Abstract
In healthy human subjects, the relative contribution of cortical regions to motor performance varies with the task parameters. Additionally, after stroke, recruitment of cortical areas during a simple motor task varies with corticospinal system integrity. We investigated whether the pattern of motor system recruitment in a task involving increasingly forceful hand grips is influenced by the degree of corticospinal system damage. Nine chronic subcortical stroke patients and nine age-matched controls underwent functional magnetic brain imaging whilst performing repetitive isometric hand grips. Target grip forces were varied between 15% and 45% of individual maximum grip force. Corticospinal system functional integrity was assessed with transcranial magnetic stimulation. Averaged across all forces, there was more task-related activation compared with rest in the secondary motor areas of patients with greater corticospinal system damage, confirming previous reports. However, here we were primarily interested in regional brain activation, which covaried with the amount of force generated, implying a prominent executive role in force production. We found that in control subjects and patients with lesser corticospinal system damage, signal change increased linearly with increasing force output in contralateral primary motor cortex, supplementary motor area and ipsilateral cerebellum. In contrast, in patients with greater corticospinal system damage, force-related signal changes were seen mainly in contralesional dorsolateral premotor cortex, bilateral ventrolateral premotor cortices and contralesional cerebellum, but not ipsilesional primary motor cortex. These findings suggest that the premotor cortices might play a new and functionally relevant role in controlling force production in patients with more severe corticospinal system disruption.
Keywords: fMRI, human, motor cortex, premotor, stroke, transcranial magnetic stimulation
Introduction
Motor system reorganization after focal brain damage has been demonstrated using functional brain imaging techniques. Compared with healthy volunteers, patients with stroke have more task-related brain activation in secondary motor regions such as premotor cortex, supplementary motor area (SMA) and cingulate motor areas, particularly in the contralesional hemisphere (Chollet et al., 1991; Weiller et al., 1993; Cramer et al., 1997; Seitz et al., 1998; Newton et al., 2002; Ward et al., 2003; Gerloff et al., 2006). Furthermore, secondary motor system overactivity is particularly prominent in more impaired patients (Ward et al., 2003).
There are two ways to investigate the functional relevance of secondary motor region recruitment. One approach uses transcranial magnetic stimulation (TMS) to disrupt activity transiently in targeted cortical regions whilst measuring differential behavioural effects in patients and healthy controls. In patients with subcortical stroke, TMS to the dorsolateral premotor cortex (PMd) in either hemisphere increases simple reaction times (Johansen-Berg et al., 2002; Fridman et al., 2004) and impairs timing of complex finger movements when delivered to the contralesional side (Lotze et al., 2006). TMS to the contralesional primary motor cortex (M1), however, affects only the timing of complex finger movements (Lotze et al., 2006). These effects are more prominent in patients with greater motor impairment, suggesting that the relative contribution of each brain region to recovered function depends on the degree of motor system damage as well as the demands of the task.
A second way of assessing the functional contribution of brain regions is to measure how task-related activity covaries with modulation of task parameters. In healthy humans, for example, functional imaging experiments have shown that increasing force production is associated with linear increases in blood oxygen level-dependent (BOLD) signal in contralateral M1 and medial motor regions, implying that they have a functional role in force production (Dettmers et al., 1995; Thickbroom et al., 1999; Ward & Frackowiak, 2003).
We recently showed that when patients with a subcortical stroke grip with their affected hands there is a shift in average motor system recruitment from primary to secondary networks in those with greater corticospinal system disruption (Ward et al., 2006). This result suggests that average task-related activity in a region is related to individual corticospinal integrity, but does not indicate whether this activity contributes towards recovered motor function. Here we assess each cortical motor region’s contribution to recovered grip by using functional magnetic resonance imaging (fMRI) to examine how activity in each area covaries with increasing force production. We then tested how the pattern in individual patients was related to a measure of corticospinal integrity assessed independently using TMS.
We hypothesized that, as in healthy controls, activity in ipsilesional M1 would covary positively with the amount of force output in patients with lesser degrees of corticospinal system damage. In contrast we expected in patients with greater corticospinal disruption, who were nevertheless still able to modulate grip force, that activity in secondary motor areas would show novel force-modulated characteristics, suggesting a behaviourally relevant role in the post-stroke functional motor network.
Materials and methods
Subjects
Patients were recruited from the National Hospital for Neurology and Neurosurgery, Queen Square, London. The patient group comprised nine male patients (range 22–69 years, mean 48.1 ± 14.3 years). Patient characteristics are listed in Table 1. All patients had suffered from first-ever stroke resulting in weakness of at least wrist and finger extensors and hand interossei (to ≤ 4 + on the Medical Research Council scale) for at least 48 h after onset of symptoms. Exclusion criteria consisted of: (1) cortical infarction; (2) carotid artery occlusion or stenosis ≥ 70%; (3) language or cognitive deficits sufficient to impair co-operation in the study; (4) inability to perform the motor task; (5) previous seizures.
Table 1.
Patient characteristics
| Patient | Age (years) |
Sex | Site of lesion | Time since stroke (months) |
Initial severity |
Grip strength (%)* |
NHPT (%)* |
Past medical history |
|---|---|---|---|---|---|---|---|---|
| 1 | 62 | M | R striatocapsular | 5 | 0 | 24.9 | 0 | Hhypertension |
| 2 | 46 | M | R internal capsule | 8 | 0 | 87.3 | 100.5 | Nil |
| 3 | 69 | M | L striatocapsular | 9 | 4 | 61.5 | 69.7 | Hypertension |
| 4 | 44 | M | R striatocapsular | 8 | 0 | 43.6 | 5.1 | Nil |
| 5 | 33 | M | L pons | 46 | 0 | 68.7 | 48.9 | Nil |
| 6 | 51 | M | L corona radiata | 6 | 4 | 85.7 | 89.6 | Hypertension |
| 7 | 58 | M | R internal capsule | 7 | 0 | 93.3 | 94.5 | Hypertension |
| 8 | 22 | M | L pons | 4 | 0 | 86.6 | 85.8 | Nil |
| 9 | 48 | M | R striatocapsular | 116 | 0 | 92.6 | 60.8 | Nil |
L, left; M, male; R, right. Initial severity represents strength of finger extension (Medical Research Council scale) at time of stroke as recorded in the medical notes. NHPT, nine-hole peg test.
Values measured at the time of study.
Age-matched control subjects were also recruited, comprising nine male subjects (range 21–74 years, mean 49.3 ± 15.6 years). They reported no history of neurological or psychiatric illness, and were not taking regular medication.
All control subjects and patients were right-handed according to the Edinburgh handedness scale (Oldfield, 1971). Full written consent was obtained from all subjects in accordance with the Declaration of Helsinki. The study was approved by the Joint Ethics Committee of the Institute of Neurology, UCL and National Hospital for Neurology and Neurosurgery, UCL Hospitals NHS Foundation Trust, London.
Behavioural evaluation
Subjects were evaluated with the nine-hole peg test (NHPT) and maximum grip strength (GRIP). Maximum grip strength was measured using the same manipulandum as used for MRI scanning. Scores for the affected hand were divided by scores for the unaffected hand and expressed as a percentage.
TMS
The functional integrity of the corticospinal system was measured in each patient using motor cortical stimulus/response curves (Devanne et al., 1997; Ridding & Rothwell, 1997; Boroojerdi et al., 2001), as described previously (Ward et al., 2006). At least 10 resting motor-evoked potentials were obtained at each of four stimulus intensities (90%, 110%, 130% and 150% of resting motor threshold, or up to a maximum of 100% of stimulator output). Maximum amplitude M-waves were elicited from first dorsal interosseus by supramaximal electrical stimulation of the ulnar nerve at the wrist. Peak-to-peak motor-evoked potential amplitudes were expressed as a fraction of the M-wave for each patient and were plotted against stimulus intensity. The gradient of the line of best fit (RCAH) was obtained for each patient using the least-squares method, and used as a measure of the functional integrity of the corticospinal system originating primarily from that hemisphere (Devanne et al., 1997; Ridding & Rothwell, 1997; Boroojerdi et al., 2001).
fMRI scanning
Motor paradigm
A 3T Siemens ALLEGRA system (Siemens, Erlangen, Germany) was used to acquire both T1-weighted anatomical images and T2*-weighted MRI transverse echo-planar images (EPI) (64 × 64, 3 × 3 mm pixels, TE = 30 ms) with BOLD contrast, as previously described (Ward et al., 2006). During scanning, all patients performed a series of dynamic isometric hand grips of different forces with their impaired hand (five left, four right) using a MRI-compatible manipulandum. The age-matched control group used either their right or left hand (five left and four right, to match the patient group). Continuous visual feedback about the force exerted was provided. Prior to scanning, but whilst lying in the scanner, subjects were asked to grip the manipulandum with their affected hand using maximum force to generate a maximum voluntary contraction (MVC). A single scanning session comprised 30 visually cued hand grips interspersed with 30 null events in a randomized and counterbalanced order (intertrial interval = 5.72 s, scanning time = 6 min 14 s). Each hand grip was visually cued. The target force was varied such that 10 grips at each of 15%, 30% and 45% of MVC were performed (30 in total). All subjects performed the motor task outside the scanner to look for the presence of associated or mirror movements. To look for bilateral movements during scanning, patients held identical hand grip manipulanda in both hands while carrying out the task unimanually. Simultaneous recordings from both hands enabled detection of true mirror movements (Nelles et al., 1998). After scanning, a 100-mm visual analogue scale (where 0 = ‘no effort’ and 100 = ‘maximum effort’) was used to assess the perceived effortfulness of the task.
Data preprocessing
Imaging data were analysed using Statistical Parametric Mapping (SPM5, Wellcome Department of Imaging Neuroscience, http://www.fil.ion.ucl.ac.uk/spm/) implemented in Matlab 6 (The Mathworks, USA) (Friston et al., 1995b; Worsley & Friston, 1995). All volumes were realigned, unwarped (Andersson et al., 2001) and slice-time corrected. The resulting volumes were then normalized to a symmetrical EPI template (the average of the EPI template and the EPI template flipped about the mid-sagittal line) based on the Montreal Neurological Institute (MNI) reference brain in Talairach space (Talairach & Tournaux, 1998) and resampled to 3 × 3 × 3 mm voxels. All normalized images were then smoothed with an isotropic 8-mm full-width half-maximum Gaussian kernel to account for intersubject anatomical differences and allow valid statistical inference according to Gaussian random field theory (Friston et al., 1995a). The time series in each voxel were high-pass filtered at 1/128 Hz to remove low-frequency confounds and scaled to a grand mean of 100 over voxels and scans within each session.
Images from those subjects using their right hand were flipped about the midsagittal plane so that all subjects were assumed to have performed the task with the left hand, consistent with previous published work (Ward et al., 2006).
Statistical analysis
Imaging data from each single subject were modelled using two orthogonal covariates. Firstly, all hand grips were defined as a single event type and modelled as delta functions (grip covariate). A second covariate (force covariate) comprised a delta function scaled by the peak force exerted for each hand grip. The force covariate was mean corrected and orthogonalized with respect to the first covariate ensuring that parameter estimates derived from each covariate are independent. Both covariates were convolved with a canonical synthetic haemodynamic response function and used in a general linear model (Friston et al., 1995b, 1998) together with a single covariate representing the mean (constant) term over scans. Thus, for each subject, voxel-wise parameter estimates for each covariate resulting from the least mean squares fit of the model to the data were generated. Parameter estimates (or ‘betas’) for the grip covariate reflect the size of increase in the BOLD signal during all hand grips compared with rest (BG). Parameter estimates for the force covariate represent the partial correlation coefficient of BOLD signal plotted against hand grip force (BF), i.e. the degree to which BOLD signal changes linearly with hand grips of different force (Buchel et al., 1998). For the control group, statistical parametric maps of the t-statistic (SPM{t}) resulting from a linear contrast of each covariate (Friston et al., 1995b, 1998) were generated to determine: (1) the main effects of hand grip; and (2) brain regions in which signal varies linearly with hand grip force The resulting SPM{t}s were thresholded at P < 0.05 (family wise error), corrected for multiple comparisons.
The experimental question in the patient group was whether an interaction between corticospinal system integrity (RCAH) and BG or BF can be detected. In a previous experiment, a subset of the current patients was scanned to examine for the main effects of hand grip (BG) only (Ward et al., 2006), but not the parametric modulation of BOLD signal with increasing force output (BF) as in this study. In the previous study, owing to the lack of comparable data, we assumed that the relationship between brain activity and corticospinal system integrity would be linear as a first-pass approximation. Although the correlation was significant, it was clear that the relationship was not linear. A post hoc analysis of those data found that using log RCAH rather than RCAH as the independent variable resulted in a stronger linear correlation. Thus, we chose a priori to use log RCAH as the independent variable in this study for the correlation analysis with BF.
We used a multisubject fixed effects model for our patient group. The fixed effects model allows ‘task by corticospinal integrity’ interactions to be examined, although the results are specific to the group studied. Thus, the specific contrasts across each covariate were weighted according to the mean corrected log RCAH scores for each patient (either positively or negatively). When applied to the model these contrast weightings identified voxels in which the correlation between BG or BF and log RCAH was significant. The resulting SPM{t}s for positive and negative correlations were thresholded at P < 0.05, corrected for multiple comparisons.
Anatomical identification was carefully performed by superimposing the maxima of activation foci both on the MNI brain and on the normalized structural images of each subject, and labelling with the aid of the atlas of Duvernoy (1991).
Results
Clinical data
All subjects were able to perform the task adequately. The duration of hand grips for low (target 15% MVC), medium (target 30% MVC) and high (target 45% MVC) hand grips are given in Table 2, along with the mean force and range of forces exerted for each patient. There was no significant correlation between log RCAH and duration of hand grip for low (r2 = 0.16, P = ns), medium (r2 = 0.03, P = ns) or high (r2 = 0.05, P = ns) target forces.
Table 2.
Hand grip performance
| Grip duration (ms) |
Mean force (% MVC) |
Range (% MVC) |
|||
|---|---|---|---|---|---|
| Patient | Low grip | Medium grip | High grip | ||
| 1 | 1309 ± 204 | 1675 ± 179 | 2199 ± 216 | 27.9 ± 9.6 | 8.4–45.6 |
| 2 | 1047 ± 78 | 1305 ± 137 | 1567 ± 194 | 26.3 ± 10.5 | 14.7–47.7 |
| 3 | 1179 ± 207 | 1540 ± 155 | 2030 ± 190 | 27.1 ± 10.4 | 12.1–43.0 |
| 4 | 1116 ± 215 | 1360 ± 209 | 2129 ± 172 | 31.9 ± 11.3 | 13.4–57.0 |
| 5 | 981 ± 130 | 1345 ± 158 | 1789 ± 192 | 29.5 ± 9.9 | 13.2–45.5 |
| 6 | 937 ± 173 | 1236 ± 263 | 1606 ± 253 | 25.5 ± 10.8 | 8.6–44.7 |
| 7 | 1206 ± 171 | 1761 ± 204 | 2238 ± 249 | 29.2 ± 11.6 | 12.3–51.4 |
| 8 | 1066 ± 141 | 1307 ± 146 | 1620 ± 153 | 32.6 ± 14.3 | 10.8–58.2 |
| 9 | 1093 ± 106 | 1457 ± 101 | 1896 ± 134 | 28.8 ± 12.4 | 8.7–51.9 |
Data are presented as means ± SD or range.
No subject displayed mirror movements or synergistic flexor movements in more proximal joints as assessed outside the scanner by direct observation, and during scanning by inspection of the force recordings from the unaffected hand during movement of the affected hand.
TMS results
M-wave, resting motor threshold and RCAH values for each patient are given in Table 3.
Table 3.
TMS data
| Patient | M-wave (mV) | rMTAH | RCAH |
|---|---|---|---|
| 1 | 6.86 | 80 | 0.003 |
| 2 | 13.34 | 41 | 0.048 |
| 3 | 18.2 | 39 | 0.055 |
| 4 | 11.04 | 85 | 0.016 |
| 5 | 10.81 | 52 | 0.031 |
| 6 | 18.04 | 65 | 0.078 |
| 7 | 13.42 | 44 | 0.324 |
| 8 | 16.79 | 54 | 0.216 |
| 9 | 15.45 | 47 | 0.716 |
M-waves are from the paretic hand first dorsal interosseus muscle. RCAH, affected hemisphere stimulus/response gradient from first dorsal interosseus (corrected for M-wave amplitude); rMTAH, resting motor threshold for first dorsal interosseus from affected hemisphere.
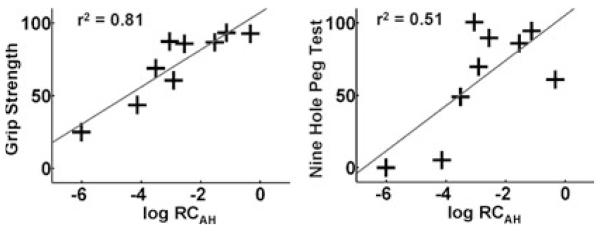
A significant positive correlation was found between log RCAH and GRIP (r2 = 0.81, P < 0.001) and between log RCAH and NHPT (r2 = 0.51, P = 0.03) (Fig. 1). No correlation was found between log RCAH and the visual analogue scale rating for effort by each patient (r2 = 0.03, P = ns).
Fig. 1.
Maximum hand grip (GRIP) and nine-hole peg test (NHPT) scores for the affected hand (expressed as percentage of the values for the unaffected hand) plotted against log RCAH.
Imaging results
Control subjects
The main effects of hand grip were consistent with previous reports using this paradigm (Ward & Frackowiak, 2003) and so are not reported here. Regions in which the magnitude of task-related signal covaried positively with grip force output in this visuo-motor task were seen in contralateral M1, SMA, ipsilateral cerebellum (lobule VI), and primary visual cortex (Table 4). There were no regions in which task-related signal decreased with increasing grip force.
Table 4.
Increasing signal change with increasing hand grip force in healthy controls
| Talairach coordinates in MNI space |
|||||
|---|---|---|---|---|---|
| Region | Side | x | y | z | Z-value |
| Primary motor cortex | C | 40 | −16 | 56 | > 8.0 |
| Supplementary motor area | C | 0 | −6 | 46 | 7.71 |
| Cerebellum (lobule VI) | I | −30 | −70 | −22 | 7.29 |
| Primary visual cortex | C | 0 | −90 | 6 | > 8.0 |
Brain voxels in which hand grip-related signal change increases linearly with increasing hand grip force. For the group analysis, control subjects are assumed to have used their left hands, and brains flipped about the mid-sagittal line accordingly. Voxels are significant at P < 0.05, corrected for multiple comparisons. C, contralateral; I, ipsilateral.
Stroke patients
A positive correlation between the parameter estimates for grip covariate (BG) and log RCAH was seen in ipsilesional M1 and contralesional cerebellum (lobule VI) (Table 5). A negative correlation between BG and log RCAH was seen in contralesional M1, bilateral premotor cortices (both dorsal and ventral), SMA, parietal and prefrontal cortices, contralesional insula cortex, posterior cingulate sulcus, and in both cerebellar hemispheres; ipsilesional lobule VI and contralesional crus I (Table 5).
Table 5.
Correlation between the main effects of hand grip and corticospinal system integrity in chronic stroke patients
| Talairach coordinates in MNI space |
||||||
|---|---|---|---|---|---|---|
| Region | Side | x | y | z | Z-value | (r2) |
| Positive correlation | ||||||
| Primary motor cortex | I | 40 | −16 | 64 | 4.31 | 0.45 |
| Cerebellum (lobule VI) | C | −16 | −46 | −24 | 4.22 | 0.45 |
| Negative correlation | ||||||
| Primary motor cortex | C | −32 | −26 | 66 | > 8.0 | 0.74 |
| Premotor cortex (dorsal) | C | −36 | −12 | 66 | > 8.0 | 0.63 |
| C | −26 | −10 | 56 | > 8.0 | 0.86 | |
| I | 42 | −2 | 54 | > 8.0 | 0.73 | |
| Premotor cortex (ventral) | C | −52 | 4 | 18 | 5.56 | 0.36 |
| I | 50 | 12 | 18 | 7.17 | 0.57 | |
| Supplementary motor area | I | 2 | 0 | 64 | > 8.0 | 0.69 |
| C | −6 | 0 | 54 | 7.39 | 0.75 | |
| Cingulate sulcus | C | −6 | −24 | 58 | 6.87 | 0.55 |
| Intraparietal sulcus | C | −36 | −54 | 56 | > 8.0 | 0.49 |
| I | 40 | −52 | 56 | > 8.0 | 0.72 | |
| Inferior parietal cortex | C | −44 | −42 | 24 | > 8.0 | 0.76 |
| Superior parietal cortex | I | 24 | −40 | 70 | 5.28 | 0.49 |
| Prefrontal cortex (dorsolateral) | I | 38 | 38 | 36 | 6.91 | 0.61 |
| C | −36 | 34 | 40 | 5.35 | 0.54 | |
| Insula cortex | C | −28 | 16 | 10 | 5.19 | 0.69 |
| Cerebellum (lobule VI) | I | 28 | −70 | −28 | 5.85 | 0.61 |
| I | 32 | −40 | −42 | 5.73 | 0.73 | |
| Cerebellum (Cr I) | C | −36 | −72 | −34 | 5.95 | 0.66 |
Brain voxels in which there is a correlation between log RCAH and the parameter estimates for the grip covariate (BG) within the patient group. Voxels are significant at P < 0.05, corrected for multiple comparisons. C, contralesional; I, ipsilesional.
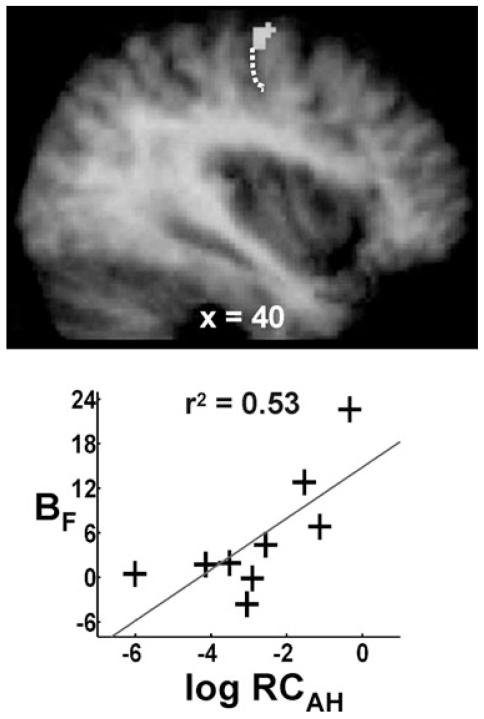
There were no suprathreshold voxels in which a positive correlation between log RCAH and the parameter estimates for the force covariate (BF) was found. However, we had hypothesized that we would observe a positive correlation between log RCAH and BF in brain regions in which task-related activity covaried positively with grip force output in the control group. In other words we expected more ‘normal’ behaviour of the motor system with greater functional integrity of the corticospinal system. Our a priori anatomical hypothesis therefore allowed us to restrict our search volume for a positive correlation between log RCAH and BF. We used spheres of 10 mm diameter centred on the coordinates of the four peak regions described in Table 4 to define the search volumes. Using this approach, the only region in which there was a positive correlation between log RCAH and BF in the patient group was in ipsilesional M1 (Table 6 and Fig. 2). Trends towards a significant positive correlation were seen in contralesional cerebellum (lobule VI, x = −30, y = −62, z = −22, Z-score = 2.89, P = 0.091) and SMA (x = 0, y = −2, z = 52, Z-score = 3.00, P = 0.064), but not in the visual cortex.
Table 6.
Correlation between size of force modulation effect and corticospinal system integrity in chronic stroke patients
| Talairach coordinates in MNI space | Correlation analysis | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Region | Side | x | y | z | Z-value | (r2) |
| Positive correlation | ||||||
| Primary motor cortex | I | 40 | −16 | 62 | 3.92‡ | 0.53 |
| Negative correlation | ||||||
| Premotor cortex (ventral) | C | −46 | 8 | 38 | 5.49* | 0.81 |
| I | 44 | 6 | 40 | 5.17* | 0.76 | |
| Premotor cortex (dorsal) | C | −26 | −4 | 60 | 4.88* | 0.57 |
| Cerebellum (crus I) | C | −34 | −76 | −32 | 4.77* | 0.82 |
Brain voxels in which there is a correlation between log RCAH and the parameter estimates for the force covariate (BF) within the patient group. Voxels are significant at P < 0.05, corrected for multiple comparisons
across the whole brain
across a restricted search volume defined by spheres of 10-mm diameter centred on the coordinates of the four peak regions described in Table 4. C, contralesional; I, ipsilesional.
Fig. 2.
A positive correlation between RCAH and BF was seen in ipsilesional primary motor cortex on the anterior bank of central sulcus (x = 40, y = −16, z = 62, r2 = 0.53). Results are overlaid onto the average T1-weighted structural scan obtained from all stroke patients. The position of the central sulcus is marked by a dotted white line. The plot of BF vs. RCAH for the peak voxel in the cluster is also shown.
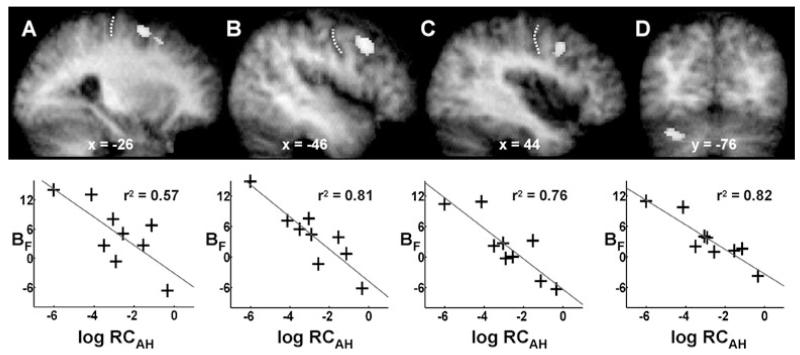
Negative correlations between log RCAH and BF were found in contralesional PMd and cerebellum (crus I), and in the middle frontal gyrus (within the ventral precentral sulcus) bilaterally at the threshold P < 0.05, corrected for multiple comparisons across the whole brain (Table 6 and Fig. 3). In the ipsilesional hemisphere, the significant cluster (P < 0.05 corrected) extended from z = 32 to 42, and in the contralesional hemisphere from z = 30 to 46. A location within the precentral sulcus below z = 50 is likely to represent the ventral premotor cortex (PMv) (Rizzolatti et al., 2002).
Fig. 3.
Brain regions in which there is a negative correlation between RCAH and BF. (A) Contralesional PMd (x= −26, y = −4, z = 60, r2 = 0.57). (B) Contralesional PMv (x = −46, y = 8, z = 38, r2 = 0.81). (C) Ipsilesional ventral premotor cortex (x = 44, y = 6, z = 40, r2 = 0.76). (D) Contralesional cerebellum (crus I) (x = −34, y = −76, z = −32, r2 = 0.82). Results are overlaid onto the average T1-weighted structural scan obtained from all stroke patients. The position of the central sulcus is marked by a dotted white line in (A–C). The plot of BF vs. RCAH for the peak voxel in each cluster is shown underneath each brain slice.
Discussion
In this study we have characterized two aspects of motor system activity during hand grip in nine subcortical stroke patients. Thus, for each brain region we have determined: (1) the average increase in activity during hand grip with the affected hand; and (2) the degree to which brain activity covaries with the amount of force produced. These two parameters can vary independently from one another. Thus, activity in one region might be high on average during hand grip, but might not vary greatly when hand grips of different forces are used. Conversely, activity in another region might be moderate on average, but vary a great deal depending on the amount of force produced.
We have previously reported a negative correlation between average brain activity in secondary motor regions during affected hand grip and a measure of corticospinal system integrity (RCAH) in chronic subcortical stroke patients (Ward et al., 2006). Here we have repeated this finding and confirmed that the shift from primary to secondary motor area recruitment is non-linear, occurring predominantly in the face of marked corticospinal disruption. We have previously interpreted this recruitment of secondary motor areas as an attempt to generate motor output to spinal cord motoneurons in the face of increasing disconnection of ipsilesional M1. However, secondary motor regions are less efficient at generating motor output so this reorganization can only be considered partially successful in reducing motor impairment after stroke.
In this study we have extended these findings by demonstrating for the first time that after subcortical stroke, the degree to which activity in brain regions covaries with the amount of force produced is related to the extent of corticospinal system damage. In patients with less corticospinal damage (and in normal subjects) brain activity in ipsilesional (contralateral) M1covaries positively with the amount of force produced, as expected. In other words, M1 is behaving more normally in these patients. However, in patients with greater damage to the corticospinal system this force-related modulation of activity is seen in bilateral PMv and contralesional PMd, but less so in ipsilesional M1. This shift suggests that premotor regions have taken on some of the executive properties of M1, which supports the notion that they are contributing to recovered function. Increasing executive properties of contralesional PMd in patients with greater corticospinal damage is consistent with the finding that TMS-induced disruption of this region has greater behavioural effect in more impaired patients (Johansen-Berg et al., 2002). Force-related modulation of brain activity in non-primary motor regions has been demonstrated previously in chronic stroke patients (Dettmers et al., 1997), but here we demonstrate that the relationship between changes in force production and brain activity within the distributed motor system is modulated by the functional integrity of the major motor output pathway. Taken together, the results of these studies indicate that our subcortical stroke patients with greater damage to the corticospinal tract are left with a motor system that is not only configured differently, but one that behaves differently in response to demands to increase relative force output.
Our results must be viewed in the context of the patients studied. We were interested in characterizing differences in motor system organization in a wide range of subcortical stroke patients. This variability does not lend itself to examining for average group effects, but is essential for a correlation analysis of the type we have employed. However, our cohort is by no means representative and thus it is difficult to generalize the results to all stroke patients.
We characterized the variability in the patient group using both TMS and fMRI. TMS was used to obtain motor cortical stimulus/response curves, which plot the size of the motor potential evoked by magnetic stimulation of a fixed site on the scalp across a range of intensities (Ridding & Rothwell, 1997). For an intrinsic hand muscle the relationship is sigmoidal (Devanne et al., 1997) and most likely reflects increasing recruitment of the elements comprising the corticospinal system, namely cortical circuitry, the motoneuron pool and spinal interneuronal relays (Burke et al., 1994). Although the exact contribution of each of these elements is not well understood, the resulting stimulus/response gradient is a sensitive reflection of the functional integrity of the corticospinal system (Devanne et al., 1997; Ridding & Rothwell, 1997; Boroojerdi et al., 2001). Our measure of corticospinal system integrity accounted for over 80% of the variability in grip strength and over 50% of the variability in a measure of manual dexterity (NHPT) in our patient group. Thus, although there is some variability in subcortical lesion location, our metric of corticospinal system integrity captures a large proportion of clinically relevant anatomical damage in our patient group.
When using fMRI, a potential problem that arises from the variation in motor impairment seen in the patient group is that of performance confounds in the experimental design (Baron et al., 2004). It is increasingly recognized that several cortical motor regions contribute to motor planning and execution via neurons that can encode multiple parameters of the task (Riehle & Requin, 1989; Alexander & Crutcher, 1990; Fu et al., 1993, 1995; Johnson et al., 1999; Kakei et al., 2001; Xiao et al., 2006). For example, the discharge of PMd neurons can be influenced by kinematic (Fu et al., 1993; Scott et al., 1997; Johnson et al., 1999; Gomez et al., 2000), visuospatial (di Pellegrino & Wise, 1993; Johnson et al., 1996; Shen & Alexander, 1997) and contextual (Hepp-Reymond et al., 1999) aspects of the movement. Thus, any differences in the way a task was performed in patients with marked impairment in comparison to those with less impairment could account for our results. Our experiment was therefore carefully designed to minimize this possibility. There were no differences between patients in terms of the range or average of relative forces exerted or the visual feedback provided. There was no relationship between the duration of hand grips and log RCAH, suggesting that the small differences in time on task between patients could not have explained our results. Neither was there a correlation between the perceived effort involved in the task and log RCAH or impairment (as measured by grip strength or NHPT). Thus, in terms of perceived effort our design ensured equivalence of task across all subjects, so that the changes in force modulatory areas observed are likely to be a consequence of differences in corticospinal system integrity rather than task difficulty. Our results reflect differences in brain activity over the relative range of forces available to each individual patient (i.e. 15–45% of maximum voluntary contraction).
However, the range and average of absolute force levels were smaller in patients with greater corticospinal damage. An alternative explanation of our result lies in the finding that premotor regions, particularly ventral, are more active during precision compared with power grip (Ehrsson et al., 2000, 2001). It is possible that the more impaired patients were performing the force modulation task more like a precision grip task, thus accounting for increased modulatory behaviour in ventral premotor cortices. Nevertheless, whether it is the change in absolute or relative force levels that is more important, it still suggests that these regions become increasingly functionally useful, in the face of greater corticospinal damage.
Single-cell recordings in macaque monkeys have demonstrated positive linear correlations between firing rates of cortical neurons and force during grip tasks in contralateral M1 (Evarts, 1968; Smith et al., 1975; Hepp-Reymond et al., 1978; Evarts et al., 1983; Wannier et al., 1991; Georgopoulos et al., 1992). Experiments in humans using fMRI have found similar results (Dettmers et al., 1995; Thickbroom et al., 1999; Ward & Frackowiak, 2003). We observed a linear increase in BOLD signal with increasing force in contralateral M1 in our control group and in patients with greater functional integrity of the corticospinal system. In patients with greater damage, however, force modulatory behaviour in ipsilesional (contralateral) M1 diminished, suggesting reduced functional utility. These patients generally exhibit greater motor impairment (Fig. 1), and yet are still able to modulate hand grip force. Modulation of grip force in the face of increasing corticospinal damage must therefore be mediated by non-M1 sites. Our results suggest the premotor cortices are able to take on this role.
In single-cell recordings, a quarter of PMd cells demonstrated force-related activity in an isometric task (Werner et al., 1991). A small proportion of PMv cells may either increase or decrease neuronal firing rates with increasing precision grip force (Hepp-Reymond et al., 1994). The fMRI signal from a region is effectively an average of the local responses, which might explain why force modulation was not seen in premotor cortices in our control group using fMRI. However, our results demonstrate that a consistently positive correlation between force and BOLD signal was more likely to be seen in premotor cortices rather than M1 as corticospinal integrity diminished, indicating an increase in the number of premotor neurons involved in force-related activity.
The adopted role of the premotor regions within the residual functional architecture is less clear. In primates, M1, PMd and PMv form a densely interconnected network subserving hand motor function (Dum & Strick, 2005). Although the laminar distribution of the corticocortical connections suggests these regions operate at the same hierarchical level, their corticospinal projections are significantly different (Dum & Strick, 2005). Spinal cord motor neurons to the hand originate from lower cervical/upper thoracic cord. A large proportion of direct cortico-motoneuronal fibres to the lower cervical cord originate from M1 (He et al., 1993; Porter & Lemon, 1993; Maier et al., 2002). PMd provides projections to the upper and lower cervical cord, whilst PMv provides projections mainly to the upper cervical cord (He et al., 1993). Thus, premotor projections are unlikely to completely substitute for those from M1 to hand muscles. However, the ability to modulate applied forces, even if those forces are lower, will increase the functionality of hand grip. Our results suggest that the premotor regions play a role in maintaining this capability.
It is possible that premotor regions are exerting their modulatory effect directly on M1 by increasing the gain of residual M1 output. When higher grip forces are required, partial damage to the corticospinal system may render existing inputs to M1 insufficient to increase output to spinal cord motor neurons. In this situation, additional premotor cortex input to M1 could facilitate an increase in residual M1 output. In normal primates, rostral PMv (area F5) is able to facilitate motor cortex output to upper limb motor neurons (Cerri et al., 2003; Shimazu et al., 2004). The human homologue of primate area F5 remains controversial but is likely to be close to Brodmann area (BA)44 (Rizzolatti et al., 2002). Our ventral premotor clusters are situated just dorsal and caudal to this region in the normal human brain (Tomaiuolo et al., 1999), although the border between BA44 and ventral BA6 remains uncertain (Amunts et al., 1999). Enlargement of the PMv hand area has been observed in primates after M1 damage (Frost et al., 2003; Dancause et al., 2005), together with the appearance of novel connections between PMv and primary somatosensory cortex (Dancause et al., 2005). The possibility of shifts in PMv/BA44 representation in some of our patients cannot therefore be excluded.
An alternative explanation is that premotor regions exert their modulatory effect through non-monosynaptic pathways to spinal cord motor neurons. In stroke patients with poorer recovery (and greater damage to the corticospinal system) a greater proportion of the descending motor command is mediated through propriospinal projections (Mazevet et al., 2003; Stinear & Byblow, 2004). Both PMd and PMv have direct and indirect (via bilateral reticulospinal pathways) projections to the propriospinal premotoneurons in the upper cervical cord (Benecke et al., 1991; He et al., 1993). Movements elicited through the propriospinal system tend to involve multiple joints rather than fractionated finger movements (Mazevet & Pierrot-Deseilligny, 1994; Mazevet et al., 2003). Thus, the appearance of flexor synergistic movements in some patients when gripping harder could be explained by increased activation through propriospinal pathways.
The shift in executive force modulation from M1 to premotor regions is reflected in changes in cerebellar activation. In control subjects, force modulation was seen in lobule VI, but in patients with increasing corticospinal disruption appeared more ventrally in crus I. Cerebellar outputs from the dentate nucleus to premotor regions are ventral to those targeting M1 (Middleton & Strick, 1997). Thus, our result may reflect increased force-related activity in contralesional premotor-cerebellar loops as a consequence of increased task-related connectivity in contralesional motor loops (Gerloff et al., 2006).
In conclusion, we have previously demonstrated that increasing corticospinal damage results in less dependence on ipsilesional M1, but greater dependence on secondary motor regions (Ward et al., 2006). This is likely to reflect recruitment of surviving motor regions that are able to influence spinal cord motoneurons and thus motor output. Our current results characterize this response to corticospinal injury further by demonstrating that motor-related activity in contralesional PMd and bilateral PMv is not only increased in patients with more damage to the corticospinal system, but that this activity now covaries with force output in a way not seen in healthy controls or in patients with less damaged corticospinal systems. Furthermore, covariation between force output and brain activity in ipsilesional M1 diminishes with increasing corticospinal system damage. Thus, there is a shift in brain regions involved in modulating grip force from contralateral (ipsilesional in the patients) M1 towards premotor regions in this group of patients. Our finding suggests that premotor regions might therefore play a new and functionally relevant role in optimizing motor output in the face of major corticospinal disruption. Thus, reconfiguration of functional neural networks post-stroke is a dynamic process dependent on both the anatomy of the damage and the demands of a task.
Acknowledgements
N.S.W., L.L. and R.S.J.F. are supported by the Wellcome Trust. J.M.N. is supported by Action Medical Research. J.R. is supported by the Medical Research Council. A.J.T. holds the Garfield Weston Chair of Clinical Neurology and Neurological Rehabilitation. We would like to thank Peter Aston and Eric Featherstone (Wellcome Department of Imaging Neuroscience) for the design and programming involved in creating the hand grip manipulandum. We would also like to thank the staff of the Acute Brain Injury Unit and Neurorehabilitation Unit at the National Hospital for Neurology and Neurosurgery, Queen Square, London, for their assistance.
Abbreviations
- BA
Brodmann area
- BOLD
blood oxygen level-dependent
- EPI
echo-planar image
- fMRI
functional magnetic resonance imaging
- M1
primary motor cortex
- MNI
Montreal Neurological Institute
- MVC
maximum voluntary contraction
- NHPT
nine-hole peg test
- PMd
dorsal lateral premotor cortex
- PMv
ventral lateral premotor cortex
- RCAH
recruitment curve gradient – affected hemisphere
- SMA
supplementary motor area
- TMS
transcranial magnetic stimulation
References
- Alexander GE, Crutcher MD. Neural representations of the target (goal) of visually guided arm movements in three motor areas of the monkey. J. Neurophysiol. 1990;64:164–178. doi: 10.1152/jn.1990.64.1.164. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HB, Zilles K. Broca’s region revisited: cytoarchitecture and intersubject variability. J. Comp. Neurol. 1999;412:319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Andersson JL, Hutton C, Ashburner J, Turner R, Friston K. Modeling geometric deformations in EPI time series. Neuroimage. 2001;13:903–919. doi: 10.1006/nimg.2001.0746. [DOI] [PubMed] [Google Scholar]
- Baron JC, Cohen LG, Cramer SC, Dobkin BH, Johansen-Berg H, Loubinoux I, Marshall RS, Ward NS. Neuroimaging in stroke recovery: a position paper from the First International Workshop on Neuroimaging and Stroke Recovery. Cerebrovasc. Dis. 2004;18:260–267. doi: 10.1159/000080293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benecke R, Meyer BU, Freund HJ. Reorganisation of descending motor pathways in patients after hemispherectomy and severe hemispheric lesions demonstrated by magnetic brain stimulation. Exp. Brain Res. 1991;83:419–426. doi: 10.1007/BF00231167. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Battaglia F, Muellbacher W, Cohen LG. Mechanisms influencing stimulus-response properties of the human corticospinal system. Clin. Neurophysiol. 2001;112:931–937. doi: 10.1016/s1388-2457(01)00523-5. [DOI] [PubMed] [Google Scholar]
- Buchel C, Holmes AP, Rees G, Friston KJ. Characterizing stimulus-response functions using nonlinear regressors in parametric fMRI experiments. Neuroimage. 1998;8:140–148. doi: 10.1006/nimg.1998.0351. [DOI] [PubMed] [Google Scholar]
- Burke D, Gracies JM, Mazevet D, Meunier S, Pierrot-Deseilligny E. Non-monosynaptic transmission of the cortical command for voluntary movement in man. J. Physiol. (Lond.) 1994;480:191–202. doi: 10.1113/jphysiol.1994.sp020352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerri G, Shimazu H, Maier MA, Lemon RN. Facilitation from ventral premotor cortex of primary motor cortex outputs to macaque hand muscles. J. Neurophysiol. 2003;90:832–842. doi: 10.1152/jn.01026.2002. [DOI] [PubMed] [Google Scholar]
- Chollet F, DiPiero V, Wise RJ, Brooks DJ, Dolan RJ, Frackowiak RS. The functional anatomy of motor recovery after stroke in humans: a study with positron emission tomography. Ann. Neurol. 1991;29:63–71. doi: 10.1002/ana.410290112. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Nelles G, Benson RR, Kaplan JD, Parker RA, Kwong KK, Kennedy DN, Finklestein SP, Rosen BR. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997;28:2518–2527. doi: 10.1161/01.str.28.12.2518. [DOI] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, Plautz EJ, Chen D, Zoubina EV, Stowe AM, Nudo RJ. Extensive cortical rewiring after brain injury. J. Neurosci. 2005;25:10167–10179. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmers C, Fink GR, Lemon RN, Stephan KM, Passingham RE, Silbersweig D, Holmes A, Ridding MC, Brooks DJ, Frackowiak RS. Relation between cerebral activity and force in the motor areas of the human brain. J. Neurophysiol. 1995;74:802–815. doi: 10.1152/jn.1995.74.2.802. [DOI] [PubMed] [Google Scholar]
- Dettmers C, Stephan KM, Lemon RN, Frackowiak RSJ. Reorganization of the executive motor system after stroke. Cerebrovasc. Dis. 1997;7:187–200. [Google Scholar]
- Devanne H, Lavoie BA, Capaday C. Input-output properties and gain changes in the human corticospinal pathway. Exp. Brain Res. 1997;114:329–338. doi: 10.1007/pl00005641. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Frontal lobe inputs to the digit representations of the motor areas on the lateral surface of the hemisphere. J. Neurosci. 2005;25:1375–1386. doi: 10.1523/JNEUROSCI.3902-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM. The Human Brain: Surface, Blood Supply, and Three-Dimensional Anatomy. Springer; New York: 1991. [Google Scholar]
- Ehrsson HH, Fagergren E, Forssberg H. Differential frontoparietal activation depending on force used in a precision grip task: an fMRI study. J. Neurophysiol. 2000;85:2613–2623. doi: 10.1152/jn.2001.85.6.2613. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Fagergren E, Jonsson T, Westling G, Johansson RS, Forssberg H. Cortical activity in precision- versus power-grip tasks: an fMRI study. J. Neurophysiol. 2001;83:528–536. doi: 10.1152/jn.2000.83.1.528. [DOI] [PubMed] [Google Scholar]
- Evarts EV. Relation of pyramidal tract activity to force exerted during voluntary movement. J. Neurophysiol. 1968;31:14–27. doi: 10.1152/jn.1968.31.1.14. [DOI] [PubMed] [Google Scholar]
- Evarts EV, Fromm C, Kroller J, Jennings VA. Motor cortex control of finely graded forces. J. Neurophysiol. 1983;49:1199–1215. doi: 10.1152/jn.1983.49.5.1199. [DOI] [PubMed] [Google Scholar]
- Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG. Reorganization of the human ipsilesional premotor cortex after stroke. Brain. 2004;127:747–758. doi: 10.1093/brain/awh082. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner L, Poline JB, Frith CD, Frackowiak RS. Spatial registration and normalization of images. Hum. Brain Mapp. 1995a;2:165–189. [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general linear approach. Hum. Brain Mapp. 1995b;2:189–210. [Google Scholar]
- Frost SB, Barbay S, Friel KM, Plautz EJ, Nudo RJ. Reorganization of remote cortical regions after ischemic brain injury: a potential substrate for stroke recovery. J. Neurophysiol. 2003;89:3205–3214. doi: 10.1152/jn.01143.2002. [DOI] [PubMed] [Google Scholar]
- Fu QG, Flament D, Coltz JD, Ebner TJ. Temporal encoding of movement kinematics in the discharge of primate primary motor and premotor neurons. J. Neurophysiol. 1995;73:836–854. doi: 10.1152/jn.1995.73.2.836. [DOI] [PubMed] [Google Scholar]
- Fu QG, Suarez JI, Ebner TJ. Neuronal specification of direction and distance during reaching movements in the superior precentral premotor area and primary motor cortex of monkeys. J. Neurophysiol. 1993;70:2097–2116. doi: 10.1152/jn.1993.70.5.2097. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Ashe J, Smyrnis N, Taira M. The motor cortex and the coding of force. Science. 1992;256:1692–1695. doi: 10.1126/science.256.5064.1692. [DOI] [PubMed] [Google Scholar]
- Gerloff C, Bushara K, Sailer A, Wassermann EM, Chen R, Matsuoka T, Waldvogel D, Wittenberg GF, Ishii K, Cohen LG, Hallett M. Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain. 2006;129:791–808. doi: 10.1093/brain/awh713. [DOI] [PubMed] [Google Scholar]
- Gomez JE, Fu Q, Flament D, Ebner TJ. Representation of accuracy in the dorsal premotor cortex. Eur. J. Neurosci. 2000;12:3748–3760. doi: 10.1046/j.1460-9568.2000.00232.x. [DOI] [PubMed] [Google Scholar]
- He SQ, Dum RP, Strick PL. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the lateral surface of the hemisphere. J. Neurosci. 1993;13:952–980. doi: 10.1523/JNEUROSCI.13-03-00952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepp-Reymond MC, Husler EJ, Maier MA, Qi HX. Force-related neuronal activity in two regions of the primate ventral premotor cortex. Can. J. Physiol. Pharmacol. 1994;72:571–579. doi: 10.1139/y94-081. [DOI] [PubMed] [Google Scholar]
- Hepp-Reymond M, Kirkpatrick-Tanner M, Gabernet L, Qi HX, Weber B. Context-dependent force coding in motor and premotor cortical areas. Exp. Brain Res. 1999;128:123–133. doi: 10.1007/s002210050827. [DOI] [PubMed] [Google Scholar]
- Hepp-Reymond MC, Wyss UR, Anner R. Neuronal coding of static force in the primate motor cortex. J. Physiol. (Paris) 1978;74:287–291. [PubMed] [Google Scholar]
- Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc. Natl Acad. Sci. USA. 2002;99:14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MT, Coltz JD, Hagen MC, Ebner TJ. Visuomotor processing as reflected in the directional discharge of premotor and primary motor cortex neurons. J. Neurophysiol. 1999;81:875–894. doi: 10.1152/jn.1999.81.2.875. [DOI] [PubMed] [Google Scholar]
- Johnson PB, Ferraina S, Bianchi L, Caminiti R. Cortical networks for visual reaching: physiological and anatomical organization of frontal and parietal lobe arm regions. Cereb. Cortex. 1996;6:102–119. doi: 10.1093/cercor/6.2.102. [DOI] [PubMed] [Google Scholar]
- Kakei S, Hoffman DS, Strick PL. Direction of action is represented in the ventral premotor cortex. Nat. Neurosci. 2001;4:1020–1025. doi: 10.1038/nn726. [DOI] [PubMed] [Google Scholar]
- Lotze M, Markert J, Sauseng P, Hoppe J, Plewnia C, Gerloff C. The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. J. Neurosci. 2006;26:6096–6102. doi: 10.1523/JNEUROSCI.4564-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier MA, Armand J, Kirkwood PA, Yang HW, Davis JN, Lemon RN. Differences in the corticospinal projection from primary motor cortex and supplementary motor area to macaque upper limb motoneurons: an anatomical and electrophysiological study. Cereb. Cortex. 2002;12:281–296. doi: 10.1093/cercor/12.3.281. [DOI] [PubMed] [Google Scholar]
- Mazevet D, Meunier S, Pradat-Diehl P, Marchand-Pauvert V, Pierrot-Deseilligny E. Changes in propriospinally mediated excitation of upper limb motoneurons in stroke patients. Brain. 2003;126:988–1000. doi: 10.1093/brain/awg088. [DOI] [PubMed] [Google Scholar]
- Mazevet D, Pierrot-Deseilligny E. Pattern of descending excitation of presumed propriospinal neurones at the onset of voluntary movement in humans. Acta Physiol. Scand. 1994;150:27–38. doi: 10.1111/j.1748-1716.1994.tb09656.x. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Cerebellar output channels. Int. Rev. Neurobiol. 1997;41:61–82. doi: 10.1016/s0074-7742(08)60347-5. [DOI] [PubMed] [Google Scholar]
- Nelles G, Cramer SC, Schaechter JD, Kaplan JD, Finklestein SP. Quantitative assessment of mirror movements after stroke. Stroke. 1998;29:1182–1187. doi: 10.1161/01.str.29.6.1182. [DOI] [PubMed] [Google Scholar]
- Newton J, Sunderland A, Butterworth SE, Peters AM, Peck KK, Gowland PA. A pilot study of event-related functional magnetic resonance imaging of monitored wrist movements in patients with partial recovery. Stroke. 2002;33:2881–2887. doi: 10.1161/01.str.0000042660.38883.56. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Wise SP. Visuospatial versus visuomotor activity in the premotor and prefrontal cortex of a primate. J. Neurosci. 1993;13:1227–1243. doi: 10.1523/JNEUROSCI.13-03-01227.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R, Lemon RN. Corticospinal Function and Voluntary Movement. Oxford University Press; Oxford, UK: 1993. [Google Scholar]
- Ridding MC, Rothwell JC. Stimulus/response curves as a method of measuring motor cortical excitability in man. Electroencephalogr. Clin. Neurophysiol. 1997;105:340–344. doi: 10.1016/s0924-980x(97)00041-6. [DOI] [PubMed] [Google Scholar]
- Riehle A, Requin J. Monkey primary motor and premotor cortex: single-cell activity related to prior information about direction and extent of an intended movement. J. Neurophysiol. 1989;61:534–549. doi: 10.1152/jn.1989.61.3.534. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Motor and cognitive functions of the ventral premotor cortex. Curr. Opin. Neurobiol. 2002;12:149–154. doi: 10.1016/s0959-4388(02)00308-2. [DOI] [PubMed] [Google Scholar]
- Scott SH, Sergio LE, Kalaska JF. Reaching movements with similar hand paths but different arm orientations. II. Activity of individual cells in dorsal premotor cortex and parietal area 5. J. Neurophysiol. 1997;78:2413–2426. doi: 10.1152/jn.1997.78.5.2413. [DOI] [PubMed] [Google Scholar]
- Seitz RJ, Hoflich P, Binkofski F, Tellmann L, Herzog H, Freund HJ. Role of the premotor cortex in recovery from middle cerebral artery infarction. Arch. Neurol. 1998;55:1081–1088. doi: 10.1001/archneur.55.8.1081. [DOI] [PubMed] [Google Scholar]
- Shen L, Alexander GE. Preferential representation of instructed target location versus limb trajectory in dorsal premotor area. J. Neurophysiol. 1997;77:1195–1212. doi: 10.1152/jn.1997.77.3.1195. [DOI] [PubMed] [Google Scholar]
- Shimazu H, Maier MA, Cerri G, Kirkwood PA, Lemon RN. Macaque ventral premotor cortex exerts powerful facilitation of motor cortex outputs to upper limb motoneurons. J. Neurosci. 2004;24:1200–1211. doi: 10.1523/JNEUROSCI.4731-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Hepp-Reymond MC, Wyss UR. Relation of activity in precentral cortical neurons to force and rate of force change during isometric contractions of finger muscles. Exp. Brain Res. 1975;23:315–332. doi: 10.1007/BF00239743. [DOI] [PubMed] [Google Scholar]
- Stinear JW, Byblow WD. The contribution of cervical propriospinal premotoneurons in recovering hemiparetic stroke patients. J. Clin. Neurophysiol. 2004;21:426–434. doi: 10.1097/00004691-200411000-00006. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournaux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme; Stuttgart: 1998. [Google Scholar]
- Thickbroom GW, Phillips BA, Morris I, Byrnes ML, Sacco P, Mastaglia FL. Differences in functional magnetic resonance imaging of sensorimotor cortex during static and dynamic finger flexion. Exp. Brain Res. 1999;126:431–438. doi: 10.1007/s002210050749. [DOI] [PubMed] [Google Scholar]
- Tomaiuolo F, MacDonald JD, Caramanos Z, Posner G, Chiavaras M, Evans AC, Petrides M. Morphology, morphometry and probability mapping of the pars opercularis of the inferior frontal gyrus: an in vivo MRI analysis. Eur. J. Neurosci. 1999;11:3033–3046. doi: 10.1046/j.1460-9568.1999.00718.x. [DOI] [PubMed] [Google Scholar]
- Wannier TM, Maier MA, Hepp-Reymond MC. Contrasting properties of monkey somatosensory and motor cortex neurons activated during the control of force in precision grip. J. Neurophysiol. 1991;65:572–589. doi: 10.1152/jn.1991.65.3.572. [DOI] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain. 2003;126:1430–1448. doi: 10.1093/brain/awg145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Frackowiak RS. Age-related changes in the neural correlates of motor performance. Brain. 2003;126:873–888. doi: 10.1093/brain/awg071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Newton JM, Swayne OB, Lee L, Thompson AJ, Greenwood RJ, Rothwell JC, Frackowiak RS. Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain. 2006;129:809–819. doi: 10.1093/brain/awl002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiller C, Ramsay SC, Wise RJ, Friston KJ, Frackowiak RS. Individual patterns of functional reorganization in the human cerebral cortex after capsular infarction. Ann. Neurol. 1993;33:181–189. doi: 10.1002/ana.410330208. [DOI] [PubMed] [Google Scholar]
- Werner W, Bauswein E, Fromm C. Static firing rates of premotor and primary motor cortical neurons associated with torque and joint position. Exp. Brain Res. 1991;86:293–302. doi: 10.1007/BF00228952. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited – again. Neuroimage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Xiao J, Padoa-Schioppa C, Bizzi E. Neuronal correlates of movement dynamics in the dorsal and ventral premotor area in the monkey. Exp. Brain Res. 2006;168:106–119. doi: 10.1007/s00221-005-0074-2. [DOI] [PubMed] [Google Scholar]