, USA). Pre-processing steps included motion correction (realignment to the first image of the time series), normalization to the standard SPM - EPI template (generating 2 × 2 × 2 mm resolution images) and smoothing (convolution with a 6 mm FWHM Gaussian Kernel).
First level analyses were performed using the general linear model implemented in SPM. Three regressors of interest (i.e. (1) all Go trials, (2) correct NoGo trials, and (3) failed NoGo trials) were convolved with the canonical hemodynamic response function, with event duration of 4 sec from stimulus presentation. Motion parameters were modelled as regressors of no interest. The main contrast of interest was a model of inhibition (i.e. correct NoGo trials versus Go trials), as described in previous studies.21 First level analysis was performed by linearly combining parameter estimates over all five runs of the task. Failed NoGo trials were not included in the analysis. A random effect model was used for second level analyses. The following analyses were performed:
Analyses 1a and 1b – to find statistically significant group differences in inhibition associated brain activation: ANCOVAs (Analysis 1a: two sample t-test, with age as a covariate of no interest; Analysis 1b: two sample t-test with age and anxiety as covariates of no interest).
Analyses 2a, 2b, 2c and 2d – to delineate associations between behavioural measures (pain intensity, percentage body area in pain, depression, anxiety and mental fatigue) and brain activation related to inhibition (i.e. NoGo > Go) in individuals with FM. To this end, linear regression analyses with PED, %BR, mental fatigue, depression and anxiety scores as predictors and brain activation associated with inhibition (i.e. NoGo > Go) as dependent variable were performed. Age was included as a covariate in all analyses.
Analysis 3 was performed as a post hoc analysis to investigate functional connectivity (fc) of the right inferior temporal gyrus/fusiform gyrus, a region where individuals with FM showed more inhibition associated activity than HCs (see results section below), in order to further explore possible compensating mechanisms in FM. To accomplish this, a seed region (SR, cluster of hyper-activation in FM in the right inferior temporal gyrus/fusiform gyrus, see also results of Analysis 1a and 1b) was created using MarsBaR-software (http://marsbar.sourceforge.net). Connectivity analyses were performed using SPM (Statistical Parametric Mapping) software packages, version 8 (Functional Imaging Laboratories, London, UK), as well as the functional connectivity toolbox Conn (Cognitive and Affective Neuroscience Laboratory, Massachusetts Institute of Technology, Cambridge, USA, http://www.nitrc.org/). For first level analyses, the SR signal was correlated with voxel signal throughout the whole brain, thereby creating SR-to-voxel connectivity maps (i.e. one map for each individual). Connectivity maps were then used for second level (random effects) analysis (i.e. Analysis 3), determining the interaction between task (NoGo > Go) × group (FM > HC).
All statistical maps were corrected for multiple comparisons on the cluster level (p < 0.05, derived from an uncorrected p < 0.005 on the voxel level, with a cluster extent of 92 contiguous voxels, as estimated by the AlphaSim application (http://afni.nimh.nih.gov/afni/doc/manual/AlphaSim), implemented in the Analysis of Functional NeuroImages (AFNI) software.
3 Results
3.1 Subjects and behavioural data
Table 1 displays a comparison of the FM and HC groups with respect to each of the demographic and clinical variables assessed in this study. There was no significant difference in age between the FM sample (43.6 years) and the HC group (41.1 years, p > 0.5). As would be expected, the FM group did differ statistically from the HC group on most clinical variables by demonstrating more medical symptoms, more depressive and anxiety symptoms, more mental fatigue, more somnolence and more perceived cognitive problems. For the patient sample, the mean duration of FM was 9.1 years with an average pain intensity of 52.7 on a 0–100 point scale (SD = 17.4); see Table 1 for all mean values and statistical comparisons.
Table 1.
Sample Characteristics
| FM (n = 18) |
HC (n = 14) |
Descriptive differences between groups (t-tests) | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 43.6 (9.79) | 41.13 (11.91) | t = 0.67, ns |
| Education | Chi-Square = 0.77, ns | ||
| High School | 66.7% | 64.3% | |
| Some College | 5.6% | 14.3% | |
| Bachelors degree or higher | 27.8% | 21.4% | |
| Pain | |||
| Average Pain Intensity (PED) | 55.4 (15.5) | NA | NA |
| % Body Distribution | 55.2 (21.46) | 1.5 (4.20) | t = 9.5, p < .001 |
| Allied Symptoms | |||
| Medical Symptoms (CMSI) | 20.9 (8.32) | 0.9 (0.92) | t = 9.3, p < .001 |
| Depressive Symptoms (CES-D) | 16.28 (9.31) | 0.71 (1.27) | t = 6.2, p < .001 |
| Anxiety (STPI)) | 20.1 (5.9) | 12.1 (2.13) | t = 4.9, p < .001 |
| Mental fatigue | 12.7 (4.03) | 4.7 (.96) | t = 7.5, p < .001 |
| MOS (Somnolence) | 50.0 (24.66) | 12.0 (12.90) | t = 5.4, p < .001 |
| MASQ | 95.6 (18.74) | 65.7 (14.4) | t = 5.1, p < .001 |
| Performance in the Go/NoGo Task | |||
| Accurancy (false Go) | 2.8 (2.84) | 3.2 (3.51) | t = −0.4, ns |
| Reaction time (RT) | 577.5 (151.58) | 570.6 (143.42) | t = 0.1, ns |
FM = Fibromyalgia, HC = Healthy control, CES-D = Center for Epidemiologic Studies Depression Scale, CMSI = Complex Medical Symptom Index, MASQ = Multiple Abilities Self-Report Questionnaire, MOS = Medical Outcomes Study Somnolence Questionnaire, PED = Patient experience diary, STPI = State-Trait Personality Inventory (State anxiety)
3.2 Performance in the Go-NoGo task
As shown in Table 1 and Figure 1, as we hypothesized, there were no significant differences between FM and HC groups in mean RT (577.50 vs 570.63 msec, respectively, F(1, 30) = 0.117, p = .735) and in mean false alarms (2.78 vs 3.20 false alarms, respectively, F (1,30) = 0.146, p = .705) of the Go/NoGo task. Furthermore, there was no effect of run block on RT, nor was there a run block by group interaction. Similar results were seen for false alarms, with no effect of run block and no run block by group interaction. There was a non-significant trend for false alarms to be higher during the first run block in both groups.
Figure 1. Go/no-go Reaction Times and False Alarms.
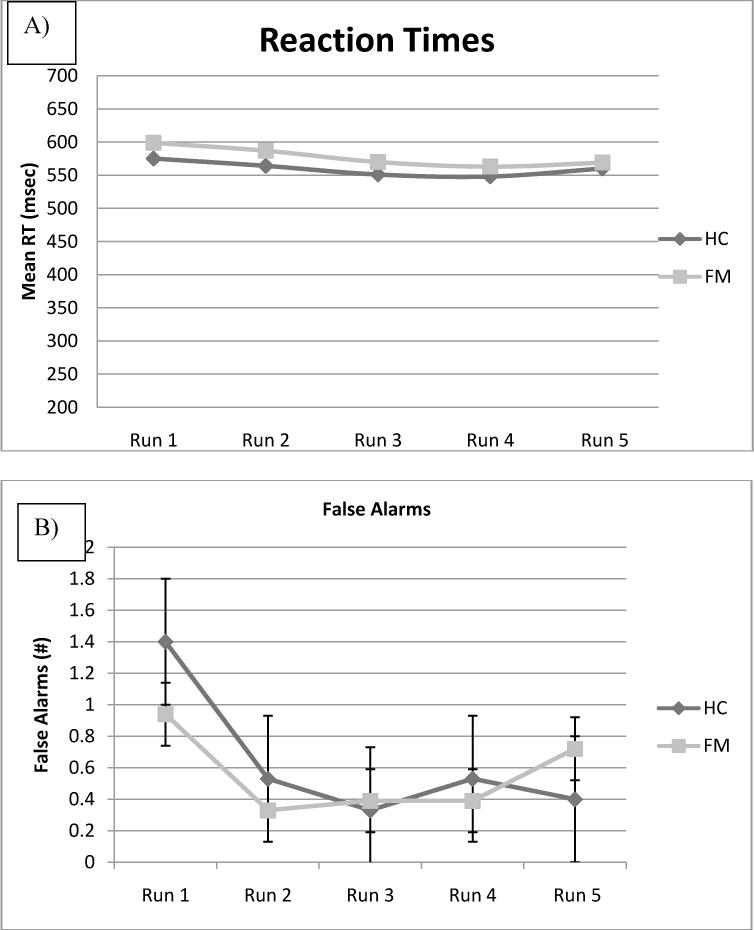
Performance measures from the Go/No-Go task for Healthy Controls and individuals with Fibromyalgia, separated by Run. Error bars reflect standard errors. Figure 1A shows reaction times for „Go“ trials: there were no significant differences between FM and HC. Figure 1B shows false alarms (incorrect responses to the „No-Go“ trials): there were no significant differences between FM and HC.
Correlations between Go/NoGo performance and symptoms of pain, depression, anxiety, sleepiness, mental fatigue and self-reported cognitive function were calculated. There were no significant correlations between RT or false alarms and any other variables.
3.3 Brain activation during response inhibition
Direct comparison of brain activation associated with inhibition (NoGo > Go) between the FM and HC groups (Analysis 1a) revealed several areas of significantly decreased activation in the FM group: mid-cingulate cortex (MCC)/SMA bilaterally, right premotor/precentral cortex (middle frontal gyrus, BA 6/9), right inferior parietal lobule, left dorsolateral prefrontal cortex (middle frontal gyrus, BA 9) and left lentiform nucleus. On the other hand, FM patients showed increased activation in the right inferior temporal gyrus/fusiform gyrus. When anxiety was controlled as a covariate (Analysis 1b), additional hypo-activations in the FM group were seen in the right insular cortex and in the left putamen. The hypoactivation in the right inferior parietal lobe, however, was no longer significant. Overall, hypo-activations were more pronounced in FM after controlling for anxiety, as was the hyper-activation in the right inferior temporal gyrus/fusiform gyrus. For details see Table 2a, as well as Figure 2a–d.
Table 2a.
Brain areas with differences in neural activation between fibromyalgia patients and healthy controls
| Region | Cluster size (# of voxels) | z-score (peak value) | Coordinates (MNI) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| HC > FM (ANCOVA with age as covariate) | |||||
| R precentral gyrus/middle frontal gyrus | 338 | 3.71 | 38 | 14 | 30 |
| R inferior parietal lobule | 111 | 3.60 | 32 | −40 | 46 |
| R/L supplemenatry motor area/mid cingulate gyrus | 309 | 3.29 | −10 | 8 | 46 |
| L middle frontal gyrus | 105 | 3.22 | −34 | 24 | 34 |
| L putamen | 102 | 3.83 | −20 | −10 | 6 |
| FM > HC (ANCOVA with age as covariate) | |||||
| R inferior temporal/fusiform gyrus | 100 | 3.14 | 44 | −36 | −14 |
| HC > FM (ANCOVA with age and anxiety as covariates) | |||||
| R precentral gyrus/middle frontal gyrus | 426 | 3.37 | 40 | −8 | 46 |
| 3.71 | 18 | 2 | 62 | ||
| R insula/putamen | 657 | 3.54 | 36 | −6 | 2 |
| 3.77 | 38 | 8 | −2 | ||
| R frontal mid Oper (MFG) | 128 | 3.64 | 40 | 12 | 30 |
| R cingulate gyrus | 300 | 3.56 | 10 | 2 | 48 |
| L middle frontal gyrus | 231 | 3.34 | −34 | 24 | 32 |
| L insula | 316 | 3.34 | −36 | −10 | −8 |
| L putamen | 316 | 3.61 | −18 | −12 | −8 |
| FM > HC (ANCOVA with age and anxiety as covariates) | |||||
| R inferior temporal gyrus/middle temporal gyrus/fusiform gyrus | 276 | 3.77 | 50 | −32 | −18 |
Figure 2. Group differences in brain activation associated with inhibition between fibromyalgia patients and healthy controls.
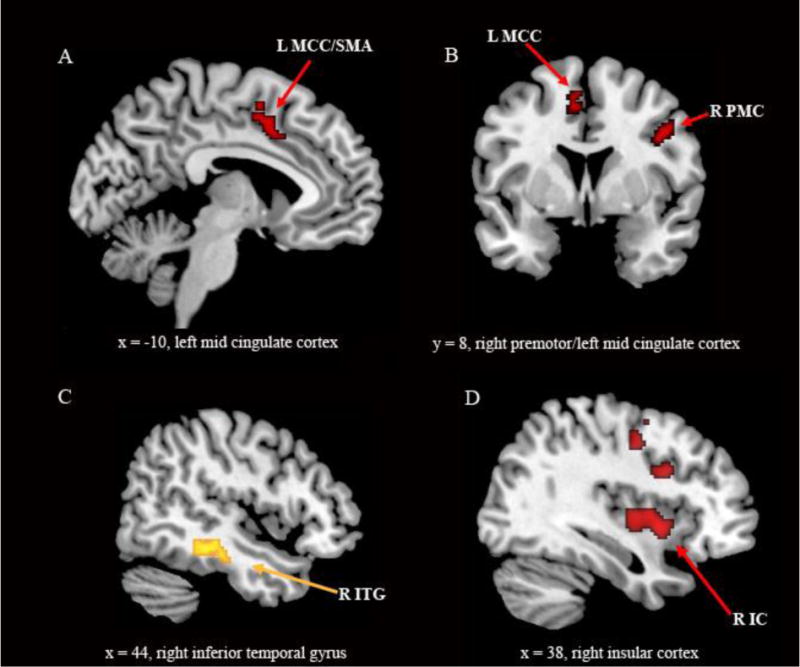
Statistical parametric maps (SPMs) demonstrating differences between groups associated with inhibition (NoGo > Go) superimposed on a template provided by MRIcron. Figure 2a, 2b, 2d: greater activation in HCs (HCs > FM). Figure 2c: greater activation in FM patients (FM > HCs): Figures 2a, 2b and 2c: two sample t-test with age as covariate of no interest. Figure 2d: two sample t-test with age and anxiety scores as covariates of no interest. IC = insular cortex, ITG = inferior temporal gyrus, MCC = mid cingulate cortex, PMC = premotor cortex, SMA = supplementary motor area, L = left, R = right. Figure 2b: the right side of the image ist he right side of the brain; SPMs are corrected for multiple comparisons (p < 0.05 cluster level).
Correlation analyses within the FM group with pain intensity, percentage body area in pain (%BR) and anxiety (Analyses 2a, 2b, 2c and 2d) yielded the following results (see Table 2b and Figure 3). At the previously determined extent threshold (92 voxels) neither pain intensity nor %BR scores showed a positive or negative association with bold response, however a negative correlation between %BR scores and BOLD response, with a cluster extent of 72 voxels, was observed in the midbrain (right lateral tegmentum) in the FM group, projecting to the nucleus cuneiformis and extending medially to the periaqueductal gray (PAG) and the nucleus coeruleus (NC) (peak voxel: × = 12, y = −28, z = −18, k = 72 voxel). Due to its high peak voxel z-value and the fact that brainstem structures, because of their small anatomical extension (compared to cortical structures) are less likely to show as many contiguous voxels, this finding is reported as a trend and will be briefly commented on in the discussion.
Table 2b.
correlation and connectivity analyses: Fibromyalgia patients
| Region | Cluster size (# of voxels) | R values | z-score (peak value) | Coordinates (MNI) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Negative correlation between pain (%BR) and BOLD response (FM group) | ||||||
| R brainstem | 72* | −.87 | 4.59 | 12 | −28 | −18 |
| Positive correlation between anxiety scores (STPI A) and BOLD response (FM group) | ||||||
| R inferior frontal gyrus/insula/medial frontal gyrus | 2165 | .86 | 4.39 | 50 | 26 | 12 |
| R superior frontal gyrus | 113 | .75 | 3.49 | 14 | 30 | 56 |
| R putamen | 230 | .82 | 4.01 | 24 | −4 | 2 |
| L superior temporal gyrus | 415 | .83 | 4.15 | −44 | −24 | −2 |
| L superior frontal gyrus | 123 | .80 | 3.85 | −12 | −10 | 70 |
| L middle frontal gyrus | 100 | .80 | 3.84 | −36 | 40 | −8 |
| L middle/inferior frontal gyrus | 302 | .77 | 3.63 | −34 | 28 | 22 |
| L putamen | 165 | .77 | 3.58 | −26 | −10 | −12 |
| Negative correlation between anxiety scores (STPI A) and BOLD response (FM group) | ||||||
| R/L rectus gyrus | 114 | −.76 | 3.55 | 0 | 50 | −20 |
| R/L superior frontal gyrus | 234 | −.85 | 4.33 | −2 | 60 | 36 |
| R temporal inferior gyrus/fusiform gyrus | 134 | −.62 | 3.94 | 50 | −38 | −26 |
| Positive correlation between MFI scores and BOLD response (FM group) | ||||||
| L insula/putamen | 187 | .74 | 3.36 | −38 | 10 | −6 |
| Negative correlation between MFI scores and BOLD response (FM group) | ||||||
| R temporal inferior gyrus/fusiform gyrus | 116 | −.76 | 3.54 | 52 | 40 | −24 |
| R orbitofrontal cortex/middle frontal gyrus | 138 | −.83 | 4.08 | 24 | 38 | −18 |
| L cerebellum, anterior lobe | 110 | −.75 | 3.48 | −20 | −44 | −30 |
| fc analysis – fc during inhibition between right inferior temporal/fusiform gyrus (SR) and TR: FM > HC | ||||||
| TR: R/L superior/medial frontal gyrus | 4.08 | / | 128 | 4 | 34 | 36 |
| TR: L supplementary motor area/superior frontal gyrus | 3.76 | / | 135 | −10 | 16 | 54 |
%BR = percentage body regions in pain, fc = functional connectivity, FM = fibromyalgia, HC = healthy controls, SR = seed region, TR = target region, L = left, R = right
Cluster fell slightly short of the apriori determined cluster extent of 92 voxels.
Figure 3. Correlation analysis between BOLD response and percentage body area in pain/ functional connectivity analysis between inhibition associated BOLD response in the right inferior temporal gyrus and medial frontal wall.
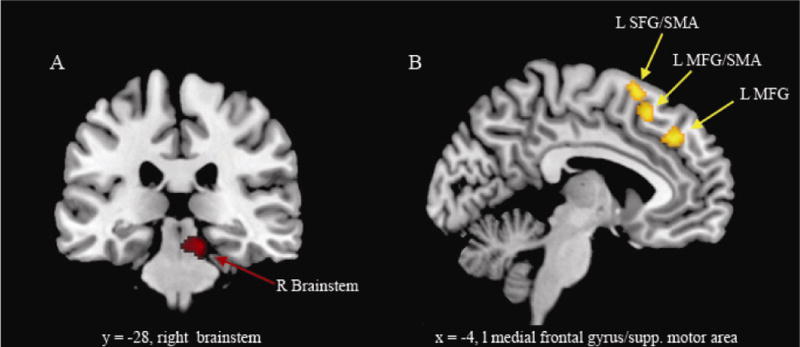
a. SPM demonstrating a negative correlation between %BR in pain and BOLD response in the brainstem (FM patients). b. greater functional connectivity in fibromyalgia patients (compared to healthy controls) between right inferior temporal/fusiform gyrus (= seed region) and MFG (= medial frontal gyrus), SFG (= superior frontal gyrus), and SMA (= supplementary motor area), L = left, R = right. Figure 2b: the right side of the image is the right side of the brain; SPMs are corrected for multiple comparisons (p < 0.05 cluster level).
Anxiety scores correlated positively with BOLD response in the right IC, right IFG, right ACC, right superior frontal gyrus, putamen bilaterally, and left middle/superior temporal gyrus. Anxiety scores were negatively correlated with inhibition associated BOLD response in the gyrus rectus bilaterally, superior frontal gyrus bilaterally (BA 9/10) and right inferior temporal gyrus/fusiform gyrus.
Mental fatigue correlated positively with BOLD response in the left IC and putamen; a negative correlation was observed in the right inferior temporal gyrus/fusiform gyrus, the right orbitofrontal cortex and the left cerebellum.
Functional connectivity analysis with the SR in the right inferior temporal gyrus/fusiform gyrus (Analysis 3) revealed an increased connectivity between this region and the ACC and medial frontal gyrus in FM patients, projecting to the preSMA, as compared to HCs during inhibition (NoGo > Go, see also Table 2b).
4 Discussion
To our knowledge this is the first study investigating neural correlates of executive functioning (i.e., inhibition) in individuals with FM using fMRI. Behaviourally, the groups showed no differences in RTs and/or accuracy. This is not surprising, given that a simple Go/NoGo paradigm was used, and deficits in performance in individuals with FM are typically only seen on very demanding tasks.18, 26 FMRI analyses on the other hand revealed significant differences between the FM and HC groups with a hypo-activation in FM in the pre-motor cortex, SMA, MCC, putamen, and after controlling for anxiety in the right IC. Hyper-activation was seen in the right inferior temporal gurys/fusiform gyrus. Overall differences were more pronounced when anxiety was controlled for as a covariate, (i.e., anxiety influenced brain activation associated with inhibition in FM in a manner more similar to that seen in HCs). Conversely, correlation analyses in FM showed a positive association between anxiety scores and brain activation in the right IC and IFG.
Medial frontal wall, premotor cortex and inferior frontal gyrus
In recent years the cerebral activation involved in re-orienting and response inhibition has been extensively investigated with a variety of brain imaging tools, such as fMRI, PET and MEG.11 The brain areas most commonly associated with response inhibition include several structures in the medial frontal wall (e.g. SMA, the pre-SMA, the anterior cingulate cortex (ACC)), the premotor cortex, the right ventrolateral cortex (especially the IFC), and subcortical structures like the caudate and the subthalamic nucleus (STN).11, 15, 30 Current research now focuses on pinpointing the brain regions that contribute specifically to certain aspects of inhibition, such as attention, decision making and motor response selection (specialisation). On the other hand it becomes increasingly clear that the brain is organized in different networks and along these lines the term inhibition network has been used (integration).11, 15 It should be noted however that depending on the methodological approach, different authors have defined networks (and their structural parts) differently, and often cortical regions participate in several networks. For example the ACC and IC are both parts of the pain system, the salience network and a presumed control network;14 the right anterior IC and IFG, together with the temporo-parietal cortex make up key components of the ventral attention system, while the (right) frontal eye field (in the premotor cortex) and the inferior parietal lobule are components of the dorsal attention system; the middle frontal gyrus in turn seems to be, at least at resting state, a link between the two attention networks.11
Based on the current literature in FM28 and on the fact that the role of the medial frontal wall in both inhibition and pain perception is well established, we had hypothesized to find altered brain activity in FM patients in this region. Indeed one of the areas showing less activation in FM patients projected to the posterior ACC, MCC and SMA. While the SMA is tightly connected (structurally) with the motor cortex and thought to be involved in planning of motor action, the pre-SMA is supposed to play a critical role in action control and response selection. Connectivity analyses (for inhibition tasks) now suggest that the pre-SMA receives input from the premotor cortex and the IFC (as part of the ventral attention system) and projects to the caudate nucleus (basal circuitry).15 Activation of the ACC on the other hand has often been attributed to task relevant parameters such as attention, control and error detection.35 Given that this area is also critically involved in pain perception, in fact being a key structure within the medial pain system with a pivotal role in stimulus evaluation,33, 45 we hypothesize that individuals with FM show decreased BOLD response associated with inhibition, because resources are taken up by ongoing neural activity associated with pain perception.
Patients also showed a decreased BOLD response in the premotor cortex, projecting close to the right frontal eye field, and the right inferior parietal cortex; both regions are part of the dorsal attention network. Interestingly, recent imaging studies have provided evidence for altered resting state networks, including the executive attention network, in chronic pain patients.6, 31 For example Napadow et al. found that pain in FM correlated with intrinsic connectivity between the IC and the right executive attention network, indicating that the executive attention network is hyper-integrated in the pain system.31 The current study is now the first to provide evidence for a task related hypo-activation of this system.
Two other regions that showed hypo-activation in FM were the IFG and the IC. As outlined above, both structures are part of the ventral attention system, which is active during the detection of targets that are both salient and behaviourally relevant (for a review, see Corbetta and Shulman, 2002).11 Strikingly, anxiety scores correlated positively with the BOLD response and differences between groups in these regions were significant only after controlling for anxiety as a covariate of no interest. Mood disturbances such as anxiety and depressive episodes are commonly associated with FM, and both threat related affect and anxiety traits have been shown to interfere with cognitive function.22 Interestingly there is evidence that anxiety is associated with a decrease in sustained activation, but an increase in transient activation in the right ventrolateral cortex when performing a working memory task,17 which has been interpreted as brain regions “working harder” to perform at equivalent levels to those of non-anxious participants. Given that pain and/or other mechanisms in FM lead to a hypo-activation in the IC and IFG, or even to the disruption of the attention network, it is conceivable that anxiety in a well defined setting, such as the Go-NoGo task, partially reverses this process. Critically it must be noted that anxiety and depression scores were highly correlated (r = 0.88) in our sample (and usually are in FM cohorts). Even though conceptually, anxiety and depression constitute different constructs, it was not possible to separate them statistically. Against this background the correlation analysis between anxiety and BOLD response in the present study should be interpreted in the light of mood disturbance (rather than anxiety alone).
Inferior temporal gyrus and fusiform gyrus
The inferior temporal gyrus and fusiform gyrus are part of the visual association cortex, and are as such critically involved in object recognition. With respect to executive functioning, activation in the fusiform gyrus has been reported in both Go-NoGo (simple and complex) (for a metanalysis see)38 and working memory paradigms.46, 51 Interestingly MCI patients have been reported to show increased activation in the fusiform gyrus within different working memory paradigms, which has been interpreted as the recruitment of additional neural resources to improve task performance.50
Along these lines we hypothesize that recruitment of the inferior temporal gyrus/fusiform gyrus might be a compensating mechanism for decreased neural resources in the medial frontal wall and premotor area. Given that this area is related to item recognition, we wonder whether individuals with FM make up for decreased resources available for response selection and motor preparation, by engaging in early/effective item recognition. Intriguingly this area showed increased functional connectivity to the medial frontal gyrus, projecting to the SMA and preSMA during inhibition (compared to HCs). Of note, the connectivity clusters were located anterior and above the cluster showing decreased activation in the MCC/SMA in the cohort analysis. Critically, it must be noted that fc analyses are based on correlations and cannot be used to determine the directedness of the signal exchange between seed and target regions. As such the increased connectivity seen in FM could reflect a top down modulation of the frontal cortex to the visual association cortex, for example in the context of attentional modulation; on the other hand it is conceivable that the increased fc is due to an increased bottom up information transfer. Interestingly, both anxiety and mental fatigue were negatively correlated with activation in the inferior temporal gyrus/fusiform gyrus, (i.e., it appears that depending on anxiety levels different neural strategies are adopted). Our data suggest that higher anxiety in FM leads to increased activity in the IC and IFG, leading to a more normal activation pattern in this area (compared to HCs). Individuals with FM with low anxiety scores on the other hand, additionally recruit the right inferior temporal gyrus/fusiform gyrus that connects to the preSMA. Our data suggest that higher anxiety/fatigue levels in FM lead to increased activity in the IC and IFG, leading to a more normal activation pattern in this area (compared to HCs). Individuals with FM with low anxiety/fatigue levels on the other hand, additionally recruit the right inferior temporal gyrus/fusiform gyrus that connects to the preSMA during inhibition.
Brainstem
Although not in the primary scope of this investigation and falling slightly short of the required extent threshold (k = 92 voxels) to correct for multiple comparisons, we would like to briefly comment on the negative correlation between the extent to which pain is wide-spread (i.e. %BR) and inhibition related BOLD response found in the right lateral tegmentum in the FM group. The peak of the cluster projected to the nucleus cuneiformis, extending medially to the PAG and the NC. For both the nucleus cuneiformis and the PAG there is strong evidence for a key role in anti-nociception. The NC on the other hand, being a major brain norepinephrine containing nucleus, is critically involved in mediating phasic alertness and attention shifting. There is evidence that pain in FM is associated with dysfunctional antinociceptive systems and it has been suggested that brainstem nuclei may play a role in this dysfunction.2, 25 Given that the underlying pathophysiology is not restricted to nociceptive/antinociceptive nuclei, but might include neighbouring and/or communicating structures involved in attention, it is conceivable that individuals with more pain, also show less attention associated BOLD response. However, at this stage assumptions concerning the underlying pathophysiology are rather speculative and further research with more advanced fMRI techniques is needed to confirm this finding with better resolution of brainstem structures.
Limitations
The current study has some limitations that may be addressed in future studies. Although our sample size is adequate for comparing group neuroimaging differences, more participants would allow for more in-depth analyses of the contributions of pain, mood, and fatigue to patterns of neural activation in fibromyalgia. Our functional connectivity analysis was post-hoc; however, these results lay the groundwork for future connectivity analyses. Finally, although it was helpful in this initial study to use a cognitive task where performance was not different between fibromyalgia patients and healthy controls, it will be more revealing to examine differences in neural activation on tasks that do show performance differences. Again, we believe this study lays the groundwork for future neuroimaging studies of cognitive function in fibromyalgia and other chronic pain disorders.
Summary
Taken together our data suggest that despite the same RTs and accuracy, activation in response to an executive function task is different in individuals with FM than in HCs. Specifically there is less activation in various cortical structures known to be part of the inhibition network, especially in the areas involved in response selection and motor preparation (SMA, premotor cortex), the dorsal attention network (IPL and premotor cortex/frontal eyefields) and the ventral attention network (IC and IFG). We hypothesize that inhibition and pain perception rely on partially overlapping networks (with an intersection in the pACC/MCC/SMA) and that resources taken up by pain processing in chronic pain patients are not available for the inhibition task. With respect to both equal performance across groups and increased activity in the inferior temporal cortex/fusiform gyrus seen in those with FM, our results also indicate a certain (functional) plasticity in FM (as far as this can be concluded from cross-sectional studies), i.e. the capability to either normalize brain activity (in the IC and IFG, a process associated with the degree of anxiety) or to recruit other brain regions to a higher extent, possibly compensating for neural resources less available elsewhere (i.e. in the medial frontal wall and premotor cortex).
Along these lines more research is needed to further examine various aspects of cognitive function in FM, and its interaction with clinical pain and other psychophysical factors such as depression, fatigue, locus of control, etc. To this end, studies that include patients with mood disorders and/or CSF as a non-pain control group could be very informative. It will be of special interest to further investigate within longitudinal study designs, whether improvement in pain leads to normalization in brain activation and re-recruitment in regions such as the medial frontal wall.
Acknowledgments
Disclosures
This study was supported in part by grant numbers R01-AR050044 (NIAMS/NIH), and DAMD 17-00-2-0018 (Department of Defense) to David Williams and Daniel Clauw and by a grant of the DFG (Deutsche Forschungsgemeinschaft, GZ: SchM 2665/1-1) to Tobias Schmidt-Wilcke. The authors of this manuscript are free of any conflict of interest associated with its content.
Abbreviations
- ACC
anterior cingulate cortex
- BA
Brodmann Area
- CFS
chronic fatigue syndrome
- fc
functional connectivity
- FM
fibromyalgia
- FWMH
full width at half maximum
- HC
healthy control
- IC
insular cortex
- MCC
mid-cingulate cortex
- IFG
inferior frontal gyrus
- NC
nucleus coeruleus
- PAG
periaqueductal grey
- SMA
supplementary motor cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Perspective: Neural activation (fMRI) during response inhibition was measured in fibromyalgia patients and controls. FM patients show lower activation in the inhibition and attention networks and increased activation in other areas. Inhibition and pain perception may use overlapping networks: resources taken up by pain processing may be unavailable for other processes.
Literature
- 1.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Bingel U, Tracey I. Imaging CNS modulation of pain in humans. Physiology (Bethesda) 2008;23:371–380. doi: 10.1152/physiol.00024.2008. [DOI] [PubMed] [Google Scholar]
- 3.Bradley LA, McKendree-Smith NL, Alberts KR, Alarcon GS, Mountz JM, Deutsch G. Use of neuroimaging to understand abnormal pain sensitivity in fibromyalgia. Curr Rheumatol Rep. 2000;2:141–148. doi: 10.1007/s11926-000-0054-2. [DOI] [PubMed] [Google Scholar]
- 4.Cappelleri JC, Bushmakin AG, McDermott AM, Dukes E, Sadosky A, Petrie CD, Martin S. Measurement properties of the Medical Outcomes Study Sleep Scale in patients with fibromyalgia. Sleep Med. 2009;10:766–770. doi: 10.1016/j.sleep.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Caseras X, Mataix-Cols D, Giampietro V, Rimes KA, Brammer M, Zelaya F, Chalder T, Godfrey EL. Probing the working memory system in chronic fatigue syndrome: a functional magnetic resonance imaging study using the n-back task. Psychosom Med. 2006;68:947–955. doi: 10.1097/01.psy.0000242770.50979.5f. [DOI] [PubMed] [Google Scholar]
- 6.Cauda F, D’Agata F, Sacco K, Duca S, Cocito D, Paolasso I, Isoardo G, Geminiani G. Altered resting state attentional networks in diabetic neuropathic pain. J Neurol Neurosurg Psychiatry. 2010;81:806–811. doi: 10.1136/jnnp.2009.188631. [DOI] [PubMed] [Google Scholar]
- 7.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 8.Congdon E, Mumford JA, Cohen JR, Galvan A, Aron AR, Xue G, Miller E, Poldrack RA. Engagement of large-scale networks is related to individual differences in inhibitory control. Neuroimage. 2010;53:653–663. doi: 10.1016/j.neuroimage.2010.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook DB, Lange G, Ciccone DS, Liu WC, Steffener J, Natelson BH. Functional imaging of pain in patients with primary fibromyalgia. J Rheumatol. 2004;31:364–378. [PubMed] [Google Scholar]
- 10.Cook DB, O’Connor PJ, Lange G, Steffener J. Functional neuroimaging correlates of mental fatigue induced by cognition among chronic fatigue syndrome patients and controls. Neuroimage. 2007;36:108–122. doi: 10.1016/j.neuroimage.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 11.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dick B, Eccleston C, Crombez G. Attentional functioning in fibromyalgia, rheumatoid arthritis, and musculoskeletal pain patients. Arthritis Rheum. 2002;47:639–644. doi: 10.1002/art.10800. [DOI] [PubMed] [Google Scholar]
- 13.Dick BD, Verrier MJ, Harker KT, Rashiq S. Disruption of cognitive function in Fibromyalgia Syndrome. Pain. 2008;139:610–616. doi: 10.1016/j.pain.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 14.Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duann JR, Ide JS, Luo X, Li CS. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J Neurosci. 2009;29:10171–10179. doi: 10.1523/JNEUROSCI.1300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ericsson A, Mannerkorpi K. Assessment of fatigue in patients with fibromyalgia and chronic widespread pain. Reliability and validity of the Swedish version of the MFI-20. Disabil Rehabil. 2007;29:1665–1670. doi: 10.1080/09638280601055782. [DOI] [PubMed] [Google Scholar]
- 17.Fales CL, Barch DM, Burgess GC, Schaefer A, Mennin DS, Gray JR, Braver TS. Anxiety and cognitive efficiency: differential modulation of transient and sustained neural activity during a working memory task. Cogn Affect Behav Neurosci. 2008;8:239–253. doi: 10.3758/cabn.8.3.239. [DOI] [PubMed] [Google Scholar]
- 18.Glass JM. Review of cognitive dysfunction in fibromyalgia: a convergence on working memory and attentional control impairments. Rheum Dis Clin North Am. 2009;35:299–311. doi: 10.1016/j.rdc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Glass JM, Park DC, Minear M, Crofford LJ. Memory beliefs and function in fibromyalgia patients. J Psychosom Res. 2005;58:263–269. doi: 10.1016/j.jpsychores.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333–1343. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 21.Heitzeg MM, Nigg JT, Yau WY, Zucker RA, Zubieta JK. Striatal dysfunction marks preexisting risk and medial prefrontal dysfunction is related to problem drinking in children of alcoholics. Biol Psychiatry. 2010;68:287–295. doi: 10.1016/j.biopsych.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koster EH, Crombez G, Verschuere B, Van Damme S, Wiersema JR. Components of attentional bias to threat in high trait anxiety: Facilitated engagement, impaired disengagement, and attentional avoidance. Behav Res Ther. 2006;44:1757–1771. doi: 10.1016/j.brat.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Landro NI, Stiles TC, Sletvold H. Memory functioning in patients with primary fibromyalgia and major depression and healthy controls. J Psychosom Res. 1997;42:297–306. doi: 10.1016/s0022-3999(96)00301-7. [DOI] [PubMed] [Google Scholar]
- 24.Lange G, Steffener J, Cook DB, Bly BM, Christodoulou C, Liu WC, Deluca J, Natelson BH. Objective evidence of cognitive complaints in Chronic Fatigue Syndrome: a BOLD fMRI study of verbal working memory. Neuroimage. 2005;26:513–524. doi: 10.1016/j.neuroimage.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Lautenbacher S, Rollman GB. Possible deficiencies of pain modulation in fibromyalgia. Clin J Pain. 1997;13:189–196. doi: 10.1097/00002508-199709000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Leavitt F, Katz RS. Distraction as a key determinant of impaired memory in patients with fibromyalgia. Journal of Rheumatology. 2006;33:127–132. [PubMed] [Google Scholar]
- 27.Lin JM, Brimmer DJ, Maloney EM, Nyarko E, Belue R, Reeves WC. Further validation of the Multidimensional Fatigue Inventory in a US adult population sample. Popul Health Metr. 2009;7:18. doi: 10.1186/1478-7954-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luerding R, Weigand T, Bogdahn U, Schmidt-Wilcke T. Working memory performance is correlated with local brain morphology in the medial frontal and anterior cingulate cortex in fibromyalgia patients: structural correlates of pain-cognition interaction. Brain. 2008;131:3222–3231. doi: 10.1093/brain/awn229. [DOI] [PubMed] [Google Scholar]
- 29.Mease PJ, Arnold LM, Crofford LJ, Williams DA, Russell IJ, Humphrey L, Abetz L, Martin SA. Identifying the clinical domains of fibromyalgia: contributions from clinician and patient delphi exercises. Arthritis Care Res. 2008;59:952–960. doi: 10.1002/art.23826. [DOI] [PubMed] [Google Scholar]
- 30.Nakata H, Sakamoto K, Ferretti A, Gianni Perrucci M, Del Gratta C, Kakigi R, Luca Romani G. Somato-motor inhibitory processing in humans: an event-related functional MRI study. Neuroimage. 2008;39:1858–1866. doi: 10.1016/j.neuroimage.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 31.Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62:2545–2555. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park DC, Glass JM, Minear M, Crofford LJ. Cognitive function in fibromyalgia patients. Arthritis Rheum. 2001;44:2125–2133. doi: 10.1002/1529-0131(200109)44:9<2125::AID-ART365>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 33.Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 34.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 35.Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt-Wilcke T, Wood P, Lurding R. Cognitive impairment in patients suffering from fibromyalgia: An underestimated problem. Schmerz. 2010;24:46–53. doi: 10.1007/s00482-009-0872-8. [DOI] [PubMed] [Google Scholar]
- 37.Seidenberg M, Haltiner A, Taylor MA, Hermann BB, Wyler A. Development and validation of a Multiple Ability Self-Report Questionnaire. J Clin Exp Neuropsychol. 1994;16:93–104. doi: 10.1080/01688639408402620. [DOI] [PubMed] [Google Scholar]
- 38.Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. Journal of Psychosomatic Research. 1995;39:315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 40.Spielberger CD. 2010 http://mindgarden.com/products/stpi.htm [Accessed 2010 March 18]
- 41.Spielberger CD, G RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 42.Spritzer KL, Hays RD. MOS sleep scale: a manual for use and scoring. Version 1.0. Los Angeles, CA, USA: 2003. [Google Scholar]
- 43.Stewart AL, Hays RD, Ware JE., Jr The MOS short-form general health survey. Reliability and validity in a patient population. Medical Care. 1988;26:724–735. doi: 10.1097/00005650-198807000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient compliance with paper and electronic diaries. Control Clin Trials. 2003;24:182–199. doi: 10.1016/s0197-2456(02)00320-3. [DOI] [PubMed] [Google Scholar]
- 45.Tracey I. Nociceptive processing in the human brain. Curr Opin Neurobiol. 2005;15:478–487. doi: 10.1016/j.conb.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 46.Veltman DJ, Rombouts SA, Dolan RJ. Maintenance versus manipulation in verbal working memory revisited: an fMRI study. Neuroimage. 2003;18:247–256. doi: 10.1016/s1053-8119(02)00049-6. [DOI] [PubMed] [Google Scholar]
- 47.Williams DA, Schilling S. Advances in the assessment of fibromyalgia. Rheum Dis Clin North Am. 2009;35:339–357. doi: 10.1016/j.rdc.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62:600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 49.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 50.Yetkin FZ, Rosenberg RN, Weiner MF, Purdy PD, Cullum CM. FMRI of working memory in patients with mild cognitive impairment and probable Alzheimer’s disease. Eur Radiol. 2006;16:193–206. doi: 10.1007/s00330-005-2794-x. [DOI] [PubMed] [Google Scholar]
- 51.Yoo SS, Paralkar G, Panych LP. Neural substrates associated with the concurrent performance of dual working memory tasks. Int J Neurosci. 2004;114:613–631. doi: 10.1080/00207450490430561. [DOI] [PubMed] [Google Scholar]


