Summary
Background
The majority of studies have reported risks of breast cancer from benign breast disease (BBD) in essentially homogenous Caucasian populations. Information on breast cancer risk factors in larger, multi-ethnic populations should facilitate the development of appropriate and targeted risk reduction strategies.
Design
Cases and controls were drawn from a parent BBD cohort of 4,970 women, 1,341 African Americans (AA) and 3,629 non-AA who were diagnosed with BBD after examination of an excisional breast biopsy. Risk factors (34 variables) included demographics, lesion types, and epidemiological variables.
Results
The final multivariable model retained significance (p<0.05) for lesion risk-level, fibroadenoma, and the interaction of age-by-race. Women with proliferative lesions (no atypia, risk level 2) were 1.7 times more likely to develop BC when compared with women with non-proliferative lesions (OR=1.7, 95% CI 1.13, 2.42, p=0.009). Women with atypia (risk level 3) were 3.75 times more likely to develop BC compared to women with non-proliferative lesions (OR= 3.75, 95% CI 1.99, 7.06, p<.001). The odds of breast cancer was approximately 35% lower among women with fibroadenoma as compared to women without fibroadenoma (OR=0.65, 95% CI 0.46, 0.94, p=0.020). African-American women with BBD who were 50 years or older were 2.28 times more likely to develop breast cancer as compared to non African-American women who were less than 50 years old (OR=2.28, 95% CI 1.34, 3.88, p=0.002).
Conclusion
Women with fibroadenoma (nonproliferative or proliferative) were less likely to progress to BC. Older African American women are at greater risk for progression to breast cancer from BBD.
Keywords: benign breast disease, progression, risk factors
Introduction
Epidemiologic studies have shown that women with proliferative epithelial disorders affecting the small ducts and terminal ductal lobular units of the breast are at increased risk of subsequent breast cancer, particularly when the epithelial proliferation is accompanied by evidence of atypia (1–4). Risk is increased approximately 1.5–2 fold for those with epithelial proliferation without atypia (risk level 2) (1–4) and 4–5 fold for those with proliferative disease with atypia (risk level 3) (1, 3–5).
Female breast cancer incidence rates vary across racial and ethnic groups. African-American (AA) women are diagnosed with breast cancer at earlier ages and at more advanced stages, and experience a 15% higher mortality than Caucasian Americans (CA) (6). Incidence rates have continued to increase in the recent past for CA women (0.4% per year for 1987–2000), but have stabilized in AA women since 1992 (7). The prevalence of several established risk factors also differs across racial and ethnic subpopulations and these risk factors may contribute to the higher cancer incidence rates in whites compared with other racial and ethnic groups (7).
Although subsequent breast cancer risk from BBD is known to vary with BBD pathologic characteristics (2, 3, 8), the interaction of other factors with BBD, excluding family history (2, 9, 10), on subsequent risk has not been well studied. Furthermore, because the majority of published studies of BBD and BC risks have been based on predominantly homogenous CA populations (2, 11–16), our understanding of risk factors in the AA population is limited by the dearth of ethnically diverse BBD cohorts. The Detroit BBD cohort, drawn from a multi-ethnic population with nearly 28% of eligible AA women, begins to address this issue (3, 4, 17).
In this report, utilizing a case control study nested within the large and diverse Detroit primary care population, we evaluated 34 demographic, histopathologic, and epidemiologic risk factors (Supplemental Table S1) as predictors for progression to BC from BBD.
MATERIALS AND METHODS
Detroit BBD Cohort
This study drew from a pool of primary care patients within the Henry Ford Health System (HFHS), one of the largest health care organizations in southeastern Michigan. It includes an HMO (Health Alliance Plan-HAP), a medical group (Henry Ford Medical Group, HFMG), and several satellite hospitals. Most patients at HFHS reside in a three-county Metropolitan Detroit area that is approximately 33% African-American.
Women were eligible for this study if they were 18 years or older, had a breast biopsy performed for diagnostic purposes, did not have a history of breast cancer, and were not diagnosed with breast cancer within 6 months of the index BBD diagnosis. Ascertainment of cohort subjects (before exclusions) was based on retrieval and review (by the primary pathologist) of all reports of biopsies with a BBD diagnosis within the Henry Ford Health system within the period from Jan 1981 to Dec 1994. Pathologists reviewed the histology for all eligible subjects within the study cohort using a detailed review form designed to capture a broad spectrum of histological findings. The primary reference pathologist re-reviewed a 10% sample of cases to assess intra-rater reliability. For women with more than 1 BBD biopsy, the earliest biopsy (index biopsy) was recorded, and the diagnosis was based on the most advanced (serious) lesion in the index biopsy.
Because multiple lesions were common (3), women were classified according to the most advanced lesion using the following classification, listed in order of probable increasing risk of cancer: nonproliferative lesion (risk level 1, reference group), proliferative lesion without atypia (risk level 2), and proliferative with atypical hyperplasia (lobular or ductal, risk level 3). Women were classified as having a non-proliferative lesion if the pathologic diagnosis was simple apocrine metaplasia, cysts, periductal mastitis/duct ectasia, mastitis, simple fibrosis, squamous metaplasia, or simple fibroadenoma. Women were classified as having a proliferative lesion if the pathologic diagnosis was simple adenosis, sclerosing adenosis, apocrine adenosis, mild or moderate/florid usual or apocrine hyperplasia, papilloma, complex fibroadenoma, or radial scar. When ADH or ALH was present with other pathologic lesions, a woman was classified in the proliferative-with-atypia category.
Ascertainment of subsequent progression to breast cancer from BBD (breast cancer cases) was accomplished through examination of the tumor registries of the Henry Ford Health system, the Detroit SEER registry, and the State of Michigan cancer registry. Follow-up on women who did not develop cancer was obtained from the National Death Index, Equifax/ChoicePoint locator service, and HFHS databases. Cancer outcome in women with BBD was defined using AJCC staging definitions and SEER summary stage. Women in any of the tumor registries with a diagnosis of carcinoma in situ (stage 0) or invasive cancer (Stage 1–4) were classified as having cancer (3).
Design of the nested case-control study
The median time from the original biopsy to the diagnosis of breast cancer for the cohort was 7.19 years (mean 7.61 years; 95% CI for mean: 6.98, 8.24) (4). Once a case (breast cancer) was identified, three controls were matched to each case using the initial BBD diagnosis date (within 30 days of the case diagnosis date) as the matching variable. Originally, selected controls that developed breast cancer (cases) remained in the study as both controls for the specific case and as cases with additional matched controls.
Risk Factor Variables
Risk factor information related to the development of breast cancer was collected for subjects in the case-control cohort through questionnaire administration and medical record abstraction. The initial working draft of the questionnaire was evaluated for its cultural appropriateness and comprehensiveness by a focus group of African-American participants (18).
Demographic, medical/clinical, and histopathologic risk factors made up the 34 variables listed in Supplemental Table S1.
Statistical Methods
Logistic regression, adjusting for case-control clusters was performed. The analysis process began with testing each individual risk factor for progression to BC, including individual risk factors by race variable interaction, followed by multivariable modeling. Any individual risk factor and/or the risk-factor-by-race interactions with p-value<0.10 was included in the multivariable model selection process. A forward step-wise model selection was used and two-way or three-way variable interactions were considered only if the variable or variable-by-race interaction was significant (p-value<0.05) in a multivariable model. The final model included variables or variable interactions with p-value<0.05. Odds ratios (OR) and the 95% confidence limits (CI) were calculated for variables or variable interactions.
Results
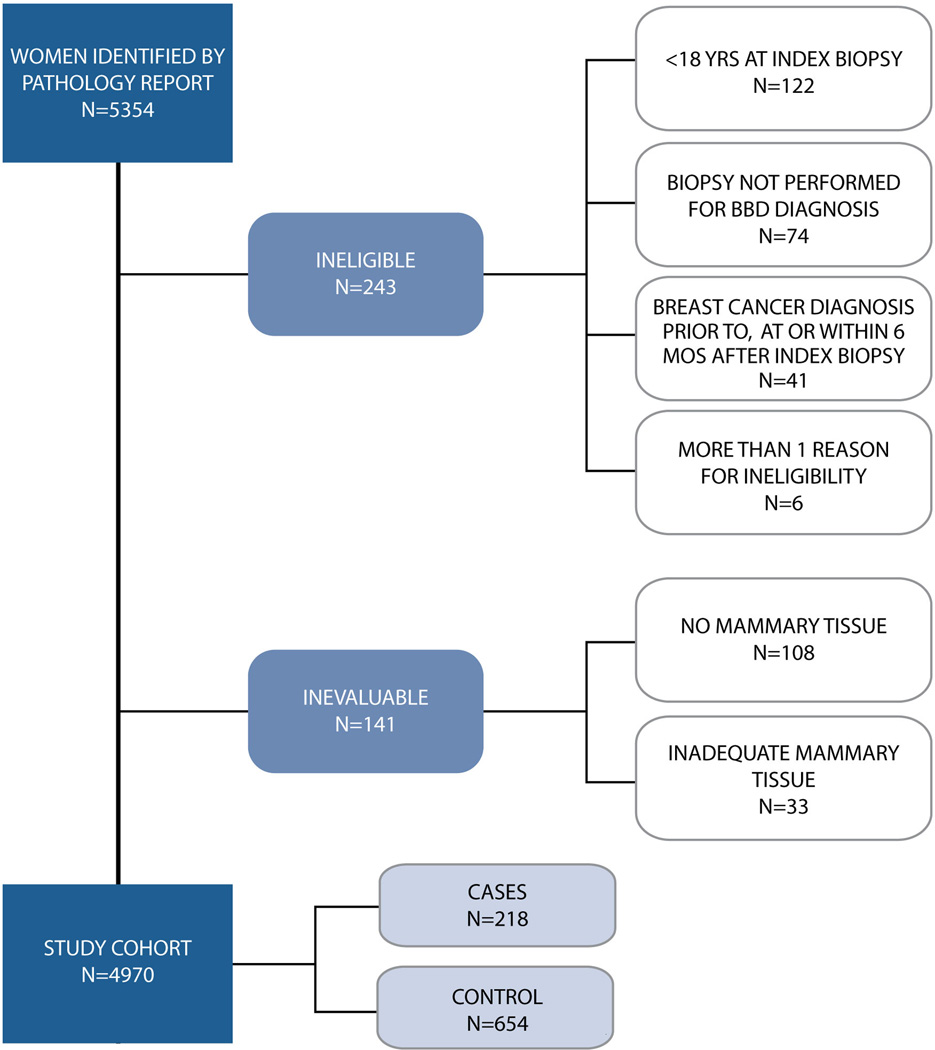
From the original cohort of 5354 women, 4970 women were eligible for pathologic assessment (Figure 1). A total of 872 subjects, drawn from the pool of 4970 were included in the case-control study of which 218 were cases. Twenty-seven percent of the cohort was AA and 70% CA. Among AA women (n=238), 58 (27%) were cases and 180 (27%) controls. Race distribution of the cohort was as follows: AA: 238 (58 cases {27%} and 180 controls {27%}), CA: 608 (156 cases {71%} and 452 controls {69%}), other: 24 (4 cases {1.8%} and 20 controls {3%}), missing race: 2 (controls). Of the 4970 women, 433 (8.7%) had no BBD lesion on histopathologic review of BBD biopsy slides. Of the 433 women, 53 (6%) were in the case-control cohort, of whom 37 were controls and 16 were cases.
Figure 1.
The overall median length of follow up for this nested case-control cohort (n=872) was 10.37 years (mean 10.32 SD=5.19), compared to 10.66 years (mean 10.6 SD=4.9) in the larger BBD cohort study (3, 4). Fourteen (2%) of the originally selected controls developed breast cancer.
Individual risk factors (34 variables), including the proportion of cases and controls by response, odds ratio, 95% CI and p-value are presented as a supplemental table. The seven individual variables-by-race interactions that showed p values <0.10 included age, hormone replacement therapy, menopausal status, fibrosis, squamous metaplasia, ADH, and phyllodes tumor (Table 1). Eight additional individual variables, (Table 1), including occupational history, contraceptive use, cysts, fibroadenoma, hyperplasia without atypia, papilloma, ALH, and lesion risk level also had p values <0.10 and were included in the initial multivariable model.
Table 1.
Significant Univariate Results from Logistic Regression Models*
| Risk Factor | Response | Control | Case | OR | Race interaction p-value |
||
|---|---|---|---|---|---|---|---|
| (N= 654) | (N= 218) | 95% CI | p-value | ||||
| Age >=50 | Yes | 243 (37%) | 117 (54%) | 1.95 | 1.43, 2.64 | <.0001 | 0.02 |
| No | 408 (63%) | 101 (46%) | |||||
| Hormone Replacement Therapy | Yes | 77 (16%) | 28 (16%) | 1.06 | 0.65, 1.74 | 0.807 | 0.024 |
| No | 418 (84%) | 143 (84%) | |||||
| Occupational History (within 10 yrs) | Yes | 316 (74%) | 110 (65%) | 0.65 | 0.45, 0.95 | 0.028 | 0.719 |
| No | 112 (26%) | 58 (35%) | |||||
| Contraceptive Use | Yes | 146 (43%) | 36 (30%) | 0.57 | 0.36, 0.90 | 0.015 | 0.126 |
| No | 192 (57%) | 83 (70%) | |||||
| Menopausal Status | Pre-menopausal | 69 (13%) | 21 (13%) | Ref | - | - | 0.0069 |
| Peri-menopausal | 250 (46%) | 103 (63%) | 1.7 | 0.95, 3.06 | 0.074 | ||
| Post-menopausal | 224 (41%) | 40 (24%) | 2.31 | 1.55, 3.44 | <.0001 | ||
| Lesion Risk Level | No BBD Lesion | 37 (6%) | 16 (7%) | 2.11 | 1.11, 3.99 | 0.022 | 0.37 |
| NP | 234 (36%) | 48 (22%) | Ref | - | - | ||
| P | 349 (54%) | 125 (57%) | 1.75 | 1.21, 2.52 | 0.003 | ||
| AH | 31 (5%) | 29 (13%) | 4.56 | 2.46, 8.47 | <.0001 | ||
| Cysts - Present | Yes | 312 (48%) | 127 (58%) | 1.52 | 1.13, 2.03 | 0.005 | 0.487 |
| No | 339 (52%) | 91 (42%) | |||||
| Fibrosis - Present | Yes | 148 (23%) | 49 (22%) | 0.99 | 0.73, 1.34 | 0.925 | 0.014 |
| No | 503 (77%) | 169 (78%) | |||||
| Squamous Metaplasia - Present | Yes | 3 (0%) | 1 (0%) | 1 | 0.10, 9.73 | 0.997 | <.0001 |
| No | 648 (100%) | 217 (100%) | |||||
| Fibroadenoma - Present | Yes | 245 (38%) | 54 (25%) | 0.55 | 0.39, 0.77 | 0.0007 | 0.108 |
| No | 406 (62%) | 164 (75%) | |||||
| Hyperplasia without atypia - Present | Yes | 214 (33%) | 95 (44%) | 1.58 | 1.15, 2.17 | 0.005 | 0.793 |
| No | 437 (67%) | 123 (56%) | |||||
| Aypical ductal hyperplasia - Present | Yes | 22 (3%) | 22 (10%) | 3.21 | 1.73, 5.96 | 0.0002 | 0.099 |
| No | 629 (97%) | 196 (90%) | |||||
| Aypical lobular hyperplasia - Present | Yes | 9 (1%) | 10 (5%) | 3.43 | 1.35, 8.70 | 0.009 | 0.662 |
| No | 642 (99%) | 208 (95%) | |||||
| Papilloma - Present | Yes | 49 (8%) | 28 (13%) | 1.81 | 1.11, 2.95 | 0.017 | 0.458 |
| No | 602 (92%) | 190 (87%) | |||||
| Phyllodes Tumor - Present | Yes | 3 (0%) | 1 (0%) | 1 | 0.10, 9.74 | 0.997 | <.0001 |
| No | 603 (100%) | 202 (100%) |
Adjusted for cluster;
Ref=reference; OR=odds ratio; CI=confidence interval.
The final multivariable model (p<0.05) retained lesion risk-level, fibroadenoma, and the interaction of race-by-age category (50 or under, and over 50) (Table 2).
Table 2.
Final multivariable logistic regression model
| Variable | OR | 95% CI | p-value |
|---|---|---|---|
| Age ≥50 | NA | NA | 0.251 |
| African American | NA | NA | 0.119 |
| Lesion Risk Level | |||
| NP | Reference | ||
| No BBD | 1.72 | 0.92, 3.19 | 0.088 |
| Proliferative | 1.66 | 1.13, 2.42 | 0.009 |
| AH | 3.75 | 1.99, 7.06 | <0.001 |
| Fibroadenoma present | 0.65 | 0.46, 0.94 | 0.02 |
| AA*Age | |||
| AA, ≥50 | 2.28 | 1.34, 3.88 | 0.002 |
| AA, <50 | 0.74 | 0.43, 1.26 | 0.262 |
| Other Race, ≥50 | 1.35 | 0.93, 1.97 | 0.113 |
| Other Race, <50 | Reference |
NP: Nonproliferative; AH: atypical hyperplasia; AA: African American
Women with proliferative lesions (no atypia, risk level 2) were 1.7 times more likely to develop BC compared to patients with non-proliferative lesions (OR=1.7, 95% CI 1.13, 2.42, p=0.009). Patients with atypia (risk level 3) were 3.75 times more likely to develop BC compared to patients with non-proliferative lesions (OR= 3.75, 95% CI 1.99, 7.06, p<.001). The average age of women with fibroadenoma was 43.8 years compared to 49.4 years for BBD women without fibroadenoma. The presence of fibroadenoma was protective against breast cancer. The odds of breast cancer were approximately 35% lower among patients with fibroadenoma as compared to women without fibroadenoma (OR= 0.65, 95% CI 0.46, 0.94, p=0.020). The interaction of age and fibroadenoma was not significant (p= 0.240). The interaction between race and age was significant after adjusting for lesion risk- level and presence of fibroadenoma. African-American patients who were 50 years or older were 2.28 times more likely to develop breast cancer than non African-American patients who were less than 50 years old (OR= 2.28, 95% CI 1.34, 3.88, p=0.002).
Discussion
The pathologic characteristics of specific types of benign breast disease have clarified our understanding of the risks of subsequent breast cancer (2, 3, 8). The interaction of other possible promotional factors of BC in the presence of BBD has been examined predominantly in homogenous CA populations (2, 9, 11–16). The availability of the Detroit BBD cohort, drawn from a multi-ethnic population with nearly 28% of AA eligible women, has begun to address the data gap in the AA population (3, 4, 17).
This breast cancer/case control study nested within a multiethnic primary care population with BBD evaluated 34 demographic, histopathologic, and epidemiologic potential risk factors. Interaction of age and race, lesion risk-level, and fibroadenoma emerged as significant predictors for progression to BC from BBD. Lesion risk-level conferred increased risk; in contrast, fibroadenoma was associated with a reduced risk for progression to BC from BBD. No three-way interaction was detected between race, age, and presence of fibroadenoma (p=0.293).
Current understanding of BBD in the AA population is limited. AA women are known to be diagnosed with breast cancer at earlier ages and at more advanced stages, and to experience a 15% higher mortality than Caucasians (6). In this multi-ethnic nested case-control study, over 27% of BBD women were AA, 70% were CA and 3% were non-AA and non-CA. There were approximately 2.7 CA cases of breast cancer for every 1 AA case and 2.5 CA controls for every 1 AA control.
To our knowledge, this is the first study to report an interaction of race and age as a promotional factor in the progression of BBD to BC. African American women with BBD who were 50 years or older were 2.28 times more likely to develop breast cancer than the reference group of non African-American BBD women who were less than 50 years old (p=0.002,Table 2). Non-AA women who were over 50 years old and predominantly Caucasian American did not differ significantly from controls. The cutoff at age 50 was an attempt to group women as premenopausal (<50 years) or postmenopausal (≥50). Our two previous reports (3, 4) on the parent BBD cohort from which this nested case-control cohort was drawn did not find an interaction of age and race. One explanation is that the case control selection resulted in the separation of a slightly different subpopulation and may have contributed to the age/race interaction recorded for the nested cohort.
The importance of age as a predictor of breast cancer risk for older women is supported by other recent studies (3, 4, 17, 19, 20). The current assumption that cancer results from a multi-step, progressive accumulation of destabilization events is consistent with the expectation of age-related increase in our cohort (3). The observation that older women are more likely to present with atypia can be offered as evidence of a temporal, morphologic continuum in the progression of breast cancer from precursor benign lesions. Support for the latter is also strengthened by the cumulative breast cancer incidence by type of lesion upon follow-up. Previously, for the parent BBD cohort, we reported that at 5 years of follow-up no significant difference in risk between women with nonproliferative lesions or with proliferative lesions without atypia was observed, even though the risk was 4 times higher for women with atypia (3). At 10 years of follow-up, however, cumulative breast cancer incidence was significant for both women with proliferative lesions without atypia and women with atypia.
The rate of lobular involution in women in AA women may be worthy of investigation. As women age, the lobules in their breasts undergo involution or regression (21, 22). A recent study (23) suggested that involution may contribute to slowing in the rate of increase of breast cancer among women older than 50 years, based on earlier speculation by Henson and Tarone (24). In the predominantly Caucasian-American Mayo Benign Breast Disease Cohort, lobular involution was associated with reduced risk of breast cancer. A definite increase in the process of involution was noted at approximately age 50 (with complete involution present in 5.8% of women aged 40–49 years and in 21.6% of women aged 50–59 years) (23).
Of the 4970 women in the parent BBD cohort, 433 had no BBD lesion on histopathologic review of BBD biopsy slides. The risk for progression to BC in this group of women, 53 of whom were in the case-control cohort (37 controls, 16 cases), although slightly higher (OR=1.72, 95% CI, 0.92, 3.19) than in the proliferative group (OR=1.66, 95% CI, 1.13, 2.42, p=0.009), did not attain significance at p=0.05, when compared to the nonproliferative reference group. The presence of AH in a BBD biopsy conferred higher risks (OR=3.75, 95% CI 1.99, 7.06, p<.001) which corroborates other studies indicating that the risk of developing breast cancer is directly related to the degree of epithelial atypia (2, 11–14, 16). We have previously reported that, at 10 years of follow-up in the cohort of 4970 women, cumulative breast cancer incidence was significant for both women with proliferative lesions without atypia and for women with atypia (3).
In this study, women with fibroadenoma were significantly younger than women without fibroadenoma, a finding consistent with previous studies (25–28). The average age of women with fibroadenoma and BBD women without fibroadenoma was below 50 years. We found no interaction of age and fibroadenoma (p=0.240), indicating that the effect of presence or absence of this lesion on progression to cancer in women with BBD, may not be age dependent. There is controversy over whether fibroadenomas mark an increased risk for subsequent breast cancer. In this study, the presence of fibroadenoma, regardless of its status as a nonproliferative or proliferative lesion, was protective against BC progression (OR=0.65, 95% CI 0.46, 0.94, p=0.020). A separate analysis indicated that the risks of developing cancer among women with fibroadenoma either as a proliferative or non-proliferative lesion were nearly the same when compared to women with no fibroadenoma as a reference group. Our findings are in conflict with the Nashville group report (25) of fibroadenoma as a long-term risk factor for breast cancer. That study indicated that even among women with fibroadenoma who have no evidence of proliferative disease, breast cancer risk increased 40% to 90% over an average of 22 years of follow-up. Risk was increased in women with complex fibroadenomas, proliferative disease, or a family history of breast cancer (25, 29). In our study, the median length of follow-up of 10.37 years was half that of the Nashville study. However, one recent study drawn from the New Mexico Tumor Registry, reported that fibroadenoma, grouped as a low-risk category with other proliferative lesions without atypia such as hyperplasia and adenosis, did not show an association with subsequent breast cancer development (30). Another, found a low incidence of malignancy in complex fibroadenomas at a mean follow-up of 2 years (28).
Several studies indicate that risk factors associated with promotion of breast cancer, do not operate for benign breast disease and breast cancer (20, 31–34). Several risk factors including exogenous estrogens (35, 36), postmenopausal exogenous female hormone use (37), relation to nulliparity, ages at menarche and birth of first child, age at artificial menopause (35), coffee consumption in the presence of fibrocystic breast disease (38), and alcohol consumption (20), did not appear to increase risk of breast cancer in women with benign breast disease. Therefore, the distinction of BBD subsets with differing risks is clinically important. This large multiethnic study found only the interaction of race and age, lesion risk-level, and fibroadenoma as significant predictors of BC progression. Women with fibroadenoma were less likely to progress to BC, irrespective of whether the fibroadenoma lesion was nonproliferative (simple) or proliferative (complex). Race, as a single variable, was not a factor, supporting previous findings that the risks associated with progression to BC from BBD do not differ across races.
In summary, fibroadenoma is protective against BC; age and race interactions, and BBD histology are the prime risk factors for breast cancer from BBD.
Supplementary Material
Acknowledgments
Support: NIH CA 70923 (MJW), ACS EDT-116 (MJW)
REFERENCES
- 1.Schnitt SJ. Benign breast disease and breast cancer risk: morphology and beyond. Am J Surg Pathol. 2003;27:836–841. doi: 10.1097/00000478-200306000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Hartmann LC, Sellers TA, Frost MH, Lingle WL, Degnim AC, Ghosh K, Vierkant RA, Maloney SD, Pankratz VS, Hillman DW, Suman VJ, Johnson J, Blake C, Tlsty T, Vachon CM, Melton LJ, 3rd, Visscher DW. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353:229–237. doi: 10.1056/NEJMoa044383. [DOI] [PubMed] [Google Scholar]
- 3.Worsham MJ, Abrams J, Raju U, Kapke A, Lu M, Cheng J, Mott D, Wolman SR. Breast cancer incidence in a cohort of women with benign breast disease from a multiethnic, primary health care population. Breast J. 2007;13:115–121. doi: 10.1111/j.1524-4741.2007.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Worsham MJ, Raju U, Lu M, Kapke A, Cheng J, Wolman SR. Multiplicity of benign breast lesions is a risk factor for progression to breast cancer. Clin Cancer Res. 2007;13:5474–5479. doi: 10.1158/1078-0432.CCR-07-0928. [DOI] [PubMed] [Google Scholar]
- 5.Marshall LM, Hunter DJ, Connolly JL, Schnitt SJ, Byrne C, London SJ, Colditz GA. Risk of breast cancer associated with atypical hyperplasia of lobular and ductal types. Cancer Epidemiol Biomarkers Prev. 1997;6:297–301. [PubMed] [Google Scholar]
- 6.Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and white American patients with breast cancer: ethnicity compared with socioeconomic status. J Clin Oncol. 2006;24:1342–1349. doi: 10.1200/JCO.2005.03.3472. [DOI] [PubMed] [Google Scholar]
- 7.Ghafoor A, Jemal A, Ward E, Cokkinides V, Smith R, Thun M. Trends in breast cancer by race and ethnicity. CA Cancer J Clin. 2003;53:342–355. doi: 10.3322/canjclin.53.6.342. [DOI] [PubMed] [Google Scholar]
- 8.Sanders ME, Page DL, Simpson JF, Schuyler PA, Dale Plummer W, Dupont WD. Interdependence of radial scar and proliferative disease with respect to invasive breast carcinoma risk in patients with benign breast biopsies. Cancer. 2006;106:1453–1461. doi: 10.1002/cncr.21730. [DOI] [PubMed] [Google Scholar]
- 9.Degnim AC, Visscher DW, Berman HK, Frost MH, Sellers TA, Vierkant RA, Maloney SD, Pankratz VS, de Groen PC, Lingle WL, Ghosh K, Penheiter L, Tlsty T, Melton LJ, 3rd, Reynolds CA, Hartmann LC. Stratification of breast cancer risk in women with atypia: a Mayo cohort study. J Clin Oncol. 2007;25:2671–2677. doi: 10.1200/JCO.2006.09.0217. [DOI] [PubMed] [Google Scholar]
- 10.Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312:146–151. doi: 10.1056/NEJM198501173120303. [DOI] [PubMed] [Google Scholar]
- 11.Krieger N, Hiatt RA. Risk of breast cancer after benign breast diseases. Variation by histologic type, degree of atypia, age at biopsy, and length of follow-up. Am J Epidemiol. 1992;135:619–631. doi: 10.1093/oxfordjournals.aje.a116341. [DOI] [PubMed] [Google Scholar]
- 12.Carter CL, Corle DK, Micozzi MS, Schatzkin A, Taylor PR. A prospective study of the development of breast cancer in 16,692 women with benign breast disease. Am J Epidemiol. 1988;128:467–477. doi: 10.1093/oxfordjournals.aje.a114995. [DOI] [PubMed] [Google Scholar]
- 13.London SJ, Connolly JL, Schnitt SJ, Colditz GA. A prospective study of benign breast disease and the risk of breast cancer. Jama. 1992;267:941–944. [PubMed] [Google Scholar]
- 14.Dupont WD, Parl FF, Hartmann WH, Brinton LA, Winfield AC, Worrell JA, Schuyler PA, Plummer WD. Breast cancer risk associated with proliferative breast disease and atypical hyperplasia. Cancer. 1993;71:1258–1265. doi: 10.1002/1097-0142(19930215)71:4<1258::aid-cncr2820710415>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 15.Dupont WD, Page DL, Parl FF, Plummer WD, Jr, Schuyler PA, Kasami M, Jensen RA. Estrogen replacement therapy in women with a history of proliferative breast disease. Cancer. 1999;85:1277–1283. doi: 10.1002/(sici)1097-0142(19990315)85:6<1277::aid-cncr9>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs TW, Byrne C, Colditz G, Connolly JL, Schnitt SJ. Radial scars in benign breast-biopsy specimens and the risk of breast cancer. N Engl J Med. 1999;340:430–436. doi: 10.1056/NEJM199902113400604. [DOI] [PubMed] [Google Scholar]
- 17.Cheng J, Qiu S, Raju U, Wolman SR, Worsham MJ. Benign breast disease heterogeneity: association with histopathology, age, and ethnicity. Breast Cancer Res Treat. 2008;111:289–296. doi: 10.1007/s10549-007-9775-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ford ME, Hill DD, Blount A, Morrison J, Worsham M, Havstad SL, Johnson CC. Modifying a breast cancer risk factor survey for African American women. Oncol Nurs Forum. 2002;29:827–834. doi: 10.1188/02.ONF.827-834. [DOI] [PubMed] [Google Scholar]
- 19.Dupont WD, Page DL. Relative risk of breast cancer varies with time since diagnosis of atypical hyperplasia. Hum Pathol. 1989;20:723–725. doi: 10.1016/0046-8177(89)90063-4. [DOI] [PubMed] [Google Scholar]
- 20.Tamimi RM, Byrne C, Baer HJ, Rosner B, Schnitt SJ, Connolly JL, Colditz GA. Benign breast disease, recent alcohol consumption, and risk of breast cancer: a nested case-control study. Breast Cancer Res. 2005;7:R555–R562. doi: 10.1186/bcr1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cowan DFHT. Involution of the breast in women aged 50 to 104 years: a histopathological study of 102 cases. Surg Pathol. 1989;2:323–333. [Google Scholar]
- 22.Hutson SW, Cowen PN, Bird CC. Morphometric studies of age related changes in normal human breast and their significance for evolution of mammary cancer. J Clin Pathol. 1985;38:281–287. doi: 10.1136/jcp.38.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milanese TR, Hartmann LC, Sellers TA, Frost MH, Vierkant RA, Maloney SD, Pankratz VS, Degnim AC, Vachon CM, Reynolds CA, Thompson RA, Melton LJ, 3rd, Goode EL, Visscher DW. Age-related lobular involution and risk of breast cancer. J Natl Cancer Inst. 2006;98:1600–1607. doi: 10.1093/jnci/djj439. [DOI] [PubMed] [Google Scholar]
- 24.Henson DE, Tarone RE. Involution and the etiology of breast cancer. Cancer. 1994;74:424–429. doi: 10.1002/cncr.2820741330. [DOI] [PubMed] [Google Scholar]
- 25.Dupont WD, Page DL, Parl FF, Vnencak-Jones CL, Plummer WD, Jr, Rados MS, Schuyler PA. Long-term risk of breast cancer in women with fibroadenoma. N Engl J Med. 1994;331:10–15. doi: 10.1056/NEJM199407073310103. [DOI] [PubMed] [Google Scholar]
- 26.Foxcroft LM, Evans EB, Porter AJ. The diagnosis of breast cancer in women younger than 40. Breast. 2004;13:297–306. doi: 10.1016/j.breast.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 27.Markopoulos C, Kouskos E, Mantas D, Kontzoglou K, Antonopoulou K, Revenas Z, Kyriakou V. Fibroadenomas of the breast: is there any association with breast cancer? Eur J Gynaecol Oncol. 2004;25:495–497. [PubMed] [Google Scholar]
- 28.Sklair-Levy M, Sella T, Alweiss T, Craciun I, Libson E, Mally B. Incidence and management of complex fibroadenomas. AJR Am J Roentgenol. 2008;190:214–218. doi: 10.2214/AJR.07.2330. [DOI] [PubMed] [Google Scholar]
- 29.McDivitt RW, Stevens JA, Lee NC, Wingo PA, Rubin GL, Gersell D. Histologic types of benign breast disease and the risk for breast cancer. The Cancer and Steroid Hormone Study Group. Cancer. 1992;69:1408–1414. doi: 10.1002/1097-0142(19920315)69:6<1408::aid-cncr2820690617>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 30.Ashbeck EL, Rosenberg RD, Stauber PM, Key CR. Benign breast biopsy diagnosis and subsequent risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:467–472. doi: 10.1158/1055-9965.EPI-06-0394. [DOI] [PubMed] [Google Scholar]
- 31.Wang DY, Fentiman IS. Epidemiology and endocrinology of benign breast disease. Breast Cancer Res Treat. 1985;6:5–36. doi: 10.1007/BF01806008. [DOI] [PubMed] [Google Scholar]
- 32.Rohan TE, Jain M, Miller AB. Alcohol consumption and risk of benign proliferative epithelial disorders of the breast: a case-cohort study. Public Health Nutr. 1998;1:139–145. doi: 10.1079/phn19980023. [DOI] [PubMed] [Google Scholar]
- 33.Rohan TE, Jain M, Miller AB. A case-cohort study of diet and risk of benign proliferative epithelial disorders of the breast (Canada) Cancer Causes Control. 1998;9:19–27. doi: 10.1023/a:1008841118358. [DOI] [PubMed] [Google Scholar]
- 34.Brinton LA. Relationship of benign breast disease to breast cancer. Ann N Y Acad Sci. 1990;586:266–271. doi: 10.1111/j.1749-6632.1990.tb17815.x. [DOI] [PubMed] [Google Scholar]
- 35.Thomas DB, Persing JP, Hutchinson WB. Exogenous estrogens and other risk factors for breast cancer in women with benign breast diseases. J Natl Cancer Inst. 1982;69:1017–1025. [PubMed] [Google Scholar]
- 36.Dupont WD, Page DL, Rogers LW, Parl FF. Influence of exogenous estrogens, proliferative breast disease, and other variables on breast cancer risk. Cancer. 1989;63:948–957. doi: 10.1002/1097-0142(19890301)63:5<948::aid-cncr2820630527>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 37.Byrne C, Connolly JL, Colditz GA, Schnitt SJ. Biopsy confirmed benign breast disease, postmenopausal use of exogenous female hormones, and breast carcinoma risk. Cancer. 2000;89:2046–2052. doi: 10.1002/1097-0142(20001115)89:10<2046::aid-cncr3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 38.Rosenberg L, Miller DR, Helmrich SP, Kaufman DW, Schottenfeld D, Stolley PD, Shapiro S. Breast cancer and the consumption of coffee. Am J Epidemiol. 1985;122:391–399. doi: 10.1093/oxfordjournals.aje.a114120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.