Abstract
Objective
Computerized decision support systems (CDSS) are commonly deployed to support prescribing, although over-riding of alerts by prescribers remains a concern. We aimed to understand how general practitioners (GPs) interact with prescribing CDSS in order to inform deliberation on how better to support prescribing decisions in primary care.
Materials and methods
Quantitative and qualitative analysis of interactions between GPs, patients, and computer systems using multi-channel video recordings of 112 primary care consultations with eight GPs in three UK practices.
Results
132 prescriptions were issued in the course of 73 of the consultations, of which 81 (61%) attracted at least one alert. Of the total of 117 alerts, only three resulted in the GP checking, but not altering, the prescription. CDSS provided information and safety alerts at the point of generating a prescription. This was ‘too much, too late’ as the majority of the ‘work’ of prescribing occurred prior to using the computer. By the time an alert appeared, the GP had formulated the problem(s), potentially spent several minutes considering, explaining, negotiating, and reaching agreement with the patient about the proposed treatment, and had possibly given instructions and printed an information leaflet.
Discussion
CDSS alerts do not coincide with the prescribing workflow throughout the whole GP consultation. Current systems interrupt to correct decisions that have already been taken, rather than assisting formulation of the management plan.
Conclusions
CDSS are likely to be more acceptable and effective if the prescribing support is provided much earlier in the process of generating a prescription.
Keywords: Clinical Computerised Decision Support (CDSS), Electronic Health Record, Prescribing Safety, Primary Care, Safety Alerts
Background and significance
Clinical computerized decision support systems (CDSS) are increasingly being used globally in the policy drive to improve the safety of prescribing.1–5 Although an increasing evidence base6–8 has demonstrated that CDSS has the potential to improve clinical outcomes,9 10 11 prevent adverse events from drug–drug interactions,12 and offer prescribing efficiency for healthcare professionals,13 overall effectiveness in clinical practice is unclear.14 Despite early promise in the context of bespoke CDSS in US secondary care,15 16 a recent systematic review found few studies showing substantial improvements in prescribing behavior.17
A decade after concerns were raised that only a minority of primary care systems provided alerts about penicillin allergies,18 CDSS is universal in UK primary care and has broadened to include warnings of real and potential adverse drug effects.5 8 However, inaccurate coding of allergies,9 spurious alerts incurring user frustration and inappropriate clinical responses,10 19 and workarounds which bypass intrinsic safety features15 have been identified.
A particular concern is that excessive alerts, exacerbated in contemporary practice when procedural information and cost advice are provided in addition to safety alerts, may be counterproductive as they lead to user fatigue.12 20 Responses to this concern have included restricting alerts, tailoring them towards specialists/generalists, and modifying the ways in which the information is presented, including incorporating ‘hard stop’ blocks for particularly high risk treatments.21–25 Alerts that offer the prescriber choice, and require reasons for deviation from protocol may be more effective than those that can simply be over-ridden.20 26 These solutions, however, are minor adjustments to the existing model of CDSS: the more fundamental question is how CDSS and the alerts they generate integrate into prescribing workflows within the consultation (or clinic visit).27–29
Crucially, designers must understand the ‘typical’ workflow within a consultation22 30 31 in order to align the operation of medical safety alerts24 32 with the tasks of the responsible clinician19 both efficiently and effectively.13 33 Hence, a holistic view of how medication decisions are made throughout the consultation is needed that not only takes into account what and when ‘prescribing-related talk’ occurs and what prescribing guidelines are followed,5 but also interprets the decision-making processes34 and how clinical narratives are constructed.35 36
Our study (INTERACT-IT), one of a series commissioned by the National Health Service (NHS) Connecting for Health Evaluation Programme,37–40 investigated the impact of technology on the interactions between a patient and their healthcare professional, offering a unique opportunity to understand how prescribing and CDSS operate within the context of a general practice consultation.
Materials and methods
A detailed account of the methodology of the INTERACT-IT study is available in the final report.41 The methods pertaining to our analysis of CDSS and general practitioner (GP) prescribing are described below.
Ethics
We obtained ethics approval from the Leeds East Multicentre Research Ethics Committee (MREC: 09/H1306/60) and governance approval from all NHS trusts. All participants provided informed consent in three stages: (i) consent to record the consultation; (ii) consent to view and analyze the recorded consultation; and (iii) consent to be contacted at a later date for permission to use the video recording or still images for dissemination or further research.
Practice, GP, and patient recruitment
We purposively sampled GP practices to ensure a spread of demography, size, and clinical software, and invited up to three GPs in each practice to participate. Arrangements were made to record whole clinics for each participating GP during two site visits approximately 6 months apart. GP clinics consist of a block of appointments, typically booked at 10 min intervals over 2–3 h. Patients were advised about the research when booking an appointment in a recorded clinic. On arrival for their consultation, patients were directed to a researcher who took informed consent (or halted recording if the patient did not wish to participate).
Multi-channel video recording
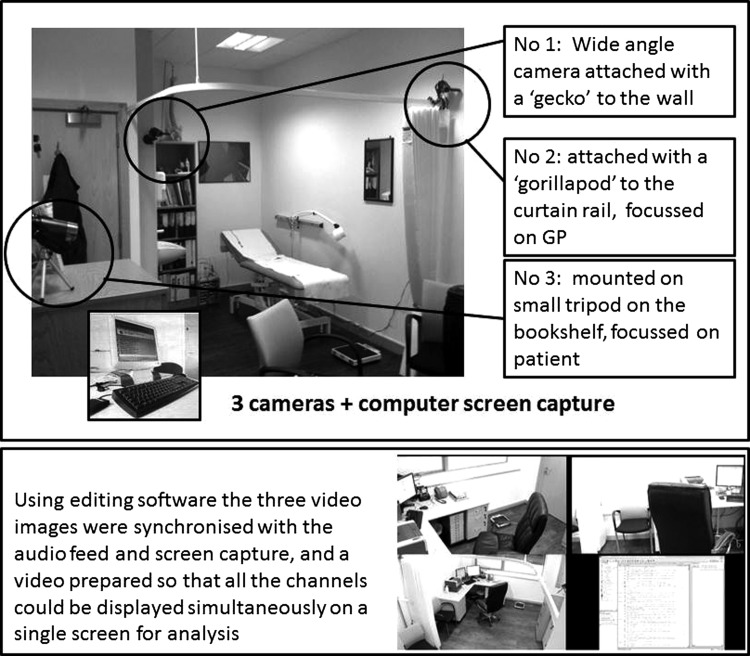
We used established methodology to undertake multi-channel video (MCV) recordings of consultations.42–44 Three cameras recorded the clinician's head and upper body, the patient's upper body, and a wide angle view of the consultation (see figure 1). A video output recorder captured the computer screen and data entry. Using editing software, the three video images were later synchronized with the audio feed and screen capture, and a video prepared so that all the channels could be displayed simultaneously on a single screen for analysis.
Figure 1.
Multi-channel video recording.
Data analysis
Timing computer tasks
We defined the ‘patient consultation’ as starting with the patient's entrance and ending with their exit from the room. The timing of computer based activities was recorded using OBSWIN software (V.3; Antam, London, UK) which enables trained raters to mark occurrences and durations of user-defined activities within a consultation. We defined ‘prescribing using the computer’ as occurring in one or more bouts which began when the GP turned to use the computer for prescribing and ended when they turned away to the patient or other task or picked up the last prescription for signing. Other computer tasks observed are given in online supplementary table S1.
Roter Interaction Analysis System coding of talk
The validated Roter Interaction Analysis System (RIAS)45 is a widely used tool which codes the verbal ‘utterances’ of both the patient and clinician into 41 mutually exclusive categories (40 clinician and 29 patient categories) designed to reflect the content and context of routine medical dialogue. Pertinent to our analysis of prescribing were doctor and patient talk about on-going or future treatment plans coded as the RIAS category ‘thera’ for ‘therapeutic talk.’
Data from RIAS coding were amalgamated with the OBSWIN time data using an MS Access database so that we could identify when the therapeutic talk occurred relative to the computer task of prescribing.
Analysis of prescribing alerts and responses
We classified each prescription as a ‘new acute,’ ‘copied acute’ (when an acute prescription is copied from the medication history), ‘new repeat’ (when a new drug is authorized as a repeat prescription), ‘re-authorization of existing repeat prescription’ or ‘issuing of previously authorized repeat prescriptions.’ Two researchers (JH and HP) independently observed the number, type of alert (see table 1 for definitions) and associated dialogue boxes presented by the system in connection with each prescription. We classified the clinician as having responded to an alert (if they took action such as selecting an alternative drug, changing a dose, checking with the patient, or seeking information from a formulary) or dismissed an alert (if they over-rode it with no apparent attention).
Table 1.
Definitions of prescribing information and safety alerts
| Classification of alerts generated by the CDSS systems on GP computers | |
|---|---|
| Background information | These highlight a patient's allergies, intolerances, and adverse drug events on the prescribing screen. The information may be re-presented as a safety alert if the prescriber selects, say, a drug to which the patient is allergic. |
| Safety alerts | These are allergies or previous adverse reactions or significant clinical warnings which interrupt prescribing and demand a response from the prescriber. |
| Process warnings | These provide practical information about packs and availability, frequency of prescribing, warnings about authorization of repeat prescriptions, etc. They interrupt prescribing and demanded a response from the prescriber. |
| ‘Add-ins’ | These are presentations of information provided by local managing agents (eg, PCT prescribing advisors) which recommended cheaper alternatives. They also interrupt prescribing and demand a response from the prescriber. |
CDSS, computerized decision support systems; GP, general practitioner; PCT, primary care trust.
We focused our detailed analysis on the ‘new acute’ prescriptions because these offered an opportunity to study the whole process of deciding on treatment and prescribing a new drug, as opposed to repeat prescriptions where much of this process may have occurred in previous consultations.
Qualitative analysis of the work of prescribing
All consultations were transcribed verbatim and transcripts of consultations which included one or more new acute prescriptions were examined for all conversation relating to prescribing. This could include deliberation about the potential benefits and side effects of proposed treatment, exploration of experience with previously prescribed medications (including allergies), and giving of instructions about the proposed treatment regime. All dialogue related to prescribing was coded into a framework developed inductively by JH and HP (see online supplementary table S2). Emerging themes were discussed with the multidisciplinary team.
Results
We observed a total of 112 GP consultations from the clinics of eight GPs in three practices (identified by pseudonym in this report) (table 2). Three GPs in one practice (Seaside) used INPS Vision software and five GPs in two practices (Church and Hills) used EMIS: these are the two most widely used clinical software systems in the UK, accounting for three-quarters of the market share (EMIS: 55%, INPS Vision: 19%).41 All the practices were ‘paperless,’ and both EMIS and INPS Vision provide a complete electronic health record (EHR) for registered patients, including details of GP and practice nurse consultations, administrative tasks, prescriptions, investigation results, and electronic versions of correspondence. Detailed descriptions of the practices and their computer systems are in the final report.42
Table 2.
Characteristics of GPs and practices
| Parameter | Characteristic | GPs (n=8) |
|---|---|---|
| GP practice | Church: medium sized urban practice | 3 |
| Seaside: large practice in a coastal town | 3 | |
| Hills: small rural practice | 2 | |
| GP sex | Male | 6 |
| Female | 2 | |
| Age bands | 25–44 years | 6 |
| 45–64 years | 2 | |
| Computer system | EMIS | 5 |
| INPS Vision | 3 |
GP, general practitioner.
Frequency of alerts
Overall, 132 prescriptions were issued in the course of 73 of the consultations, of which 81 (61%) attracted at least one alert. Multiple alerts occurred in 29 (22%) prescriptions, with five interrupted by three or more alerts, each of which could require two or more mouse clicks and (depending on the system) an obligatory entry of a comment to explain why the alert was being over-ridden. In addition, background information about the presence/absence of known allergies, interactions, or adverse events was presented for all new prescriptions. Table 3 gives details of the number of alerts occurring in the process of generating different types of prescriptions.
Table 3.
Prescriber alerts and warnings in general practice
| Prescription mode | New acute | New acute (copied) | New repeat | Re-authorize repeat | Issue existing repeat | Total |
|---|---|---|---|---|---|---|
| Number of prescriptions | 62 | 16 | 14 | 28 | 12 | 132 |
| Number of prescriptions with background information | 62 | 16 | 14 | 6 | 0 | 98 |
| Number of prescriptions interrupted by an alert or warning, n (%) | 31 (50) | 11 (69) | 3 (21) | 4 (14) | 0 | 49 (37) |
| Number of different types of alerts and warnings interrupting prescribing and requiring a response | ||||||
| Safety alerts | 35 | 13 | 3 | 4 | 0 | 55 |
| Process warnings | 15 | 4 | 8 | 25 | 5 | 57 |
| Add-ins | 3 | 0 | 2 | 0 | 0 | 5 |
| Total interruptions | 53 | 17 | 13 | 29 | 5 | 117 |
Copied acute: a ‘one-off’ prescription which is copied from the medication history; Issuing existing repeat: a prescription is issued from a previously authorized repeat prescription; New acute: a prescription issued as a ‘one-off’ typically in response to a presenting problem in a consultation; New repeat: a new drug authorized to be issued on a defined number of occasions; Re-authorize repeat: an existing repeat is re-authorized to be issued on a further defined number of occasions.
Of the total of 117 alerts, 55 were safety alerts and 57 were process warnings, with an further five generated by ‘ScriptSwitch’ (an additional system funded by the local healthcare trust to advise on cost-effective prescribing) in one of the practices. Only three (2%) of the alerts resulted in apparent action on the part of the GP: in each case the GP checked the prescribing information (in a formulary or computer database) but did not subsequently alter the prescription.
New acute prescriptions and work of prescribing
Our subsequent analysis focuses on the 62 new acute prescriptions observed in the course of 49 consultations. The characteristics of the patients in these consultations are given in table 4. We present our results in two stages:
Quantitative analysis of the duration and timing of therapeutic talk within the consultation (as defined by the RIAS code ‘thera’46) in relation to the timing of computer tasks (OBSWIN)
Qualitative analysis of the transcribed consultation to explore the ‘work’ of prescribing.
Table 4.
Characteristics of the patients receiving new acute prescriptions
| Parameter | Characteristic | n=47 |
|---|---|---|
| Sex | Male | 12 |
| Female | 35 | |
| Age bands (years) | 0–4 | 2 |
| 5–15 | 0 | |
| 16–24 | 4 | |
| 25–44 | 4 | |
| 45–64 | 19 | |
| 65–74 | 7 | |
| 75+ | 10 |
Therapeutic talk in the consultation
Analysis of RIAS-coded utterances (table 5) revealed that over half the therapeutic talk by doctor and patient occurred before the computer was used to generate the prescription. Therapeutic talk accounted for 40% of all GP utterances in consultations resulting in a new acute prescription, 53% of which occurred before the computer was used to process the prescription and 72% before the prescription was printed. Similarly, therapeutic talk contributed 16% of the patient's conversation, with 79% occurring before the prescription was printed.
Table 5.
RIAS analysis
| Before prescribing | During prescribing | After prescribing | Total | |
|---|---|---|---|---|
| Mean duration of consultation in seconds (minutes and seconds) | 347 (5 min 47 s) | 107 (1 min 47 s) | 90 (1 min 30 s) | 543 (9 min 3 s) |
| Mean number of utterances in phase (percentage of total consultation) | ||||
| All GP utterances | 80.0 (62) | 24.7 (19) | 24.4 (19) | 129.1 (100) |
| All patient utterances | 63.3 (67) | 15.3 (16) | 16.5 (17) | 95.0 (100) |
| GP ‘therapy’ utterances | 27.4 (53) | 9.7 (19) | 14.6 (28) | 51.7 (100) |
| Patient ‘therapy’ utterances | 7.4 (49) | 4.4 (30) | 3.1 (21) | 14.9 (100) |
Mean duration, mean number, and type of utterances before, during, and after the computer is used to generate a prescription are shown.
‘All utterances’ includes all types of talk including those about therapy. ‘Therapy utterances’ include any talk about medication or treatment.
GP, general practitioner; RIAS, Roter Interaction Analysis System.
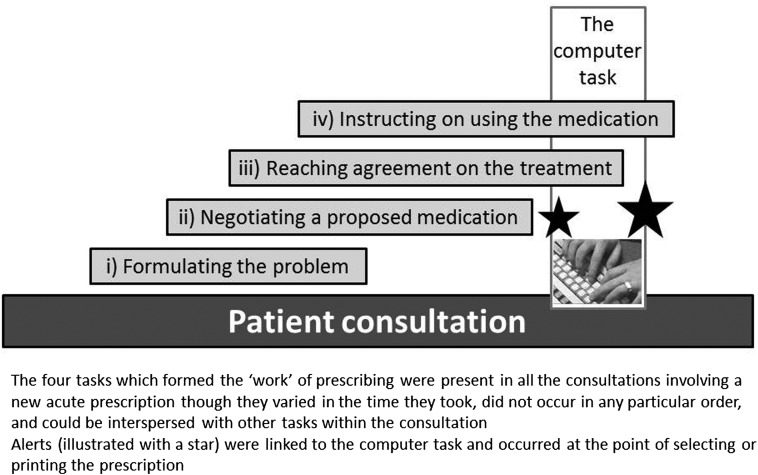
The ‘work’ of prescribing
In addition to the computerized process of generating a prescription, we identified four tasks which formed the ‘work’ of prescribing: (i) formulating the problem such that a prescription was justified; (ii) negotiating a proposed medication; (iii) reaching agreement on the treatment; and (iv) instructing the patient on using the medication. Figure 2 offers a schema of this process. These tasks were present (implicitly or explicitly) in all the consultations involving a new acute prescription, although they varied in the time they took, did not occur in any particular order, and could be interspersed with other tasks within the consultation. Online supplementary table S3 sequences all tasks for a single case and online supplementary table S4 provides additional examples of the tasks which we describe below.
Figure 2.
Schema of the work of prescribing.
Formulating the problem such that a prescription was justified
The GP typically formulated the problem after taking the history and performing an examination. If a prescription were to be proffered, formulation was done in a way that led naturally to treatment:
GP: Alright, definitely a patch at the bottom of that right lung, definitely a patch of infection on the actual chest, rather than just a bronchitis actually… you're definitely going to need some antibiotics, you know… [Seaside.GP1.P3]
On occasion the formulation was contested, or further information was forthcoming and the problem subsequently re-formulated.
If the patient initially formulated the problem, different narratives were used. A few patients presented the formulation as a direct request:
I've got this cough and I've got this chest. And I'm going away next week so I wanted some antibiotics or something [Seaside.GP2.P7]
More often, patients’ formulations were phrased tentatively, or as a question, and sometimes the patient presented credentials (eg, previous experience) to support their own formulation. For example, a patient prefaced her request for antibiotics for diverticulitis:
I've had this problem for ages… I'm not a doctor I know… I had a similar problem but not quite as bad as this [Seaside.GP1f.P4]
Patient formulations usually led to a discussion in which the situation was clarified prior to the GP reformulating the problem leading (or not) to a prescription.
Negotiating a proposed medication
We observed great variety in how negotiations between the GP and patient unfolded, and the range of stratagems or tactics employed. Negotiation could precede formulation as the GP explored previous experience with a drug, or ‘thought out loud’ offering the opportunity to ‘test’ a patient's attitude to a prescription before formally suggesting it.
… your blood pressure isn't perfect. Em, the question is whether or not we persevere with it and try and get it down lower [Church.GP2.P2]
Usually, once the GP had formulated the problem, agreement was reached, although sometimes this involved several minutes of clarifying indications, perhaps with reference to test results, explaining potential benefits and side effects, outlining practical advantages, or discussing side effects. We saw occasional examples of direct refusals, although more often the negotiation was about seeking and giving information. For example, a patient with concerns about side effects spent several minutes discussing the rationale for taking simvastatin:
Patient: But it does make (SIGHS)—I cannae really describe what it makes us feel like but not as good as normal
GP: Yeah. It's [taking a statin] a good way of doing it, em, eh, and the cholesterol blocks up your arteries basically… and makes your, makes your heart worse and causes strokes and that sort of thing.
Patient: Ah, yeah. Well, I wouldn't really like that… [Hills.GP1.P8]
Patient formulation was usually followed by extensive negotiation as the GP gathered clinical information and then re-formulated the problem. This might reiterate the patient's formulation (eg, after the patient stated a need for an antibiotic for a chest infection, the GP explicitly reformulated the problem when he discovered clinical signs on chest examination (Seaside.GP2.P7) or entail the GP proffering an alternative course of action.
Reaching agreement on the treatment
The amount of time taken to reach the point of concordance varied greatly and in some cases was instantaneous:
GP: … in the meantime we've stopped your tablet for the acid, the lansoprazole…. I want to give you something different
Patient: Thank you, that's what we want, so that's that [Hills.GP2.P2]
In other cases the decision making was much more protracted:
GP: It [a possible adverse reaction] might be a coincidence but it might not be so we can try and start it [a statin] again and see if it happens again
Patient: Well I wouldn't like to take them again [Church.GP1.P1]
After about a minute of discussion about side effects and cholesterol levels and risks, the GP suggested an alternative statin and the patient agreed to ‘give it a go.’
Instructing the patient on using the medication
Commonly, instructions (sometimes supported by printed information leaflets) about the use of the medication and follow-up arrangements were given while the computer was being used to generate the prescription or after the prescription had been printed and signed. However, practical information about how a drug was taken could also occur as part of the earlier negotiations. For example, a discussion of the twice daily administration of a long-acting β2 agonist inhaler was cited by the GP as an advantage of adding in the new treatment (Hills.GP2.P8).
The processing of generating the prescription
Structure of the task
We observed three phases in the prescribing task of generating a prescription: (i) selecting drug(s); (ii) responding to options; and (iii) printing the prescription (see examples in table 6). Turning to the computer could offer a break in conversation to concentrate on treatment options, although at times GPs talked throughout this process, giving instructions on dosage, mode, and timing, and sometimes explaining to the patient what they were doing.
Table 6.
Examples of the process of generating prescriptions using the EMIS or INPS Vision system
| EMIS (Hills.GP1.P09) | Vision (Seaside.GP1f.P16) | |||||||
|---|---|---|---|---|---|---|---|---|
| Time (minutes into the consultation) | Processing | Information and alerts | Conversation | Time (minutes into the consultation) | Processing | Information and alerts | Conversation | |
| 1. Selecting drugs | 06:19 | ‘Add Drugs’ dialogue box called up GP begins typing drug name |
Background information on bottom section of ‘Add Drugs’ dialogue box indicates ‘ADVERSE REACTION TO PENICILLIN’ | GP: Some Naspetin cream | 08.02 | From Top menu, GP picks drop-down menu; ‘Acute therapy add’ dialogue box displayed (in middle bottom screen work area) |
Background information: ‘No Drug Allergies recorded’ (highlighted in red) | Patient: It's the fact that it never goes down…you know, it's not GP: You want to do one or the other, do the diet or do the colpermin because you won't know which is helping otherwise, will you? So try the tablets first and then try your diet. Patient: Right, right, yes please. Yeah |
| 06:29 | NASEPTIN appears in Drug Name bar GP clicks option button <no action required> to dismiss the warning |
Process warning: ‘Your selection has a generic equivalent which is more appropriate’ | 08:12 | GP types in drug name to the ‘Acute therapy add’ dialogue box. Form self-populates with default details | ||||
| 2. Choosing options (working through system dialogue) | 06:33 | Still within ‘Add Drugs’ dialogue box GP clicks option button <no action required> to dismiss the warning GP types in dosage GP types in quantity |
General warning: ‘This product contains Arachis (PEANUT) oil’ | GP: Three times a day Patient: Right |
08:13 | GP clicks switch to generic option GP selects ‘Information: pack details’ which displays options; pack size selected and appears in form |
GP: Can I do your blood pressure? Patient: Please. I think I'll need a prescription soon of my three tablets, I don't know if you'd rather do them today or not. GP: I can do it yeah, yeah. It's no problem. I can give you enough for a few packets worth 'cause that'll give you enough time to try. Patient: …and if it goes down again I'll start with the em Movicol again. GP: Yeah |
|
| 08:30 | GP clicks option button <no action required> to dismiss the warning | Add-in (‘Pharmacy Support’) on options for Peppermint oil | ||||||
| 06.38 | GP turns away from computer to instruct patient GP completes the systems dialogue while continuing to give instructions |
GP: First aid measures. If you do get a nose bleed squeeze your nose. | 08.34 | GP clicks option button <proceed> to dismiss the warning |
Process warning (on quantity) | |||
| 08:36 | GP clicks option buttons <proceed> and <OK> to dismiss the warning |
Safety alert: Two advice warnings to advise patients with IBS to consult a doctor if symptoms change or persist for more than 2 weeks | ||||||
| 3. Printing | 07:17 | GP moves cursor to <Issue> button which is clicked, highlighting the Issue Medication; (Dispense) option | GP: If it doesn't settle down, then you do need to get it packed sometimes, but I think this will all settle down. | 08:43 | Consultation Manager Form Clicks option button <Print> |
Patient: Before it's not bothered me when I took the Movicol, but this time I think I've been so tender… | ||
GP, general practitioner; IBS, irritable bowel syndrome.
Information, alerts, and GP responses
In both Vision and EMIS, background information on allergies and adverse reactions was presented as soon as the prescribing dialogue box was activated.
Examples of the presentation of safety alerts in the two systems are given in table 6. Safety alerts relating to patient-specific allergies and drug interactions, and general information about contra-indications interrupted the clinician's workflow (ie, requiring a response) either at the point of selecting the drug (in EMIS) or just before sending the prescription to print (EMIS and Vision). Process alerts (eg, warnings about prescribing frequency, duplicated prescriptions) also requiring a response from the GP, pre-empted printing especially in Vision. Add-ins such as ‘ScriptSwitch’ interrupted Vision users on one site with further alerts.
Discussion
Summary of findings
CDSS alerts do not integrate with the flow of prescribing work within the consultation. Current systems interrupt to correct decisions already made rather than to assist earlier deliberations. By the time an alert appears the GP will have formulated a problem, potentially spent several minutes considering, explaining, negotiating, and reaching agreement with the patient, possibly given instructions, and printed an information leaflet about the proposed treatment. An alert in the final seconds of the task of generating the paper prescription is likely to be regarded as intrusive and unwelcome, and increases the probability of it being ignored.
Strengths and limitations
Our use of MCV recording enabled an in-depth understanding of the process of prescribing as we were able to integrate the conversation, patient, professional actions, and computer use within the consultation. Although both patient and GP were aware of the recording, there is evidence that this does not significantly affect the process of the consultation,47 48 and our use of discrete cameras ensured that the process was as unobtrusive as possible (see figure 1). Our mixed method analysis enabled us both to quantify the issue and to use qualitative analysis to understand the process of prescribing which the CDSS seeks to influence.
The eight participating GPs may not be representative of the range of professionals, although we recruited clinicians with diverse personal and practice demographics. Their willingness to be videoed may reflect relative confidence in their consulting skills and computer use, although the particular task of interest (computer prescribing) is routine within primary care. We may not have captured the full range of prescribing scenarios, although by recording whole clinics we hoped to capture a typical profile of primary care consultations. To enable assessment of transferability to other healthcare contexts,49 we have provided a description of the practices, clinicians, and computer systems (table 2) and characteristics of the patients (table 4) and the process of prescribing using the two computer systems (table 6).
Our study was UK-based, although the challenge of implementing effective decision support for prescribing, and specifically the problem of alert fatigue, is international.12 24 50
Designing to support prescribing workflow
Studies undertaken in a secondary care setting have suggested the importance of presenting alerts at the point of selecting drugs (as opposed to the moment of printing),22 however, our data from a primary care setting suggest that this is already too late. Decisions about prescribing were made by the GPs, often in negotiation with the patient, long before the GP turned to the computer to generate the paper prescription.
The implication of this observation is that information about potential treatments, ideally personalized to the individual clinical scenario, need to be presented at the point in the consultation when the GP is considering prescribing, not when a negotiated prescription is about to be printed. For example, background information about allergies and previous adverse reactions might be made available as part of ‘summary pages’ in the computer system and visible at all times. Acute prescriptions are not uncommonly copied from previous episodes, exploration of which is often part of history taking. ‘Hovering’ over the previous entry could reveal the associated clinical entry and any relevant adverse reactions. A coded diagnosis could trigger provision of context and patient specific information—and possibly guideline-based advice—that could be incorporated into the decision-making process. Rather than just blocking a chosen prescription (eg, to avoid an interaction with warfarin), a system might offer alternative safer suggestions. Recognized user frustration with alert mechanisms12 and the perception that CDSS is a threat to physician autonomy22 51 might be diminished if these functions were perceived to be supporting the natural workflow of prescribing within the consultation.
Currently, once a clinician has opened the prescribing dialogue box, access to other pages in the EHR containing crucial information (such as latest renal function) may not be possible. Adapting the process to enable access to core information from the history throughout the process of prescribing could facilitate better decision making. Clinicians may learn to adapt their computer use habits if they perceive benefit. Entering a diagnosis before processing a prescription, as required by the EMIS software, was perceived as ‘annoying,’ but if entering a coded diagnosis (at the point of formulating the problem) gave access to relevant guidance tailored to the individual patient, it might be more likely to be adopted.
The work of prescribing is tacit, while that required for processing the prescription involves explicit, codified rules and procedures.52 Balancing these two modes of knowledge underpins safe prescribing and CDSS needs to be configured to support the entire work of prescribing rather than as a block task often competing for attention with instruction and planning needs for the patient.
Conclusion
CDSS has become the norm for GP prescribing in the UK and many other countries but is concentrated in the form of information and safety alerts issued at the point of prescribing. Our data, from observation of real-life consultations, can help designers and users understand how to align CDSS functionality with the GP perspective on the work of prescribing. Crucially, the natural flow of clinical decision making should be facilitated, not interrupted by the pre-ordained computer workflow unless a major threat to patient safety has arisen. The aim of an effective CDSS should be to prevent the occurrence of safety alerts at the time of prescribing by providing all necessary information and support earlier in the process.
Acknowledgments
We are grateful to the officers of the Primary Care Research Networks in supporting practice recruitment and to the practices, GPs, and administrative staff for their active participation, and the patients who agreed to have their consultations videoed. We thank Professor Simon de Lusignan who chaired the Independent Steering Committee supported by Antony Chuter (lay advisor), Stephen Corbett (Connecting for Health sponsor), and Lee Priest (representing the funder). We also acknowledge the contributions of Dr Guro Huby (an investigator on the project until her retirement), Elizabeth Neill (study secretary), Dr John Harries (researcher in the early stages of the project), and Andy Pryde (technical advisor on video recording).
Footnotes
Contributors: HP, AS, KC, BF, and RW conceived the idea for the study, developed the protocol, and secured funding. HP was the Principal Investigator and with AS, KC, BF, and RW led study administration, data analysis, and interpretation of results. JH, FT, and HM undertook the data collection, handling of data, and data analysis. SB assisted with RIAS coding and analysis. All authors had full access to all the data, and were involved in interpretation of the data. JH and HP wrote the initial draft of the paper, to which all the authors contributed. HP is the study guarantor.
Funding: This work was funded by the National Health Service Connecting for Health Evaluation Programme (NHS CFHEP 010). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NHS CFH Evaluation Programme or the Department of Health. HP is supported by a Primary Care Research Career Award from the Chief Scientist's Office of the Scottish Government.
Competing interests: All authors have completed the Unified Competing Interests form at http://www.icmje.orge/coi_disclosure.pdf (available on request from the corresponding author) and declare that (1) all authors have support in the form of a grant from the National Health Service (NHS) Connecting for Health Evaluation Program for the submitted work; (2) no author has a relationship with any company that might have an interest in the submitted work in the previous 3 years; (3) no author's spouse, partner, or children have financial relationships that may be relevant to the submitted work; and (4) no author has non-financial interests that may be relevant to the submitted work.
Ethics approval: The Leeds East Multicentre Research Ethics Committee supported this study (MREC: 09/H1306/60).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Department of Health Building a safer NHS for patients: improving medication safety. London: DH, 2004 [Google Scholar]
- 2.Corrigan JM, Donaldson MD, Kohn LT, et al. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academy Press, 2001 [Google Scholar]
- 3.Stroetmann EN, Thierry J-P, Stroetmann KAA, et al. eHealth for safety. Impact of ICT on patient safety and risk management. Luxembourg: Office for Official Publications of the European Communities, 2007 [Google Scholar]
- 4.Safety and Quality Council Second national report on patient safety, improving medication safety. Canberra: Australian Council for Safety and Quality in Health Care, 2002 [Google Scholar]
- 5.Duerden M, Millson D, Avery A, et al. The quality of GP prescribing. An inquiry into the quality of general practice in England. London: The Kings Fund, 2011 [Google Scholar]
- 6.Pearson SA, Moxey A, Robertson J, et al. Do computerised clinical decision support systems for prescribing change practice? A systematic review of the literature (1990–2007). BMC Health Serv Res 2009;9:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avery T. Avoidable prescribing errors: communication and monitoring. Prescriber 2010;21:44–6 [Google Scholar]
- 8.Kaushal R, Shojania KG, Bates DW. Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review. Arch Intern Med 2003;163:1409–16 [DOI] [PubMed] [Google Scholar]
- 9.Kaelber DC, Bates DW. Health information exchange and patient safety. J Biomed Inform 2007;40(6 Suppl):S40–5 [DOI] [PubMed] [Google Scholar]
- 10.Karnon J, McIntosh A, Dean J, et al. A prospective hazard and improvement analytic approach to predicting the effectiveness of medication error interventions. Saf Sci 2007;45:523–39 [Google Scholar]
- 11.Avery AJ, Barber N, Ghaleb M, et al. Investigating the prevalence and causes of prescribing errors in general practice: The PRACtICe Study. 2012. A report for the GMC. http://www.gmc-uk.org/about/research/12996.asp (accessed Sep 2012). [Google Scholar]
- 12.Kuperman GJ, Bobb A, Payne TH, et al. Medication-related clinical decision support in computerized provider order entry systems: a review. J Am Med Inform Assoc 2007;14:29–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schade CP, Sullivan FM, de Lusignan S, et al. e-Prescribing, efficiency, quality: lessons from the computerization of UK family practice. J Am Med Inform Assoc 2006;13:470–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Black AD, Car J, Pagliari C, et al. The impact of eHealth on the quality and safety of health care: a systematic overview. PLoS Med 2011;8:e1000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koppel R, Metlay JP, Cohen A, et al. Role of computerized physician order entry systems in facilitating medication errors. JAMA 2005;293:1197–203 [DOI] [PubMed] [Google Scholar]
- 16.Sittig DF, Wright A, Osheroff JA, et al. Grand challenges in clinical decision support. J Biomed Inform 2008;41:387–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schedlbauer A, Prasad V, Mulvaney C, et al. What evidence supports the use of computerized alerts and prompts to improve clinicians’ prescribing behavior? J Am Med Inform Assoc 2009;16:531–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernando B, Savelyich BS, Avery AJ, et al. Prescribing safety features of general practice computer systems: evaluation using simulated test cases. BMJ 2004;328:1171–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cresswell KM, Sheikh A. Information technology-based approaches to reducing repeat drug exposure in patients with known drug allergies. J Allergy Clin Immunol 2008;121:1112–17 [DOI] [PubMed] [Google Scholar]
- 20.van der Sijs H, Aarts J, Vulto A, et al. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc 2006;13:138–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kesselheim AS, Cresswell K, Phansalkar S, et al. Clinical decision support systems could be modified to reduce ‘alert fatigue’ while still minimizing the risk of litigation. Health Aff (Millwood) 2011;30:2310–17 [DOI] [PubMed] [Google Scholar]
- 22.Miller RA, Waitman LR, Chen S, et al. The anatomy of decision support during inpatient care provider order entry (CPOE): Empirical observations from a decade of CPOE experience at Vanderbilt. J Biomed Inform 2005;38:469–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsieh TC, Kuperman GJ, Jaggi T, et al. Characteristics and consequences of drug allergy alert overrides in a computerized physician order entry system. J Am Med Inform Assoc 2004;11:482–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phansalkar S, Edworthy J, Hellier E, et al. A review of human factors principles for the design and implementation of medication safety alerts in clinical information systems. J Am Med Inform Assoc 2010;17:493–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zachariah M, Phansalkar S, Seidling HM, et al. Development and preliminary evidence for the validity of an instrument assessing implementation of human-factors principles in medication-related decision-support systems—I-MeDeSA. J Am Med Inform Assoc 2011;18(Suppl 1):i62–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasley SK. Decision support and patient safety: the time has come. Am J Obstet Gynecol 2011;204:461–5 [DOI] [PubMed] [Google Scholar]
- 27.Shah NR, Seger AC, Seger DL, et al. Improving acceptance of computerized prescribing alerts in ambulatory care. J Am Med Inform Assoc 2006;13:5–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niazkhani Z, Pirnejad H, Berg M, et al. The impact of computerized provider order entry systems on inpatient clinical workflow: a literature review. J Am Med Inform Assoc 2009;16:539–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawamoto K, Houlihan CA, Balas EA, et al. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ 2005;330:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Virk P, Bates DW, Halamka J, et al. Analyzing transaction workflows in an ePrescribing system. AMIA Annu Symp Proc 2006:1129. [PMC free article] [PubMed] [Google Scholar]
- 31.Russ AL, Zillich AJ, McManus MS, et al. A human factors investigation of medication alerts: barriers to prescriber decision-making and clinical workflow. AMIA Annu Symp Proc 2009;2009:548–52 [PMC free article] [PubMed] [Google Scholar]
- 32.Khajouei R, Jaspers MW. The impact of CPOE medication systems’ design aspects on usability, workflow and medication orders: a systematic review. Methods Inform Med 2010;49:3–19 [DOI] [PubMed] [Google Scholar]
- 33.Garg AX, Adhikari NKJ, McDonald H, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes. J Am Med Inform Assoc 2005;293:1223–38 [DOI] [PubMed] [Google Scholar]
- 34.Baysari M, Westbrook J, Day R. Narrative review: errors in selecting medicines for prescription and the role of computerized decision support. Drug Safety 2011;34:289–98 [DOI] [PubMed] [Google Scholar]
- 35.Pearce C, Arnold M, Phillips CB, et al. The many faces of the computer: An analysis of clinical software in the primary care consultation. Int J Med Inform 2012;81:475–84 [DOI] [PubMed] [Google Scholar]
- 36. Britten N, Stevenson FA, Barry CA, et al. Misunderstandings in prescribing decisions in general practice: qualitative study. BMJ 2000;320:484–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheikh A, Cornford T, Barber N, et al. Implementation and adoption of nationwide electronic health records in secondary care in England: final qualitative results from prospective national evaluation in “early adopter” hospitals. BMJ 2011;343:d6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robertson A, Cresswell K, Takian A, et al. Implementation and adoption of nationwide electronic health records in secondary care in England: qualitative analysis of interim results from a prospective national evaluation. BMJ 2010;341:c4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greenhalgh T, Stramer K, Bratan T, et al. Adoption and non-adoption of a shared electronic summary record in England: a mixed-method case study. BMJ 2010;340:c3111. [DOI] [PubMed] [Google Scholar]
- 40.Greenhalgh T, Stramer K, Bratan T, et al. Introduction of shared electronic records: multi-site case study using diffusion of innovation theory. BMJ 2008;337:a1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davies E. English GP systems market. Woodcote Consulting, 2011. http://www.woodcote-consulting.com/?p=29 (accessed 8 Dec 2012). [Google Scholar]
- 42.Pinnock H, Cresswell K, Fernado B, et al. Evaluation of the effect of IT on interactions between healthcare workers and patients (NHS CFHEP 010) Connecting for Health Evaluation Programme. 2011. http://www.birmingham.ac.uk/research/activity/mds/projects/HaPS/PHEB/CFHEP/reports/projects/010.aspx (accessed Sep 2012).
- 43.de Lusignan S, Kumarapeli P, Chan T, et al. The ALFA (Activity Log Files Aggregation) toolkit: a method for precise observation of the consultation. J Med Internet Res 2008;10:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Lusignan S, Pearce C, Kumarapeli P, et al. Reporting observational studies of the use of information technology in the clinical consultation. A position statement from the IMIA Primary Health Care Informatics Working Group (IMIA PCI WG) . Yearb Med Inform 2011;6:39–47 [PubMed] [Google Scholar]
- 45.Pflug B, Kumarapeli P, van Vlymen J, et al. Measuring the impact of the computer on the consultation: an open source application to combine multiple observational outputs. Inform Health Soc Care 2010;35:10–24 [DOI] [PubMed] [Google Scholar]
- 46.Roter D, Larson S. The Roter interaction analysis system (RIAS): utility and flexibility for analysis of medical interactions. Pat Ed Counsel 2002;46:243–51 [DOI] [PubMed] [Google Scholar]
- 47.Pringle M, Stewart-Evans C. Does awareness of being video recorded affect doctors’ consultation behaviour? Br J Gen Pract 1990;40:455–8 [PMC free article] [PubMed] [Google Scholar]
- 48.Coleman T. Using video-recorded consultations for research in primary care: advantages and limitations. Fam Pract 2000;17:422–7 [DOI] [PubMed] [Google Scholar]
- 49.Murphy E, Dingwall R, Greatbatch D, et al. Qualitative research methods in health technology assessment: a review of the literature. London: Health Technology Assessment, 1998 [PubMed] [Google Scholar]
- 50.Ludwick DA, Doucette J. Adopting electronic medical records in primary care: Lessons learned from health information systems implementation experience in seven countries. Int J Med Inform 2009;78:22–31 [DOI] [PubMed] [Google Scholar]
- 51.Bates DW, Kuperman GJ, Wang S, et al. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J Am Med Inform Assoc 2003;10:523–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wyatt JC. Management of explicit and tacit knowledge. J R Soc Med 2001;94:6–9 [DOI] [PMC free article] [PubMed] [Google Scholar]