Abstract
Background
In 2010, the US Drug Enforcement Administration issued regulations allowing electronic prescribing of controlled substances (EPCS), a practice previously prohibited.
Objective
To carry out a survey of the experience of prescribers in the nation's first study of EPCS implementation.
Materials and methods
Prescribers were surveyed in a community setting before and after implementation of EPCS, to assess adoption, attitudes, and challenges.
Results
Of the 102 prescribers enabled to use EPCS and who responded to surveys before and after implementation, 70 had sent at least one controlled substance prescription electronically. Most users reported that EPCS was significantly less burdensome than expected. Over half reported that EPCS was easy to use and improved work flow, accuracy of prescriptions (69.5%), monitoring of medications (59.3%), and coordination with pharmacists, though high prior expectations for improved efficiency were not met. EPCS users reported a significant decrease in the perceived frequency of medication errors and drug diversion, compared with controls. Barriers to use of EPCS included limited pharmacy participation and instances of unreliability of the technology.
Discussion
Interest in adoption of EPCS is considerable among providers, pharmacies, and vendors. The results suggest that while most EPCS security features may be more acceptable to providers than expected, barriers such as the limited participation by pharmacies may also partly explain slow adoption rates for EPCS nationally.
Conclusions
EPCS was a better experience for many providers than they had expected, but related improvements in practice efficiency and quality of care will depend upon implementation strategies.
Keywords: electronic prescribing, controlled medications, opioids
Introduction
Electronic prescribing has the potential to significantly improve patient safety and clinician practice by enhancing medication management and reducing risks.1–3 Electronic prescribing for non-federally controlled medications is an important component of health information systems,4 and the technical capability to implement such electronic prescribing exists in the USA. Although rates of use of electronic prescribing were initially low,5 58% of US office-based physicians e-prescribed during 2011.6 However, a significant remaining barrier to use of electronic prescribing have been restrictions on electronic transmission of prescriptions for federally controlled substances (CS). Federally, CS represent only 11% of all prescriptions, but they are an important component of practice, issued by 90% of prescribers.7
CS, categorized as schedules II–V, such as narcotics, stimulants, and anxiolytics, are those prescription pharmaceutical agents determined by the US Drug Enforcement Administration (DEA) and the US Food and Drug Administration to have the highest potential for abuse and dependence. This is a national problem, with the number of opioid prescriptions increasing fourfold between 1997 and 2007, and associated abuse also increasing.8 9 To reduce opportunities for drug diversion and abuse, DEA recognizes that ‘It is essential that the rules governing the electronic prescribing of CS (EPCS) do not inadvertently facilitate diversion and abuse,’ and that ‘before CS prescriptions are issued electronically, the process is adequately secure to protect both DEA registrants and society.’10 Therefore, electronic prescribing systems are required to implement additional security measures for dispensing federally CS beyond those required for federally non-controlled prescription medications (eg, antibiotics, anticoagulants, etc).
In June 2010, a DEA Interim Final Rule (IFR) allowing for EPCS became effective.10 The regulation applies to providers, pharmacies, prescribing and pharmacy system vendors, and electronic prescribing networks. Security measures required by the IFR include a process for identity-proofing prescribers; a specified format for the networking software necessary to securely transmit a digital signature; the capability of the pharmacy systems to digitally sign, receive, and archive records; completion of third-party audits of prescribing and pharmacy systems; daily audits; access controls; reporting of security breaches; and ceasing use if a component becomes non-compliant.10 The industry has been adapting prescribing software and information systems to comply with the IFR, but as of December 2012, adoption by health systems and providers was limited. Reasons include delays in completion of certification audits and prescriber identity proofing; and the need in some states to promulgate new regulations allowing EPCS.
This paper presents the experiences of prescribers in a study of EPCS implementation conducted in Berkshire County, Massachusetts. This project was the only effort to introduce EPCS in a community-based, non-governmental setting in the USA preceding the IFR. The project operated under DEA issued waivers to the Controlled Substances Act (CSA) regulations starting 6 months before the IFR became effective, though with similar requirements. In this research, prescribers were surveyed before and after implementation of EPCS, as part of an evaluation of its impact on patient safety and work flow.
Background and significance
The Centers for Disease Control and Prevention have declared prescription drug abuse an epidemic.11 In 2011, approximately 20% of the US population aged ≥12 years (51.2 million Americans) reported having used prescription pain relievers, tranquilizers, stimulants, or sedatives non-medically.12 Consequences of prescription drug abuse include unintentional drug poisonings, which have now become the leading cause of unintentional injury deaths in the USA, quadrupling between 1999 and 2008.8 From 2004 to 2009, emergency department visits related to the misuse or abuse of pharmaceutical agents doubled. More than 75% of those who reported non-medical use of pain relievers said they obtained them from prescriptions prescribed for someone else.13 Although pain medications are prescribed by a range of specialties, about 80% are prescribed by 10–20% of practitioners.14 15
The potential benefits of EPCS include improved patient management and reduced prescription fraud associated with paper prescriptions, which account for a small but significant proportion of opioid medications diverted for abuse.16 With the growing number of prescriptions for all medications, it is increasingly important to identify multiple prescribers at the point of prescribing and dispensing. Knowing about other prescribers can improve quality, limit supply, and curb abuse.17–19 Electronic systems also allow for direct reconciling of medications prescribed with those dispensed in order to identify gaps in patient adherence, a source of poor health outcomes and increased health costs.20
Expectations about the impact of EPCS on quality and patient safety, and use of current functionalities of electronic prescribing systems among prescribers in a community setting have been documented.21 Common problems associated with CS prescribing included lost or stolen prescriptions, medication interactions, incorrect medication or dose prescribed, and alteration of a prescription by a patient. Prescribers expected EPCS to improve management with other prescribers and pharmacists improve the accuracy of prescriptions, and help identify prescription diversion or misuse. Security measures associated with EPCS were expected to be burdensome, potentially discouraging adoption. Understanding whether these expectations were realized, and additional experiences with EPCS, will inform future strategies for implementation.
Materials and methods
Population and setting
The study was conducted at ambulatory sites affiliated with Berkshire Health Systems (BHS), the primary provider of healthcare services in Berkshire County, Massachusetts. BHS includes over 300 physicians, dentists, nurse practitioners, physicians’ assistants, and other clinicians. Thirty-two pharmacies are located in Berkshire County, including 22 chain outlets and eight independents.
In 2008–2009, nine pharmacies and 185 BHS prescribers representing a range of specialties joined a research and demonstration project on EPCS. These prescribers were chosen as they were large multispecialty provider groups already using an electronic prescribing system required for the study. Thirty-five providers withdrew within the first month or did not participate owing to system incompatibility (n=27), relocation or job change (n=6), and health problems (n=2). One hundred and fifty providers were issued security tokens and trained in the use of EPCS.
The process developed for electronically creating and transmitting CS prescriptions in the study reflects the IFR, with some variation. Requirements for the project included reviewing the credentials of prescribers and verifying their identity; two-factor authentication (two methods of identifying one's self) for sending each e-prescription; transmission to the pharmacy through an e-prescribing network (intermediary); weekly DEA database provider verification; and confirmatory faxes to the pharmacies for each EPCS. Provider identities were verified by the electronic prescribing vendor as they entered the project; registered in the EPCS system by the research project liaison as being authorized to use EPCS; oriented to the EPCS technology; and authenticated to the system. Two-factor authentication was achieved by providers using a passcode, plus an assigned security token (resembling a small flash drive). The latter had to be secured by the provider at all times, and was inserted into the computer USB drive with each CS prescription transmitted electronically. Orientation included the security requirements of the system and the process of using two-factor authentication. Differences between the DEA study requirements and the IFR include the following: the study identity proofing used a system representative rather than a certification authority; and weekly provider checks and a confirmatory fax transmitted by the intermediary to the pharmacy with each CS prescription were required by the project, but not the IFR.
All pharmacies in Berkshire County were invited to participate, and nine pharmacies chose to do so: four independent pharmacies, two grocery pharmacies, two hospital employee pharmacies, and one national chain. Comparison of state-reported data with prescribing software showed that these pharmacies accounted for 22% of schedule II CS prescriptions dispensed in Berkshire County during the study period.
Implementation of EPCS was staged to facilitate comparison of EPCS prescribers with controls over time. Of the 150 prescribers who were trained, 89 were deployed to start EPCS immediately (group 1), and 6 months later, a second group of 61 were deployed to start EPCS (group 2). Groups were selected based on convenience and practice clusters, starting with the largest group practice. Group 2 physicians were practices that were matched to some extent with group 1 prescribers for practice size and specialty. All participants were surveyed before training, and at 6 months after the start of EPCS. Group 2 prescribers were re-surveyed before being deployed and at the same time as group 1 6 months after implementation, to serve as concurrent controls for group 1's post-implementation survey. Group 2 physicians were again asked about their experience 6 months after beginning EPCS. For analysis, experiences of group 1 and group 2 prescribers at 6 months after implementation were combined. During the study, there were two periods of 6 and 8 weeks during which EPCS was deactivated owing to failure of one of the security measures (ie, CS prescription was transmitted electronically without full authentication) and was reactivated when a security consultant confirmed that the processing problems had been resolved. These delays were taken into account in timing surveys.
Survey instrument
All BHS prescribers were surveyed at baseline before the project and asked about use of electronic prescribing, prescribing practices for CS, and expectations for EPCS, as described in an earlier paper.21 Questions were developed de novo for the survey to deal with expectations for EPCS specifically, and to correspond to components related to the Rogers model of technology adoption22 and the technology acceptance model to assess familiarity with use of health technology.23 The final survey had five domains: (i) current prescribing practice; (ii) current e-prescribing activities; (iii) current prescribing of CS: potential problems with patient safety, convenience, and identifying non-medical use; (iv) expectations for the EPCS system (eg, effect on workflow, patient safety, and potential barriers); and (v) perceptions about proposed security measures for use of EPCS.
The 6-month follow-up survey was an abbreviated form of the initial survey, and was sent to those who were enabled to conduct EPCS. Prescribers were asked to rate on an ordinal scale their experience with e-prescribing activities, concerns about patient safety in the previous 6 months, current use of EPCS and experience with security measures (to compare expectations with experience), and overall satisfaction with the system.
Data analysis
Descriptive statistics were generated on the survey categories of interest, including provider characteristics, degree of burden of each security measure, experience with particular features of EPCS, and overall satisfaction with the system. Experience with EPCS was compared with expectations at the prescriber level, using the McNemar test of paired proportions for non-parametric data.24 The impact of EPCS on perceived patient safety was measured by comparing responses to a question asking prescribers how often several safety issues occurred in the previous 6 months. After implementation, responses were compared with baseline replies before implementation using the Wilcoxon sign rank test for pairs for non-parametric data.24
Exploratory factor analysis was conducted on 16 items related to expectations for EPCS to identify conceptual themes, using the Varimax with Kaiser normalization method.25 Factors that emerged from the post-implementation survey were compared with those identified in analysis of the pre-implementation survey. As noted in an earlier paper,21 the baseline survey before introduction of EPCS identified important factors related to the expected impact of EPCS: (1) improvement in patient management; (2) risk of EPCS technology to patient care; and (3) practice efficiency. The factors were then used to create a composite score for each factor that was used in models predicting overall satisfaction with EPCS.
A logistic regression model estimating predictors of overall experience with EPCS was fitted based on theoretical concepts associated with diffusion of innovation, including provider characteristics, ease of use, and familiarity with the technology. The analysis assessed whether certain provider or medical practice characteristics (such as gender, age, reliability of, and comfort with, technology, and the number of patients seen in a typical week) and factors associated with experience with EPCS during the study were associated with respondents’ overall satisfaction with EPCS. The Statistical Package for the Social Sciences (SPSS) Predictive Analytic SoftWare (PASW Statistic) V.19 was used for analyses.
Results
Survey respondents
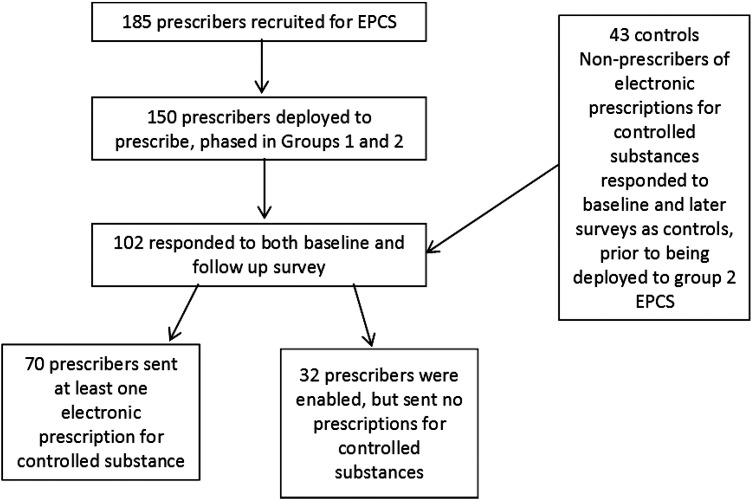
One hundred and two prescribers in the study (group 1 and group 2 combined) completed surveys both before and after implementation (68% response rate), figure 1. Respondents were most often in internal medicine/family practice (45.1%) and neurology or psychiatry (18.6%). Non-respondent prescribers (of the 150 deployed, 48 did not respond to the surveys before and after implementation) did not differ from respondents in age, gender, and specialty, but fewer sent at least one EPCS. The 43 controls (group 2 prescribers before implementing EPCS, response rate 69.3%) differed from cases in their practice specialty (fewer in internal medicine/primary care, more in emergency medicine). Seventy of the 102 respondents sent one or more CS prescriptions electronically. Prescribers who electronically transmitted at least one CS prescription (n=70) were not significantly different in age, gender or race from those who were enabled but did not send any prescriptions (n=32), but were more often in internal medicine or family practice.
Figure 1.
Recruitment and participation in survey. EPCS, electronic prescribing of controlled substances.
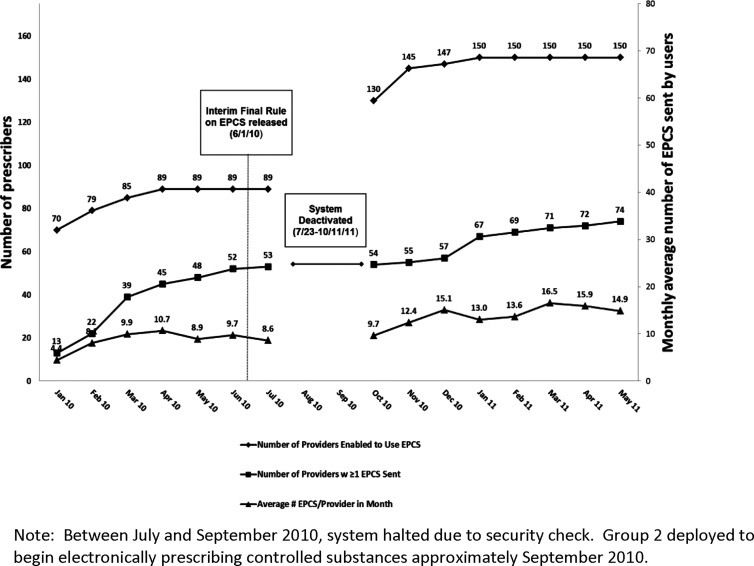
Figure 2 shows implementation of EPCS by date. Eighty-nine prescribers were initially trained and deployed between January and March 2010 (group 1), and an additional 61 after July 2010 (group 2). The average number of electronic CS prescriptions per user per month plateaued at 15 prescriptions by May 2011. The number of CS prescriptions sent electronically represented an average of 62% of total CS prescriptions (electronic and paper) sent by participating prescribers to participating pharmacies. Across prescribers, the proportion of all CS prescriptions sent electronically ranged from 1.3% to 99.7%.
Figure 2.
Number of electronic prescriptions for controlled substances (EPCS) sent by study prescribers.
Table 1 shows the degree of burden experienced by prescribers in implementing the EPCS security measures. It also compares each provider's experience during the study with their prior expectations on a parallel question in the pre-implementation survey. All security measures were less burdensome than had been expected. For instance, while 45% of providers expected that carrying a security token at all times would be a large inconvenience, only 10.3% found it to be so (p<0.001). Findings were similar for other security features, with the greatest burden experienced being the computer prescribing screen timing out, with 20% rating it so.
Table 1.
Providers’ expectations versus experience in each of the following items as a burden during the study of electronic prescribing of controlled substances (EPCS)
| Degree to which each item would be a burden | EXPECTATION E-prescribers who expected item to be a ‘large inconvenience’ n (%) |
EXPERIENCE E-prescribers who rated item a ‘large inconvenience’ during the study n (%) |
p Value† |
|---|---|---|---|
| A. Carry a token or flash drive with electronic signature to authenticate and send all controlled substances rx (n=57)** | 19 (33.3) | 6 (10.5) | 0.007 |
| B. Keep the token in possession at all times (n=58)*** | 26 (44.8) | 6 (10.3) | <0.001 |
| C. Report lost or stolen token within 12 h (n=58)** | 18 (31.0) | 4 (6.9) | 0.001 |
| D. Computer prescribing screen timing out after brief period of inactivity, I would have to re-enter the password (n=60)*** | 25 (41.7) | 12 (20.0) | 0.001 |
| E. Not able to use personal electronic device (must use laptop or desktop) (n=57)** | 10 (17.5) | 2 (3.5) | 0.008 |
| F. Authenticate identity in person one time, when receiving security token (n=58)* | 11 (19.0) | 3 (5.2) | 0.039 |
n=70 total EPCS users with at least one prescription transmitted electronically.
Difference between expectations and experience significant at *p<0.05; **p<0.01; ***p<0.001.
†McNemar's test of paired proportions with continuity correction.
Table 2 shows responses to questions about the impact of EPCS on practice work flow and efficiency, practice management, and patient safety. As in the previous table, this table compares each provider's response with expectations before implementation, and limits responses to those who used EPCS at least once. Over half of prescribers found EPCS (second response column) to be easy to use (72.9%); to improve accuracy of prescriptions (69.5%); to improve work flow (66.1%); to improve monitoring of medications in the practice (59.3%); to improve coordination with pharmacists (55.9%), and to lead to fewer calls to pharmacists (54.2%). On most dimensions, however, EPCS experience did not meet the high expectations reported before implementation. For several patient care-related effects of EPCS (eg, easier to identify diversion or misuse), expectations were much more positive than experience. While few prescribers said EPCS did not have advantages over the current system (15.3%), this is four times the number who expected no advantage.
Table 2.
Experience of users of electronic prescribing of controlled substances (EPCS) for impact on practice and patient safety
| Experience with EPCS | EXPECTATIONS Agree or strongly agree n (%) |
EXPERIENCE Agree or strongly agree n (%) |
p Value† |
|---|---|---|---|
| A. Improved work flow and efficiency of practice (n=59) | 47 (79.7) | 39 (66.1) | 0.096 |
| B. Easy to use (n=59) | 45 (76.3) | 43 (72.9) | 0.832 |
| C. Has improved accuracy of prescriptions, fewer medical errors (n=59) | 48 (81.4) | 41 (69.5) | 0.118 |
| D. Has made it easier to identify diversion or misuse of medications** (n=58) | 44 (75.9) | 25 (43.1) | 0.001 |
| E. Has improved monitoring of medication management within your practice** (n=59) | 50 (84.7) | 35 (59.3) | 0.006 |
| F. Has improved coordination of medication management with other prescribers*** (n=59) | 46 (78.0) | 23 (39.0) | <0.001 |
| G. Has improved coordination of medication management with pharmacists*** (n=59) | 50 (84.7) | 33 (55.9) | <0.001 |
| H. Has improved communication with patients regarding their controlled substance prescriptions* (n=59) | 37 (62.7) | 26 (44.1) | 0.027 |
| I. Has led to financial savings for you or your office* (n=59) | 19 (32.2) | 8 (13.6) | 0.007 |
| J. Has caused technical problems with consequences for patient care (n=59) | 13 (22.0) | 9 (15.3) | 0.424 |
| K. Has caused system breaches on patient confidentiality* (n=59) | 4 (6.8) | 0 (0) | N/A |
| L. Has led to depersonalized patient care (eg, fewer in-person visits to pick up refill prescriptions) (n=58) | 5 (8.6) | 3 (5.2) | 0.687 |
| M. Has led to fewer calls to pharmacists to clarify prescriptions* (n=59) | 43 (72.9) | 32 (54.2) | 0.035 |
| N. Has caused a learning curve that was disruptive to my practice (n=59) | 9 (15.3) | 13 (22.0) | 0.289 |
| O. Has provided no advantages over current system of prescribing controlled substances (n=59) | 2 (3.4) | 9 (15.3) | 0.039 |
| P. Has improved patient satisfaction (n=59) | 33 (55.9) | 27 (45.8) | 0.286 |
n=70 total EPCS users with at least one prescription transmitted electronically.
Difference between expectations and experience significant at *p<0.05; **p<0.01; ***p<0.001.
†McNemar's test of paired proportions with continuity correction.
Exploratory factor analysis of the 16 items in table 2 identified themes in the experience with EPCS, using the Varimax with Kaiser normalization method. The following factors were conceptualized as: (1) patient management (Cronbach's α=0.906); and (2) risk of the new technology to patient care (Cronbach's α=0.780). These factors were consistent with two of the three factors identified in an earlier analysis of baseline data in a larger sample of 246 prescribers about their expectations of EPCS, which were (1) patient management (Cronbach's α=0.904 (three out of five items emerged as the same as the current factor 1); (2) risk averse to the new technology (Cronbach's α=0.725, all items the same as the current factor 2); and (3) practice efficiency (Cronbach's α=0.774, all items).21 Practice efficiency did not emerge as a factor on the follow-up survey.
Prescribers rated the importance of barriers to consistently using EPCS for all eligible patients (table 3). The vast majority of prescribers (70.9%) said that the small number of participating pharmacies was a barrier to any use of EPCS, disrupting work flow to accommodate a small number of patients. This barrier was followed by technical challenges related to the computer program (38.4%) and security token (34.5%). Other non-technical aspects of EPCS such as patient demand, worries about system security, and difficulty of use, were rated low as barriers.
Table 3.
Barriers to use of electronic prescribing of controlled substances (EPCS), for prescribers in study
| How important is the following in limiting the number of electronic prescriptions of controlled substances you send electronically, compared with sending all electronically? | Not important or somewhat not important | Neither | Somewhat important or very important |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| A. Not comfortable because of security (N=86) | 53 (61.6) | 24 (27.9) | 9 (10.5) |
| B. Electronic prescribing of controlled substances interferes with my office work flow (N=86) | 50 (58.1) | 18 (20.9) | 18 (20.9) |
| C. Participating pharmacies are a small proportion of my practice (N=86) | 8 (9.3) | 17 (19.8) | 61 (70.9) |
| D. I like to see patients in person to assess them when they come in to pick up refills (n=87) | 42 (48.3) | 20 (23.0) | 25 (28.7) |
| E. The technology (computer program) is not always reliable (N=86) | 32 (37.2) | 21 (24.4) | 33 (38.4) |
| F. The technology (flash drive sized security token) is not always reliable (n=87) | 35 (40.2) | 22 (25.3) | 30 (34.5) |
| G. The technology (flash drive sized security token) is hard to use (N=86) | 43 (50.0) | 28 (32.6) | 15 (17.4) |
| H. I don't like carrying a security token (N=86) | 44 (51.2) | 29 (33.7) | 13 (15.1) |
| I. Patients did not want their prescriptions sent electronically (n=87) | 51 (58.6) | 27 (31.0) | 9 (10.3) |
n=102 prescribers authorized and trained to prescribe electronically.
To measure the impact of EPCS on perceived patient safety, prescribers estimated how often concerns about patient safety occurred in the 6 months before the study, and again after 6 months. Post-implementation responses were compared with responses from controls, who were re-surveyed at the same time, but before being deployed. Results for study prescribers are shown in table 4. Providers using EPCS reported that several safety problems occurred less often after implementation of EPCS, including being alerted that the pharmacy filled the prescription with the wrong drug or incorrect dose strength or instructions; and patients reporting that they had lost the prescription and required a replacement. Controls, analyzed separately, reported no significant differences in the same time period for any items (control group results not shown).
Table 4.
Prescribers’ perceived impact of electronic prescribing of controlled substances (EPCS) on medication errors and diversion (of a total of 70 EPCS users responding with at least one prescription transmitted electronically)
| Problems during the past 6 months: | N | Baseline mean | Follow-up mean | p Value† |
|---|---|---|---|---|
| A. Was alerted that the pharmacy incorrectly filled prescription with wrong drug** | 63 | 1.32 | 1.10 | 0.007 |
| B. Was alerted that incorrect dose strength or instruction was dispensed at the pharmacy** | 63 | 1.41 | 1.21 | 0.007 |
| C. Prescription you wrote was altered by a patient or someone other than yourself* | 59 | 1.27 | 1.10 | 0.029 |
| D. Prescription was not written by you, or counterfeit prescriptions discovered | 63 | 1.08 | 1.03 | 0.480 |
| E. Your prescription pads were stolen | 61 | 1.07 | 1.00 | 0.180 |
| F. Incorrect medication was prescribed by you (eg, wrote down wrong drug, dose or strength) | 66 | 1.46 | 1.42 | 0.753 |
| G. Medication interactions were unknown at time of prescribing | 63 | 1.49 | 1.48 | 0.991 |
| H. Patient reported they had lost the written prescription requiring a replacement prescription* | 67 | 2.27 | 2.05 | 0.029 |
| I. Received call from patient or pharmacy that prescribed medication was not covered by insurance | 64 | 3.03 | 2.95 | 0.785 |
Scale is from 1=never occurred in past 6 months to 5=occurred >10 times in past 6 months. No statistical significant difference between original and follow-up survey responses for control group.
Difference between baseline and follow up responses significant at *p<0.05; **p<0.01; ***p<0.001.
†Wilcoxon signed rank test for pairs.
Prescribers using the system at least once reported relative comfort in dealing with the problems related to CS misuse (results not shown in table). Fifty-seven (81.2%) felt comfortable with their ability to detect prescription drug abuse or dependence. Fifty-two (74.3%) felt comfortable with their ability to identify if a patient is attempting abuse or diversion. Fifty-one (72.7%) felt comfortable with their ability to balance the needs of their patients for maximum pain control with the risk of abuse or diversion. These responses did not differ significantly from responses provided by the same providers at baseline before implementation of EPCS.
Sixty percent of prescribers in the study who responded to the survey reported that they were somewhat or very satisfied with the use of EPCS. Logistic regression models (not shown) estimated the association of several dimensions of EPCS with overall satisfaction (somewhat or very satisfied versus very unsatisfied, somewhat unsatisfied, or neither). Independent variables tested included prescriber characteristics (age and gender); number of patients seen in a typical week; comfort with using computer for other activities such as email to patients; use of other features of electronic prescribing; and themes identified in the factor analysis (improvement in patient management and risk of the new technology). The final model indicated that adjusting for age, comfort using a computer, and number of patients per week, a belief that EPCS had improved patient management increased the odds of being satisfied overall with the system fourfold (OR=4.1, CI 1.321 to 12.668, p<0.05). Also controlling for previously mentioned variables, a belief that EPCS limited the risk to patient care increased the odds of being satisfied with the system nearly fourfold (OR=3.8, CI 1.006 to 14.051, p<0.05).
Discussion
This was the first attempt in the nation to implement EPCS in a community setting. Our study found that the impact of EPCS in this project was positive for attitudes toward adoption, but implementation features were not always as expected. Provider experience with EPCS was less burdensome than expected. However, expectations that EPCS would improve practice efficiency were not borne out in this small sample, a result seen elsewhere for larger health information technology implementation studies.26
Despite the limitations of pharmacy participation and several technical problems, over half of prescribers rated EPCS positively. Open-ended comments were provided by 40 respondents. Positive comments related mostly to the concept of EPCS, ease of implementation, and patient safety concerns, and negative comments were more related to limitations of the system used for the study, including technical aspects and implementation (eg, not enough pharmacies, unreliable system, or incompatibility with computer). None reported a concern that potential system breaches might compromise patient safety, although EPCS transmissions were suspended temporarily when a prescription was sent without the use of a security token.
Users of EPCS reported a significant perceived decrease in some of the problems associated with written CS prescriptions (eg, prescription altered, or reported lost), but not others (counterfeit prescriptions). Perceived reductions in medication errors are consistent with improvements identified with electronic prescribing of non-controlled medications,2 and with the speculations of others about EPCS.27 Security items were unexpectedly not a large burden. With the development of new approaches to two-factor authentication such as biometrics and one-time password technology, newer technologies may be even less of a perceived burden than the security token.
However, certain barriers to successful implementation for all prescribers serve as lessons for other states and systems that use EPCS. Most important, as shown in this project, if a sufficient number of community pharmacies do not have systems in place to accept EPCS, prescribers will not see the value of changing systems and office work flow to accommodate this practice. Results strongly suggest that providers are interested in using EPCS as a tool for efficient practice and patient safety. Our finding that EPCS improved patient management underscores the clinical importance of successful use of this tool.
A limitation of the study is that the impact of EPCS on quality of care is not measured directly in the patient record, but through prescriber perceptions before and after implementation. However, this was a first look at EPCS implementation, and there will be greater opportunity to measure health outcomes once there is a critical mass of EPCS activity. Another limitation is that the prescribing software was not integrated into the patient's electronic medical record or the state prescription monitoring program (PMP). Therefore, prescriptions dispensed and prescribed by other prescribers outside BHS were not known at the time of prescribing. While the trend is to move from stand-alone e-prescribing software as used in this study to electronic health record (EHR)-based e-prescribing, the study focused on the additional security features needed for EPCS, which are required regardless of the type of prescribing software used. Thus, the study made use of the predominant mode of e-prescribing at the test site, to which adding security features for study purposes was feasible, so as not to introduce additional variables beyond those being tested. Immediate accessibility of a full patient record while prescribing through integrated EHRs, prescribing systems, and health information exchanges, certainly improves the efficiency of practice and patient safety. Further, linking PMPs with EHRs (eg, having a tab in the EHR system that automatically retrieves information from the PMP system) would ensure smooth integration into practice work flow. This will improve patient care coordination, particularly coordination of medication management with other prescribers and pharmacies.
Conclusion
The findings of this study provide important lessons to those implementing EPCS in a community setting. First, prescriber adoption may be initially slow depending upon the degree of pharmacy participation. Second, since provider expectations of the burden of security measures were not realized, acceptance may increase with education. Finally, prescribers’ positive perceptions of patient care improvements with EPCS may provide an incentive for wider EPCS adoption. Differences between the study rules and the IFR were noted. However, the findings are still generalizable, as many of the differences between the waivers and IFR were not in areas affecting prescribers directly.
EPCS is a promising tool for improving public health and public safety by preventing diversion or misuse of CS. It promises to become a major tool to curb the growing substance abuse epidemic, and contribute to patient safety, practice efficiency, and positive health outcomes.
It is likely that EPCS applications and systems will be widely available within the next year.28 Adoption of EPCS will gradually occur in communities where both providers and pharmacies are using systems that have completed certification audits as required by the IFR. The extent to which this leads to widespread availability across the industry will depend, however, upon how quickly parties fulfill the requirements of the IFR. Further understanding of the barriers to adoption of these treatment enhancements can lead to successful implementation in the broader medical community.
Footnotes
Contributors: CPT designed the study, supervised all aspects of the analysis, and drafted the manuscript and revisions. MLK contributed to designing the study and survey instrument, writing the manuscript, contributed to analysis, and assisted in drafting the paper. SJK managed the overall project and contributed to survey analysis. PK designed and advised on the study and analysis. RVN managed all data and conducted statistical analyses. AM contributed to design and fielded all surveys. GMC designed the study, initiated the pilot implementation, and advised on interpretation of the data. All authors gave final approval of the version to be published. The authors would like to thank Wendy Colnon for editorial assistance.
Funding: This project was supported by the Agency for Healthcare Research and Quality (AHRQ), grant no. R18 HS017157.
Competing interests: None.
Ethics approval: Institutional Review Board, Brandeis University, Massachusetts Department of Public Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Institute of Medicine, ed. Preventing medication errors : Quality Chasm Series. WashingtonDC: National Academies, 2006:1–24. [Google Scholar]
- 2.Kaushal R, Kern LM, Barron Y, et al. Electronic prescribing improves medication safety in community-based office practices. J Gen Intern Med 2010;25:530–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abramson EL, Bates DW, Jenter C, et al. Ambulatory prescribing errors among community-based providers in two states. J Am Med Inform Assoc 2012;19:644–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halamka J, Aranow M, Ascenzo C, et al. E-prescribing collaboration in Massachusetts: early experiences from regional prescribing projects. J Am Med Inform Assoc 2006;13:239–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.California Health Care Foundation. E-prescribing in California: Why aren't we there yet? March, 2012 [Google Scholar]
- 6.SureScripts. National Progress Report on e-Prescribing and Interoperable Healthcare. 2011.
- 7.Gallagher C. Electronic Prescribing. National Association of Controlled Substances Authorities Annual Educational Conference; October, 2006; San Antonio, TX [Google Scholar]
- 8.Centers for Disease Control (CDC) Vital Signs: Overdoses of Prescription Opioid Pain Relievers—United States, 1999–2008, 2011 [PubMed] [Google Scholar]
- 9.Office of National Drug Control Policy. Epidemic: responding to America's prescription drug abuse crisis. Washington, DC, 2011 [Google Scholar]
- 10.Federal Register: Electronic Prescriptions for Controlled Substances; Final Rule, 21 CFR Parts 1300, 1304, 1306, and 1311. Sect. Vol. 75, No. 61, 2010 [Google Scholar]
- 11.Centers for Disease Control and Prevention Prescription Drug Overdoses: An American Epidemic: Centers for Disease Control and Prevention (CDC) February 17, 2011 [Google Scholar]
- 12.Substance Abuse and Mental Health Services Administration. Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD 2012 Contract No.: NSDUH Series H-44, HHS Publication no. (SMA)12-4713. [Google Scholar]
- 13.Substance Abuse and Mental Health Services Administration Results from the 2010 National Survey on Drug Use and Health: volume 1: summary of national findings. Rockville, MD, 2011 [Google Scholar]
- 14.Dhalla IA, Mamdani MM, Gomes T, et al. Clustering of opioid prescribing and opioid-related mortality among family physicians in Ontario. Can Fam Med 2011;57:392–6 [PMC free article] [PubMed] [Google Scholar]
- 15.Swedlow A, Ireland J, Johnson G. Prescribing patterns of schedule II opioids in California Workers’ Compensation: Cal. Workers’ Compensation Update March 2011. [Google Scholar]
- 16.Rosenblum A, Parrino M, Schnoll SH, et al. Prescription opioid abuse among enrollees into methadone maintenance treatment. Drug Alcohol Depend 2007;90:64–71 [DOI] [PubMed] [Google Scholar]
- 17.Simeone R, Holland L. An evaluation of prescription drug monitoring programs. Albany, NY: Simeone Associates, Inc., 2006 [Google Scholar]
- 18.Perrone J, Nelson LS. Medication reconciliation for controlled substances—an “Ideal” prescription-drug monitoring program. N Engl J Med 2012;366:2341–3 [DOI] [PubMed] [Google Scholar]
- 19.Clark T, Strickler G, Kreiner P, et al. Prescription Monitoring Programs: An Assessment of the Evidence for Best Practices Brandeis University Prescription Monitoring Program Center of Excellence Working Paper. Waltham, MA May, 2012 [Google Scholar]
- 20.Fischer MA, Stedman MR, Lii J, et al. Primary medication non-adherence: analysis of 195,930 electronic prescriptions. J Gen Intern Med 2010;25:284–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas CP, Kim M, McDonald A, et al. Prescribers’ expectations and barriers to electronic prescribing of controlled substances. J Am Med Inform Assoc 2012;19:375–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogers E. Diffusion of innovations. 5th edn New York City: The Free Press, 2003 [Google Scholar]
- 23.Tamblyn R, Huang A, Kawasumi Y, et al. The Development and evaluation of an integrated electronic prescribing and drug management system for primary care. J Am Med Inform Assoc 2006;13:150–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pagano M, Gauvreau K. Principles of biostatistics. 2nd edn Pacific Grove, CA: Duxbury Press, 2000 [Google Scholar]
- 25.SPSS, Inc. PASW Statistics Base 18.0 Core system user's guide. Chicago: Statistical Package for the Social Sciences, 2011 [Google Scholar]
- 26.Laramee AS, Bosek M, Shaner-McRae H, et al. A comparison of nurse attitudes before implementation and 6 and 18 months after implementation of an electronic health record. Comput Inform Nurs 2012;30:521–30. [DOI] [PubMed] [Google Scholar]
- 27.Figge HL, Fox BI, Tribble DA. Electronic prescribing of controlled substances. Am J Health Syst Pharm 2009;66:1311–16 [DOI] [PubMed] [Google Scholar]
- 28.Marotz D, Kaufman P, Etreby M. E-prescribing of controlled substances—the real world. Presentation to the National Council on Prescription Drug Programs Annual Meeting May 8, 2012