Abstract
Functional imaging studies of cortical motor systems in humans have demonstrated age-related reorganisation often attributed to anatomical and physiological changes. In this study we investigated whether aspects of brain activity during a motor task were influenced not only by age, but also by neurophysiological parameters of the motor cortex contralateral to the moving hand. Twenty seven right-handed volunteers underwent functional magnetic resonance imaging whilst performing repetitive isometric right hand grips in which the target force was parametrically varied between 15 and 55% of each subject’s own maximum grip force. For each subject we characterised two orthogonal parameters, BG (average task-related activity for all hand grips) and BF (the degree to which task-related activity co-varied with peak grip force). We used transcranial magnetic stimulation (TMS) to assess task-related changes in interhemispheric inhibition from left to right motor cortex (IHIc) and to perform measures relating to left motor cortex excitability during activation of the right hand. Firstly, we found that BG in right (ipsilateral) motor cortex was greater with increasing values of age2 and IHIc. Secondly, BF in left ventral premotor cortex was greater in older subjects and in those in whom contralateral M1 was less responsive to TMS stimulation. In both cases, neurophysiological parameters accounted for variability in brain responses over and above that explained by ageing. These results indicate that neurophysiological markers may be better indicators of biological ageing than chronological age and point towards the mechanisms by which reconfiguration of distributed brain networks occurs in the face of degenerative changes.
Keywords: Motor cortex, Premotor cortex, fMRI, TMS, Ageing, Hand grip, Interhemispheric inhibition
Introduction
Several recent studies have used functional brain imaging techniques to examine for age-related changes in the way the central nervous system is organised (Calautti et al., 2001; Hesselmann et al., 2001; Heuninckx et al., 2005; Hutchinson et al., 2002; Mattay et al., 2002; Naccarato et al., 2006; Riecker et al., 2006; Rowe et al., 2006; Tekes et al., 2005; Ward and Frackowiak, 2003; Wu and Hallett, 2005). In general, task-related activation appears to be focused and lateralised in younger subjects but more diffuse and bilateral with advancing age (Cabeza, 2001; Ward, 2006). Studies concentrating on the motor system have found that age-related differences are greater when complex tasks are used. These differences are usually found in non-motor brain regions which may reflect increased cognitive monitoring of performance (Heuninckx et al., 2005).
In order to study age-related changes in the cortical motor system not attributable to the cognitive effects of ageing, a recent study employed a simple hand grip paradigm and reported two main findings (Ward et al., in press). Firstly, advancing age was associated with greater task-related activation in right (ipsilateral) primary motor cortex (M1) during gripping with the right hand. This finding has been demonstrated in two previous studies (Naccarato et al., 2006; Ward et al., in press) and has been attributed to a putative reduction in interhemispheric inhibition (IHI) between the motor cortices in older subjects.
The second main finding was that the linear increase in blood oxygen level dependent (BOLD) signal normally seen with parametric increases in peak grip force in the contralateral motor cortex diminished with advancing age. However, increasing BOLD signal with increasing peak grip force in bilateral ventrolateral premotor cortices (PMv) was more prominent in older subjects. In the older brain, existing inputs to M1 may be insufficient to increase output to spinal cord motor neurons when higher grip forces are required (Oliviero et al., 2006; Pitcher et al., 2003;Sale and Semmler, 2005). In normal primates rostral PMv (area F5) has dense connections to M1 (Dancause et al., 2006) and is able to enhance M1 output to upper limb spinal cord motor neurons (Cerri et al., 2003; Shimazu et al., 2004). Thus, we have previously hypothesised that age-related flattening of stimulus–output curves might lead to brain areas with direct connections to M1, such as PMv, exerting a modulatory effect by increasing the gain of M1 output (Ward et al., in press).
We have recently used transcranial magnetic stimulation (TMS) to assess how properties of motor cortex are affected by ageing. During activation of a target muscle the modulation of IHI targeting the ipsilateral motor cortex is a function of age: younger individuals show stronger IHI whereas in older individuals this phenomenon is attenuated and often reversed (Talelli et al., 2008). We also studied the effect of age on cortical excitability by constructing active stimulus–output curves, which plot the size of motor evoked potential (MEP) in the preactivated target muscle against the stimulation intensity. We found that the area under the curve and the maximum MEP diminish with age but that the intensity of TMS stimulation required to elicit 50% of the maximal MEP (I50) did not (Talelli et al., 2008).
Biological ageing might occur at different rates across individuals and so the purpose of the current study was to assess whether variability in brain activation during the performance of a well-characterised motor task is more closely related to underlying neurophysiological changes than to chronological age. Thus, we assessed the cortical motor system of subjects using fMRI and TMS in separate sessions. Subjects were asked to perform a simple isometric hand grip task with parametric modulation of force output during fMRI. Our first prediction was that average task-related activation in ipsilateral M1 will be greater in subjects with lower IHI. Although the increase in ipsilateral M1 activation with ageing has been previously attributed to diminished IHI, it has never been measured in the same subjects. Secondly we predicted that the ability of M1 to increase its output in response to a given input, as assessed with TMS, will relate to force-dependent modulation of BOLD signal positively in M1 and negatively in PMv. Such findings would support an anatomical mechanism for ‘age-related’ reorganisation in the cortical motor system (Ward, 2006).
Methods
Subjects
Twenty seven healthy volunteers (age range 19 to 78 years, mean 42.2 years, SD 19.8 years; 18 males) participated in the study. All subjects were right handed according to the Edinburgh handedness scale (Oldfield, 1971). They reported no history of neurological illness, psychiatric history, vascular disease or hypertension. Subjects were not taking regular medication. Full written consent was obtained from all subjects in accordance with the Declaration of Helsinki. The study was approved by the Joint Ethics Committee of the Institute of Neurology, UCL and National Hospital for Neurology and Neurosurgery, UCL Hospitals NHS Foundation Trust, London.
The TMS and fMRI experiments were performed within 2 h of one another in all subjects.
Behavioural evaluation
Maximum grip strength with each hand was measured for each subject using a Jamar hydraulic hand dynamometer (Fabrication Enterprises, Inc., NY, USA). Three trials were allowed, and the maximum value was used.
Transcranial magnetic stimulation
The TMS methodology has been previously described in detail (Talelli et al., 2008). In summary, two Magstim 200 stimulators were used to stimulate the hotspot for the FDI muscle in the M1. Measures of corticospinal excitability were performed on the left M1 with a 70 mm figure-of-eight coil. The motor thresholds were calculated at rest and during background contraction of the target muscle according to standard criteria (Rothwell, 1997). A recruitment curve (RC) was then constructed by plotting the average amplitude of the MEP evoked in the right FDI contracting at 15–20% of the maximum voluntary contraction (MVC) at 8 stimulation intensities (90, 100, 110, 130, 140, 150, 160 and 170% of active motor threshold). All MEP measures were expressed as a ratio to the max peak-to-peak amplitude of the compound action potential (CMAP) evoked by supramaximal electrical stimulation of the ulnar nerve at the wrist.
A second 50 mm figure-of-eight coil was then placed over the right M1 to measure the interhemispheric inhibition (IHI). We used a standardised paired-pulse paradigm (Chen et al., 2003; Daskalakis et al., 2002; Ferbert et al., 1992) whereby a suprathreshold TMS pulse delivered over the motor hotspot (conditioning pulse) suppresses the response to a standard TMS pulse (test pulse) delivered to the contralateral homologue 7–50 ms later. In this set of experiments we concentrated on the IHI targeting the right M1; hence the conditioning pulse was given over the left hemisphere, the test pulse was delivered on the right hemisphere and MEPs were measured in the left FDI. The stimulation intensity for both the conditioning and the test stimuli was adjusted to evoke an MEP of 1–1.5 mV in the resting contralateral FDI muscle and was then kept constant throughout the experiment. IHI was defined as the conditioned/unconditioned MEP amplitude ratio, smaller values reflecting stronger IHI. Two interstimulus intervals, 10 and 40 ms were assessed in different blocks since recent evidence suggests that different pathways may be implicated (Chen et al., 2003; Irlbacher et al., 2007). IHI was initially measured at rest. Then, we repeated the measures whilst the subjects were contracting the right FDI to 15–20% MVC in response to an auditory cue preceding the conditioning stimulus by 600 ms. The absolute values of active IHI were finally expressed as a ratio to the values at rest for the respective interval (IHI10c and IHI40c). IHIc therefore reflects the change seen in the IHI targeting the right motor cortex when the right hand is active. Values <1 reflect stronger inhibition, whilst values >1 reflect less inhibition.
Data preprocessing
TMS data were processed using SPSS v.14 (SPSS Inc., USA). The recruitment curves were sigmoidal in shape and were best fitted by a 3 parameter Boltzmann sigmoidal model (r2 >0.89 coefficient of determination in all subjects) based on the modified Levenberg–Marquardt nonlinear least mean squares algorithm. This function is often used to provide parameter estimates for RCs (Capaday et al., 1999; Devanne et al., 1997). The equation is: MEP=MEPmax/ 1+ exp((I50−I)/ slope), where MEPmax is the maximum MEP amplitude; I50 represents the stimulation intensity required to get a response 50% of the maximum. The inverse of the slope parameter (1 / slope) is directly proportional to the maximal steepness of the curve occurring at I50. Finally, the area under the RC (AUC) was calculated using the method of trapezoid integration to provide a summary measure of the corticospinal output across all stimulation intensities. Correlations between age and TMS measures were assessed by computing Pearson’s product–moment correlation coefficient. Partial and part correlations were employed as appropriate. The significance level was set at P <0.05.
fMRI scanning
Motor paradigm
All subjects underwent a single scanning session. During scanning, all subjects performed a series of dynamic isometric hand grips with the dominant right hand using a MRI-compatible manipulandum as previously described (Ward and Frackowiak, 2003;Ward et al., in press). Continuous visual feedback about the force exerted was provided. Prior to scanning, but whilst lying in the scanner, subjects were asked to grip the manipulandum using maximum force to generate a maximum voluntary contraction (MVC) for each hand. A single scanning session comprised 100 visually cued hand grips interspersed with 60 null events in a randomised and counterbalanced order (SOA=3.77 s). The onset and target force of each single hand grip was visually cued. The target force was varied such that twenty grips at each of 15%, 25%, 35%, 45% and 55% of MVC were performed in a random order. Subjects were instructed to maintain hand grip for the duration of the visual cue (1.7 s). All subjects practised the motor task in two 3-minute blocks: once outside the scanner and for a second time in the scanner before scanning started. To look for bilateral movements during scanning subjects held identical hand grip manipulanda in both hands whilst carrying out the task unimanually.
Data acquisition
A 3T Siemens ALLEGRA system (Siemens, Erlangen, Germany) was used to acquire both T1-weighted anatomical images and T2*-weighted MRI transverse echo-planar images (EPI) (64×64 3×3 mm pixels, TE=30 ms) with BOLD contrast. Each echoplanar image comprised forty eight 2 mm thick contiguous axial slices taken every 3 mm, positioned to cover the whole cerebrum, with an effective repetition time (TR) of 3.12 s per volume. In total, 202 volumes were acquired during each scanning session. The first six volumes were discarded to allow for T1 equilibration effects.
Data preprocessing
Imaging data were analysed using Statistical Parametric Mapping (SPM5, Wellcome Department of Imaging Neuroscience, http://www.fil.ion.ucl.ac.uk/spm/) implemented in Matlab 6 (The Mathworks Inc., USA). All volumes were realigned, unwarped to account for task-related head motion (Andersson et al., 2001), and slice-time corrected.
The resulting volumes were then normalised to a standard EPI template based on the Montreal Neurological Institute (MNI) reference brain in Talairach space (Talairach and Tournaux, 1998). All normalised images were then smoothed with an isotropic 8 mm full-width half-maximum Gaussian kernel to account for inter-subject anatomical differences and allow valid statistical inference according to Gaussian random field theory (Friston et al., 1995a). The time series in each voxel were high pass filtered at 1/128 Hz to remove low-frequency confounds and scaled to a grand mean of 100 over voxels and scans within each session.
Statistical analysis
Statistical analysis was performed in three stages. In the first stage, data from each subject were analysed separately using a single subject fixed effects model. All hand grips were defined as a single event type and modelled as delta functions (grip covariate). A second covariate (force covariate) comprised a delta function scaled by the measured peak force exerted for each hand grip. The force covariate was mean corrected and orthogonalised with respect to the grip covariate. Both covariates were convolved with a canonical synthetic haemodynamic response function and used in a general linear model (Friston et al., 1998) together with a single covariate representing the mean (constant) term over scans. Thus for each subject, voxel-wise parameter estimates for each covariate resulting from the least mean squares fit of the model to the data were calculated. The parameter estimates (or betas) for the grip covariate reflect the magnitude of increase in the BOLD signal during all hand grips compared to rest (BG). Parameter estimates for the force covariate (BF) are equivalent to the partial correlation coefficient of BOLD signal plotted against hand grip force (Buchel et al., 1998). The statistical parametric maps of the t statistic (SPM{t}) resulting from linear contrasts of each covariate (Friston et al., 1995b) were generated and stored as separate images for each subject.
The data for the second stage of analysis comprised the pooled parameter estimates for each covariate across all subjects. Contrast images containing these data for each subject were entered into a one sample t-test for each covariate of interest. After characterizing the average group effects, we examined for the influence of neurophysiological parameters on the parameter estimates BG and BF. Thus, we performed simple linear regression analyses within SPM5, in which the two orthogonal covariates were: (i) contrast images for each subject for the effect of interest (BG or BF) and (ii) a single value representing the neurophysiological parameter of interest for each subject (mean corrected and normalised across the group). SPM{t}s representing brain regions in which there is a linear relationship between the relevant parameter estimates and neurophysiological parameter were generated. For each significant voxel the correlation coefficient for the plot of parameter estimateagainst the neurophysiological parameter was also calculated to illustrate the relationship.
A third stage of analysis was performed in order to determine whether the neurophysiological parameter of interest could explain variability in BG or BF over and above that explained by age2. Age2 rather than age was used to take account of the nonlinear nature of age-related changes, as used in our previous studies (Ward and Frackowiak, 2003;Ward et al., in press). The three serial covariates in each model comprised (i) contrast images for each subject for the effect of interest (BG or BF), (ii) age2 and (iii) the neurophysiological parameter of interest. The second and third covariates were serially orthogonalised with respect to the previous covariates. SPM{t}s for the orthogonalised neurophysiological parameter representing brain regions in which BG (or BF) can be explained by the orthogonalised neurophysiological parameter over and above any relationship with age2 were generated. SPM{F}s representing brain regions in which changes in BG (or BF) could be explained by changes in either age2 and/or the orthogonalised neurophysiological parameter were generated. The partial correlation coefficient between BG (or BF) and (i) age2 and (ii) the orthogonalised neurophysiological parameter were also calculated.
Our a priori regions of interest (ROI) were sensorimotor cortices (SMC) and PMv in both hemispheres. The ROIs consisted of 10 mm diameter spheres centred on the following coordinates derived from our previous work (Ward and Frackowiak, 2003;Ward et al., in press) contralateral SMC (x=−42, y=−18, z=58), ipsilateral SMC (x=44, y=−20, z=56), contralateral PMv (x=−46, y=20, z=22) and ipsilateral PMv (x=48, y=14, z=18).
All SPM{t}s were transformed to the unit normal Z-distribution to create a statistical parametric map (SPM{Z}). All t-tests carried out within SPM were one tailed.
Anatomical identification was carefully performed by super-imposing the maxima of activation foci both on the MNI brain and on the normalised structural images of each subject and labelling with the aid of the atlas of Duvernoy (1991).
Results
Behavioural results
The mean MVC for right hand grip was 39.1 kg, SD=9.1 kg). There was no significant correlation between age (r2=0.03, P=ns) or age2 (r2 =0.04, P=ns) and MVC.
Imaging results: Group
The average force and duration of hand grips for all trials and for each target force are given in Table 1. The average error in achieving the target force is also given for all trials and for each target force, in units of %MVC. None of these parameters correlated with age or age2.
Table 1.
In-scanner performance
| Target force |
||||||
|---|---|---|---|---|---|---|
| All trials | 15% MVC | 25% MVC | 35% MVC | 45% MVC | 55% MVC | |
| Force (%MVC) | 34.6 (1.5) | 15.8 (1.7) | 24.8 (1.3) | 34.1 (1.3) | 43.5 (1.7) | 54.9 (2.7) |
| Duration (ms) | 1918 (123) | 1811 (121) | 1865 (122) | 1929 (140) | 1973 (141) | 1995 (131) |
| Error (%MVC) | ±2.5 (0.6) | ±2.1 (1.0) | ±1.9 (0.5) | ±2.2 (0.6) | ±2.8 (0.9) | ±3.4 (1.3) |
Average values for in-scanner task performance on all trials and for each target force are given together with SEM in brackets.
Main effects of handgrip
The main effects of hand grip were consistent with previous reports using this paradigm (Ward and Frackowiak, 2003; Ward et al., in press) and so are not reported in detail here. Activations were seen in a network of regions, the most lateralised of which were in contralateral sensorimotor cortex and ipsilateral superior cerebellum. Other activations were bilaterally distributed, including dorsolateral premotor cortex (PMd) and ventrolateral premotor cortex (PMv), supplementary motor area (SMA), cingulate motor areas, inferior parietal cortex and intraparietal sulcus, insula cortex, visual cortices, cerebellar vermis, and both inferior and superior cerebellar hemispheres.
Force-related changes
Regions in which the magnitude of task-related signal increased linearly with increasing hand grip force were seen in contralateral sensorimotor cortex and superior cingulate sulcus, ipsilateral cerebellum (lobule VI), and primary visual cortex (Table 2). At a lower threshold (P<0.0001, uncorrected) the cluster centred on contralateral M1 was found to extend in the rostral–caudal direction from y=−44 to −7, and in the ventral–dorsal direction from z=48 to 78, thus additionally encompassing contralateral primary sensory cortex (S1), posterior M1 (BA 4p) and caudal PMd.
Table 2.
Force-related changes in brain activation
| Talairach coordinates in MNI space |
|||||
|---|---|---|---|---|---|
| Region | Side | x | y | z | Z-value |
| (i) Linearly increasing BOLD signal with progressively increasing force | |||||
| Primary motor cortex | C | −36 | −18 | 66 | 5.41 |
| Postcentral gyrus | C | −38 | −34 | 64 | 5.37 |
| Superior cingulate sulcus | C | −6 | −22 | 46 | 3.23* |
| Cerebellum (lobule VI) | I | 16 | −52 | −26 | 4.60 |
| Primary visual cortex | C | −2 | −94 | 16 | 6.71 |
| (ii) Linearly decreasing BOLD signal with progressively increasing force | |||||
| Ventrolateral premotor cortex/BA44 | C | −50 | 22 | 16 | 3.48‡ |
Voxels in which hand grip related signal increases linearly with increasing hand grip force. All voxels are significant at P< 0.05, corrected for multiple comparisons across whole brain.
Significant at P<0.05, corrected for multiple comparisons within 10 mm radius sphere centred on x=−4,y=−16, z=50 (from Ward et al., in press).
Significant at P< 0.05, corrected for multiple comparisons within 10 mm radius sphere centred on (x=−46, y=20, z=22) (from Ward and Frackowiak, 2003, and Ward et al., in press). Cerebellar localisation performed from Schmahmann et al. (1999). C=contralateral, I=ipsilateral to hand movement, BOLD=blood oxygen level dependent, BA=Brodmann area.
We previously reported a trend for decreasing magnitude of signal change with increasing grip force in left (contralateral) PMv (Ward et al., in press). In this study, using the contralateral PMv ROI we found a similar result in left posterior inferior frontal gyrus close to BA44 (Tomaiuolo et al., 1999) (Table 2).
Imaging results: Age-related changes
We were able to replicate our previous age-related findings (Ward et al., in press) and demonstrate (i) that BG is greater with and increasing age2 in ipsilateral M1, (ii) that BF decreases with increasing age2 in contralateral sensorimotor cortex, in a cluster encompassing both primary sensory and motor cortices, and (iii) that BF increases with increasing age2 in left (contralateral) but not right PMv/BA44 (Table 3).
Table 3.
Age-related changes
| Talairach coordinates in MNI space |
Correlation analysis |
|||||
|---|---|---|---|---|---|---|
| Region | Side | x | y | z | Z-value | (r2) |
| (i) BG and age2 | ||||||
| Primary motor cortex | I | 46 | −20 | 52 | 3.18 | (+) 0.29 |
| Primary sensory cortex | I | 38 | −24 | 62 | 3.04 | (+) 0.25 |
| (ii) BF and age2 | ||||||
| Primary motor cortex | C | −38 | −22 | 60 | 3.09 | (−) 0.25 |
| Ventrolateral premotor cortex/BA44 |
C | −52 | 20 | 18 | 2.94 | (+) 0.21 |
All regions/clusters are significant at P<0.05, corrected for multiple comparisons within a priori sensorimotor and ventrolateral premotor cortex ROIs (see Methods section). All cluster sizes <50 voxels. The direction of the correlation (+ or −) is given next to the value for the coefficient of determination, r2.
C = contralateral, I = ipsilateral to hand movement, BA=Brodmann area, BG=parameter estimate for the main effect of hand grip, BF=parameter estimate for the force covariate (see Methods section).
Imaging results: Neurophysiological-related changes
The TMS results from a slightly larger group of subjects have been reported and discussed in detail elsewhere (Talelli et al., 2008). Tables 4 and 5 summarise the mean and range of values for the measures relevant in this study as well as the correlations with age, where appropriate.
Table 4.
TMS measures of corticospinal excitability
| Mean (range) | Correlation with age2 (r2) |
|
|---|---|---|
| 1. Motor thresholds (% stimulator’s intensity) | ||
| Left M1 | ||
| Resting (RMT) | 37.7 (28–65) | ns |
| Active (AMT) | 28.4 (19–45) | ns |
| Right M1 | ||
| Resting (RMT) | 36.5 (29–50) | ns |
| Active (AMT) | 28 (22–39) | ns |
| 2. RC of active MEP amplitude‡ (CMAP corrected) | ||
| AUC | 12.1 (1.8–23) | (−) 0.42* |
| Parameter estimates (Boltzmann model) | ||
| MEPmax/CMAP | 0.39 (0.03–0.82) | ns |
| I50 (%AMT) | 137 (115–168) | ns |
| 1/ slope | 0.14 (0.05–0.37) | ns |
The direction of the correlation (+ or −) is given next to the value for the coefficient of determination, (r2).
refers to stimulation of left M1
P<0.05.
RMT=resting motor threshold; AMT=active motor threshold; RC recruitment curves; CMAP=compound motor action potential; AUC=area under the curve; MEPmax=maximum MEP amplitude estimated; I50 = stimulation intensity needed to get a response of 50% of the maximal MEP.
Table 5.
Interhemispheric inhibition targeting the right M1 assessed with paired-pulse TMS
| 10 ms | 40 ms | |
|---|---|---|
| restIHI | 0.61 (0.32–0.90) | 0.60 (0.28–1.11) |
| activeIHI | 0.54 (0.09–0.89) | 0.57 (0.20–1) |
| IHIc (activeIHI/restIHI) | 0.86 (0.42–1.23) | 0.95 (0.5–1.49)a |
IHI = interhemispheric inhibition; restIHI=IHI measured with both hands relaxed; activeIHI=IHI measured during a tonic contraction of the dominant hand at 15–20% MVC. IHIc values < 1 indicate stronger IHI in the active condition.
Correlates positively with age (r2 = 0.24, P=0.018) and age2 (r2 = 0.24, P=0.018).
Changes with interhemispheric inhibition
Neurophysiological data on interhemispheric inhibition (IHI10c and IHI40c) were available for only 23 of the 27 subjects. In 4 subjects (age 19, 28, 58 and 72) it was impossible to complete the measures due to very high thresholds to TMS. In these 23 subjects, IHI40c correlated positively with age (r2 =0.24, P=0.018) and age2 (r2=0.24, P=0.018). As noted in the Methods section, a higher value for IHIc indicates less interhemispheric inhibition from the left hemisphere towards the right during right hand grip. IHI10c did not correlate with age or age2. As reported previously in detail, we have tested for the potential confounding effect of several parameters including IHI measures at rest, stimulation intensities used for the conditioning and test stimuli, the change in the amplitude of the MEP evoked by the test and the conditioning stimuli in the rest and active conditions and the subjects’ gender. In all cases, the correlations between age (or age2) and IHIc were unchanged.
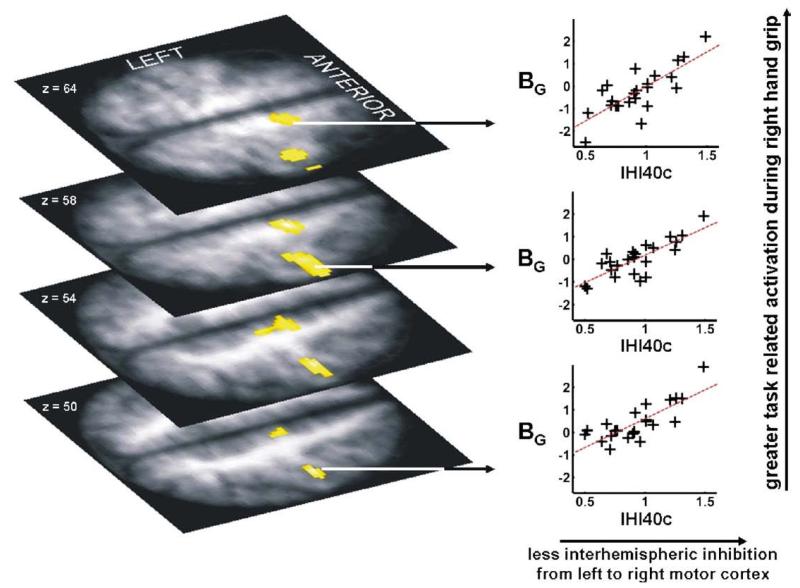
IHI40c, but not IHI10c, was found to explain regional variability in the imaging parameters BG and BF. A strong positive relationship between BG for right hand grip and IHI40c (r2 =0.63), indicating that less interhemispheric inhibition was associated with greater task-related activity, was found in ipsilateral (right) M1 and SMA (Table 6, Fig. 1). When age2 was included in the model and IHI40c was orthogonalised with respect to age2, IHI40c remained able to explain between 32 and 38% of the variability in ipsilateral M1 brain activity during hand grip, over and above that explained by age2 (which accounted for 31–32% of the variability) (Table 7). No negative correlations were found between BG and IHI40c.
Table 6.
Changes in brain activation relating to neurophysiological parameters
| Talairach coordinates in MNI space |
Correlation analysis |
|||||
|---|---|---|---|---|---|---|
| Region | Side | x | y | z | Z-value | (r2) |
| (i) BG and IHI40c | ||||||
| Primary motor cortex | I | 40 | −20 | 58 | 4.32* | (+) 0.63 |
| I | 42 | −18 | 50 | 4.22* | (+) 0.60 | |
| Supplementary motor area | I | 12 | −12 | 58 | 4.39* | (+) 0.62 |
| (ii) BF and IHI40c | ||||||
| Primary motor cortex | I | 44 | −20 | 64 | 4.03* | (+) 0.49 |
| (iii) BG and I50‡ | ||||||
| Dorsolateral | C | 42 | 8 | 52 | 4.42 | (+) 0.56 |
| premotor cortex | ||||||
| Ventrolateral premotor | C | −44 | 12 | 20 | 4.19 | (+) 0.53 |
| cortex/BA44 | I | 46 | 14 | 24 | 4.65 | (+) 0.60 |
| Pre-supplementary motor area |
I | 2 | 20 | 50 | 4.87 | (+) 0.63 |
| Frontal operculum | I | −34 | 20 | 0 | 4.34 | (+) 0.55 |
| Cerebellum | C | −38 | −62 | −50 | 4.81 | (+) 0.63 |
| (iv) BF and I50 * | ||||||
| Ventrolateral premotor | C | −46 | 22 | 20 | 3.55 | (+) 0.41 |
| cortex/BA44 | C | −52 | 20 | 26 | 3.38 | (+) 0.39 |
| I | 48 | 20 | 26 | 3.2 | (+) 0.35 | |
IHI40c reflects the change seen in the interhemispheric inhibition targeting the non-dominant motor cortex when the dominant hand is active, at an ISI of 40 ms. A higher value for IHI40c represents less transcallosal inhibition. I50 is the stimulation intensity required to get a response of 50% of the maximum MEP. A higher value for I50 indicates lower motor cortex excitability.
Voxels are significant at P<0.05, corrected for multiple comparisons within a priori ROIs (see Methods section).
Clusters are significant at P<0.05, corrected for multiple comparisons across whole brain. The direction of the correlation (+ or −) is given next to the value for the coefficient of determination, r2.
C = contralateral, I = ipsilateral to hand movement, BA=Brodmann area, BG=parameter estimate for the main effect of hand grip, BF=parameter estimate for the force covariate (see Methods section).
Fig. 1.
Brain activation during right hand grip (BG) is greater in left (ipsilateral) sensorimotor cortex and supplementary motor area in subjects with less interhemispheric inhibition (where a greater value for IHI40c represents less interhemispheric inhibition) from left to right motor cortices. The significant voxels (P<0.05, corrected) are overlaid onto the mean normalised T1-weighted structural image obtained from all subjects. Peak coordinates, Z-scores and correlation coefficients are given in Table 6.
Table 7.
Changes in brain activation relating to age and/or neurophysiological parameters
| Talairach coordinates in MNI space |
|||||||
|---|---|---|---|---|---|---|---|
| Region | Side | x | y | z | Z-value | Partial correlation with age2 (r2) |
Partial correlation with neurophysiologya (r2) |
| (i) BG and age2/IHI40c | |||||||
| Primary motor cortex | I | 40 | −20 | 56 | 3.95 | (+) 0.33 | (+) 0.38 |
| I | 42 | −18 | 50 | 3.79 | (+) 0.31 | (+) 0.32 | |
| (ii) BF and age2/IHI40c | |||||||
| Primary motor cortex | I | 42 | −20 | 64 | 3.91 | (+) 0.02 | (+) 0.46 |
| (iii) BG and age2/I50 | |||||||
| Ventrolateral premotor cortex/BA44 | C | −42 | 12 | 20 | 4.54 | (+) 0.02 | (+) 0.52 |
| I | 46 | 14 | 24 | 4.95 | (+) 0.03 | (+) 0.57 | |
| I | 50 | 16 | 16 | 4.65 | (+) 0.03 | (+) 0.45 | |
| (iv) BF and age2/I50 | |||||||
| Ventrolateral premotor cortex/BA44 | C | −46 | 22 | 20 | 3.12 | (+)0.10 | (+) 0.35 |
| C | − 54 | 22 | 22 | 3.13 | (+) 0.17 | (+) 0.27 | |
| I | 46 | 22 | 26 | 3.09 | (+) 0.05 | (+) 0.31 | |
Voxels in which the variability in parameter estimates BG or BF can be explained by age2 and/or a neurophysiological parameter (after orthogonalisation with respect to age2). The relative contribution of each explanatory variable is given as the r2. Voxels are significant at P<0.05, corrected for multiple comparisons within a priori ROIs (see Methods section). The direction of the correlation (+ or −) is given next to the value for the coefficient of determination, r2.
C = contralateral, I=ipsilateral to hand movement, BA=Brodmann area, BG=parameter estimate for the main effect of hand grip, BF=parameter estimate for the force covariate (see Methods section).
After correction for age2.
A positive relationship between BF for right hand grip and IHI40c was found in the ipsilateral (right) SMC ROI (Table 6, Fig. 2). This result demonstrates that a negative correlation between BOLD signal and peak grip force (opposite to the positive correlation seen in contralateral M1) is seen in subjects with higher interhemispheric inhibition, but that as interhemispheric inhibition diminishes, the correlation between BOLD and peak grip force tends to become positive (as seen in contralateral M1). When age2 was included in the model and IHI40c was orthogonalised with respect to age2, variability in BF in ipsilateral sensorimotor cortex was almost exclusively explained by IHI40c (46%), once again demonstrating that IHI40c was able to explain variability in BF over and above that explained by age2 (Table 7).
Fig. 2.
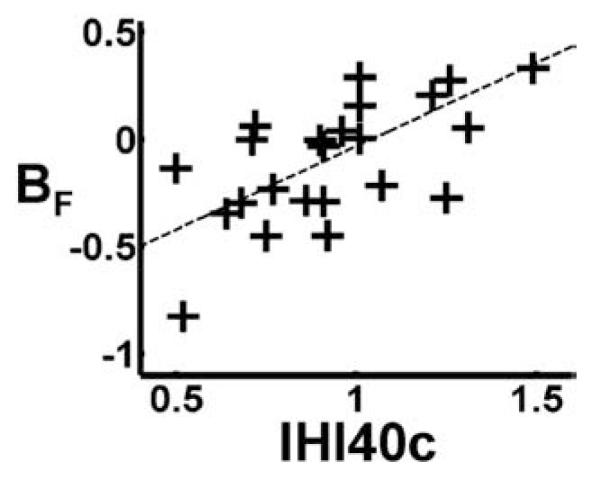
The effects of interhemispheric inhibition on force modulation properties in left (ipsilateral) sensorimotor cortex are shown. Parameter estimates for the force covariate BF (which represents the partial correlation coefficient between BOLD signal and right hand grip force) in left sensorimotor cortex are plotted against IHI40c (where a greater value for IHI40c represents less interhemispheric inhibition from left to right motor cortices).
Changes with contralateral motor cortex excitability
TMS measures were available for the 27 subjects scanned. In these, AUC but not RC-slope or I50 correlated negatively with age (r2=0.41, P<0.001) and age2 (r2=0.42, P<0.001). It is noted that the AUC and less strongly the RC-slope both correlated with MEPmax (r2=0.36, P<0.001 and r2=0.25, P<0.02) but I50 was not.
The neurophysiological parameters RC-slope or AUC were not found to explain any variability in either BG or BF. In particular, in relation to our hypothesis, neither parameter was able to account for variability in BF in contralateral sensorimotor cortex or bilateral PMv over and above age2.
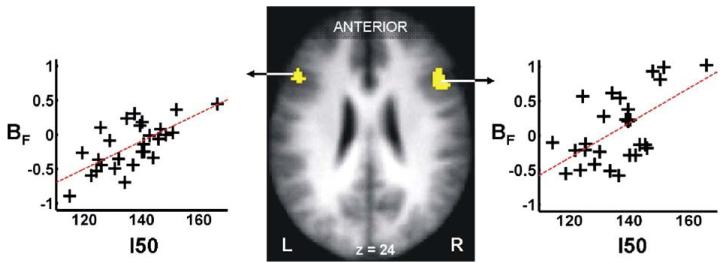
Our second hypothesis was that neurophysiological measures relating to the input–output characteristics of M1 (contralateral to moving hand) would be reflected in particular by BF in PMv bilaterally. We found a positive relationship between BF and I50 bilaterally in our a priori PMv ROIs (Table 6, Fig. 3). In other words, force modulating properties of PMv were greater in subjects requiring greater TMS intensity (in relation to the individual’s threshold) to generate 50% of the maximum MEP. When age2 was included in the model and I50 was orthogonalised with respect to age2, I50 was able to explain between 27 and 35% of the variability in BF in PMv (depending on the hemisphere) over and above that explained by age2 (1–17%) (Table 7).
Fig. 3.
The effect of stimulus–output characteristics of left sensorimotor cortex I50 (where I50 represents the TMS stimulation intensity required to get a response 50% of the maximum motor evoked potential in the right first dorsal intersosseus muscle), on force modulation properties BF (where BF represents the partial correlation coefficient between BOLD signal and right hand grip force) in the ventral premotor cortices bilaterally. The significant voxels (P<0.05, corrected) are overlaid onto the mean normalised T1-weighted structural image obtained from all subjects. Peak coordinates, Z-scores and correlation coefficients are given in Table 6.
We also hypothesised that BF would be greater in contralateral sensorimotor cortices in those with greater motor cortex excitability (lower I50 values) but were unable to demonstrate such a finding at our chosen threshold. Within the contralateral (left) SMC ROI (x=−40, y=−22, z=56) however, there was a subthreshold trend for greater BF with lower I50 values (r2=0.18).
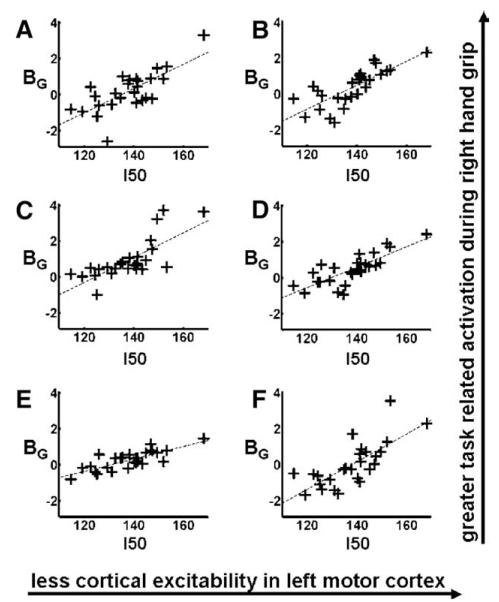
In addition, we found a relationship between I50 and BG in PMv/BA44 bilaterally, ipsilateral PMd, pre-SMA, frontal operculum and contralateral cerebellum (Table 6, Fig. 4), indicating greater average task-related activity during all hand grips in these regions in subjects with less excitable motor cortices (higher I50 values). Within our a priori PMv ROIs, I50 explained between 45 and 57% of BG variability over and above that explained by age2 (2–3%) (Table 7). There were no regions in which task-related activity decreased with lower motor cortex excitability.
Fig. 4.
The effect of stimulus–output characteristics of left sensorimotor cortex, I50 (where I50 represents the TMS stimulation intensity required to get a response of 50% of the maximum motor evoked potential in the right first dorsal intersosseus muscle), on the magnitude of brain activation during right hand grip (BG) in: (A) left ventral premotor cortex, (B) right ventral premotor cortex, (C) right dorsal premotor cortex, (D) right presupplementary motor area, (E) left cerebellar hemisphere, (F) left frontal operculum. Peak coordinates, Z-scores and correlation coefficients are given in Table 6.
Discussion
We have used fMRI to study brain activation during a simple visuomotor task in which the force levels are parametrically varied in an event-related design. We have confirmed (in a new cohort of subjects) that advancing age is associated with (i) increased task-related activity in ipsilateral M1 and (ii) a shift in the linear relationship between BOLD signal and peak grip force away from contralateral sensorimotor cortex towards rostral PMv/BA44 in keeping with previous results (Ward et al., in press). These age effects are not due to differences in performance in the task and are unlikely to be due to differences in haemodynamic response. We have previously shown that the shape of the haemodynamic response during this visuomotor task in motor and premotor areas is not affected by age (Ward et al., in press).
In addition we used TMS in the same subjects to determine a number of neurophysiological parameters relating to left motor cortex excitability and to assess interhemispheric inhibition from left to right motor cortices. We have previously demonstrated that a number of these measures are correlated with age (Talelli et al., 2008). By looking for relationships between task-related brain activity and neurophysiological parameters, we have now demonstrated that the changes in neurophysiological characteristics of the motor cortex that appear to occur with advancing age are associated with alterations in task-related brain activity in distributed and interconnected brain regions.
Firstly, we found that activity in ipsilateral M1during an active motor task is greater when the interhemispheric inhibition from the contralateral hemisphere does not increase in relation to the task. We also found that task-related activity in ipsilateral M1 became increasingly modulated by peak grip force in subjects with less task-related change in interhemispheric inhibition (IHIc). It has previously been demonstrated in this study and several others results (Dettmers et al., 1995; Thickbroom et al., 1999; Ward and Frackowiak, 2003) that by using motor paradigms in which force is parametrically varied, the BOLD signal increases linearly with peak force in some brain regions. This is typically seen in M1 contralateral to the moving hand and has been used to infer involvement in the execution of the motor task and termed executive motor behaviour (Dettmers et al., 1995). In this study we found that lower task-related change in interhemispheric inhibition was associated not only with an average increase in task-related activity (as characterised by BG) but also an increase in executive motor properties (as characterised by BF) in ipsilateral M1. Furthermore, we have demonstrated that interhemispheric inhibition is able to account for variability in ipsilateral M1 activity (both BF and BG) over and above that explained by chronological ageing. We have thus provided some evidence that modulation in the activity of the pathway mediating interhemispheric inhibition (IHI40) is at least one of the mechanisms regulating the amount and potentially the functional role of ipsilateral M1 activation during the execution of a simple grip task in the human brain; the same mechanism could be thus responsible for the changes seen in the system with advancing age.
We have previously reported that there is no correlation between age and task-related changes in IHI10 (IHI10c) (Talelli et al., 2008); we have postulated that this new finding adds to existing evidence that although IHI10 and IHI40 are both used to quantify inhibitory interactions between the two M1s, each may involve different connections (Chen et al., 2003; Irlbacher et al., 2007) and thus respond differently to dynamic changes. In line with this, there was no relationship between IHI10c and task-related changes in BOLD signal in any of the areas of interest in this study. This suggests that the pathway probing IHI10 may not be involved in task-related regulation of the inhibition targeting the motor areas ipsilateral to the moving hand. Another interesting finding was the relationship between reduced task-related change in IHI and task-related activity in the ipsilateral SMA, which was again specific for the longer latency IHI40 across the whole age range. This finding provides some support to our inference that long-latency IHI may involve circuits that expand beyond the primary motor areas.
Our second main result demonstrates that a higher I50 (indicating that greater intensity TMS pulses are required to achieve 50% of the maximal MEP) was associated with an increase in average task-related activity (BG) in a number of brain regions including bilateral PMv, contralateral PMd and cerebellum, and ipsilateral SMA and frontal operculum. That this increase in activity with increasing I50 is functionally relevant is supported by the finding that the executive motor properties (as characterised by BF) of PMv also increase with higher I50. In other words, reduced stimulus–output characteristics of motor cortex lead not only to an increase of task-related activity in secondary motor areas which have direct connections with M1 (Chen et al., 2003;Dancause et al., 2006;Dancause et al., 2007;Dum and Strick, 2005), but also to an increase in M1-like behaviour in PMv bilaterally. When higher grip forces are required, neurodegenerative changes of ageing may render existing inputs to M1 less effective at increasing output to spinal cord motor neurons. In this situation, additional premotor cortex input to M1 might be required to attempt an increase in residual M1 output. In normal primates, rostral PMv (area F5) projections to M1 can modulate output to upper limb motor neurons (Cerri et al., 2004). The human homologue of primate area F5 remains controversial but is likely to be close to BA44 (Rizzolatti et al., 2002). The results we have reported in rostral PMv/BA44 are situated very close to this region in the normal human brain(Tomaiuolo et al., 1999), although the border between BA44 and ventral BA6 remains uncertain (Amunts et al., 1999).
It is interesting that only I50, and not RC-slope or AUC, could explain the variation of BF (and BG) in PMv. The reason for that must lie in the particular information each of these provide. The AUC is derived from a gradual summation of the additional output achieved as the input to the system increases; its value depends greatly on the maximum output (at the plateau level) achieved. The RC-slope, which is measured in the steepest part of the curve, reflects the maximum gain in the input–output relation and also depends on the maximum value of input–output curve. I50 is more associated with the rate of increase in the gain at relatively low levels of input and does not depend on the maximal value. Our paradigm examined force modulation between 15 and 55% of maximal output. Thus, measures depending on the maximum output (AUC and RC-slope) will be less suited than I50 in explaining variability in force–brain activity relationships (BF) in our paradigm. On the other hand, age probably affects several different aspects of the input–output of the system, i.e. the maximum output, maximum gain and distribution of excitability, which are encapsulated in the AUC; this would explain why the AUC is a better correlate for age-related effects.
We previously described reduction in executive motor behaviour in contralateral sensorimotor cortex with advancing age (Ward et al., in press) and have replicated this finding in the current study (Table 3). We hypothesised that variability in contralateral sensorimotor cortex BF values would also be explained by differences in motor cortex excitability but were unable to demonstrate this with any of the measures relating to motor cortex excitability. This result initially appears to be in contrast with the ones of a previous study in stroke patients, where corticospinal excitability on the lesioned side (measured as the linear slope of the input–output curve) was positively correlated with BF values in the ipsilesional M1 (Ward et al., 2007). However, in stroke patients the input–output curves often do not reach a plateau and the slope of the resulting linear curve is more of a gross measure of “integrity” of the corticospinal tract rather than of the “excitability” in the system. This would explain why task-related activations were strongly correlated with TMS measures performed at rest. It appears that in normal individuals, TMS measures of corticospinal excitability do not explain the activation characteristics of the motor cortex itself, although they do explain, to some extent, the way other interconnected brain regions behave during motor execution.
Our results have some similarities to those found in subcortical stroke patients. Using a comparable hand grip paradigm, it has been demonstrated that there is a shift in the brain regions involved in modulating grip force away from contralateral M1 towards premotor regions, particularly PMv, in patients with more impairment (Ward et al., 2003) and with greater corticospinal system damage (Ward et al., 2007). Taken together, these findings suggest that premotor regions might play a role in optimising motor output not only in the face of major corticospinal disruption, but also in healthy individuals with lower motor cortex excitability. Our results do not specifically address whether these changes in cortical organisation are (partially) compensatory, although this does appear to be the case after stroke (Ward and Cohen, 2004). Thus, based on patterns of anatomical connectivity, damage or degradation to cortical motor areas and their connections appears to lead to similar patterns of reorganisation in health and disease. This result provides further specific evidence that patterns of reorganisation seen after subcortical stroke may already have been employed as part of the normal ageing process, and that subjects with ‘older’ brains may therefore have less potential for functional recovery after pathological injury.
In conclusion, this study has shown that the use of neurophysiological measures of cortical excitability and/or cortico-cortical connectivity appears to reflect the functional configuration of distributed brain networks more fully than chronological age. Examining for relationships between task-related activation and relevant neurophysiological measures will contribute to understanding the distinct pathways mediating these processes and thus help to choose the appropriate tools to investigate brain organisation in normal ageing and disease.
Acknowledgments
NW is supported by the Wellcome Trust. PTand JR are supported by the Stroke Association.
Footnotes
Disclosure statement
All authors confirm that they have no financial, actual or potential, conflicts of interest that could inappropriately influence or bias this work.
References
- Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HB, Zilles K. Broca’s region revisited: cytoarchitecture and intersubject variability. J. Comp. Neurol. 1999;412:319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Andersson JL, Hutton C, Ashburner J, Turner R, Friston K. Modeling geometric deformations in EPI time series. NeuroImage. 2001;13:903–919. doi: 10.1006/nimg.2001.0746. [DOI] [PubMed] [Google Scholar]
- Buchel C, Holmes AP, Rees G, Friston KJ. Characterizing stimulus-response functions using nonlinear regressors in parametric fMRI experiments. NeuroImage. 1998;8:140–148. doi: 10.1006/nimg.1998.0351. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Cognitive neuroscience of aging: contributions of functional neuroimaging. Scand. J. Psychol. 2001;42:277–286. doi: 10.1111/1467-9450.00237. [DOI] [PubMed] [Google Scholar]
- Calautti C, Serrati C, Baron JC. Effects of age on brain activation during auditory-cued thumb-to-index opposition: A positron emission tomography study. Stroke. 2001;32:139–146. doi: 10.1161/01.str.32.1.139. [DOI] [PubMed] [Google Scholar]
- Capaday C, Lavoie BA, Barbeau H, Schneider C, Bonnard M. Studies on the corticospinal control of human walking. I. Responses to focal transcranial magnetic stimulation of the motor cortex. J. Neurophysiol. 1999;81:129–139. doi: 10.1152/jn.1999.81.1.129. [DOI] [PubMed] [Google Scholar]
- Cerri G, Shimazu H, Maier MA, Lemon RN. Facilitation from ventral premotor cortex of primary motor cortex outputs to macaque hand muscles. J. Neurophysiol. 2003;90:832–842. doi: 10.1152/jn.01026.2002. [DOI] [PubMed] [Google Scholar]
- Chen R, Yung D, Li JY. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J. Neurophysiol. 2003;89:1256–1264. doi: 10.1152/jn.00950.2002. [DOI] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, Plautz EJ, Stowe AM, Friel KM, Nudo RJ. Ipsilateral connections of the ventral premotor cortex in a new world primate. J. Comp. Neurol. 2006;495:374–390. doi: 10.1002/cne.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, Mahnken JD, Nudo RJ. Interhemispheric connections of the ventral premotor cortex in a new world primate. J. Comp. Neurol. 2007;505(6):701–715. doi: 10.1002/cne.21531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R. The mechanisms of interhemispheric inhibition in the human motor cortex. J. Physiol. 2002;543:317–326. doi: 10.1113/jphysiol.2002.017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmers C, Fink GR, Lemon RN, Stephan KM, Passingham RE, Silbersweig D, Holmes A, Ridding MC, Brooks DJ, Frackowiak RS. Relation between cerebral activity and force in the motor areas of the human brain. J. Neurophysiol. 1995;74:802–815. doi: 10.1152/jn.1995.74.2.802. [DOI] [PubMed] [Google Scholar]
- Devanne H, Lavoie BA, Capaday C. Input–output properties and gain changes in the human corticospinal pathway. Exp. Brain Res. 1997;114:329–338. doi: 10.1007/pl00005641. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Frontal lobe inputs to the digit representations of the motor areas on the lateral surface of the hemisphere. J. Neurosci. 2005;25:1375–1386. doi: 10.1523/JNEUROSCI.3902-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM. The Human Brain: Surface, Blood Supply, and Three-Dimensional Anatomy. Springer-Verlag; New York: 1991. [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J. Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Ashburner L, Poline JB, Frith CD, Frackowiak RS. Spatial registration and normalization of images. Hum. Brain Map. 1995a:165–189. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general linear approach. Hum. Brain Map. 1995b;2:189–210. [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Hesselmann V, Zaro WO, Wedekind C, Krings T, Schulte O, Kugel H, Krug B, Klug N, Lackner KJ. Age related signal decrease in functional magnetic resonance imaging during motor stimulation in humans. Neurosci. Lett. 2001;308:141–144. doi: 10.1016/s0304-3940(01)01920-6. [DOI] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Debaere F, Peeters R, Swinnen SP. Neural basis of aging: the penetration of cognition into action control. J. Neurosci. 2005;25:6787–6796. doi: 10.1523/JNEUROSCI.1263-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson S, Kobayashi M, Horkan CM, Pascual-Leone A, Alexander MP, Schlaug G. Age-related differences in movement representation. Neuroimage. 2002;17:1720–1728. doi: 10.1006/nimg.2002.1309. [DOI] [PubMed] [Google Scholar]
- Irlbacher K, Brocke J, Mechow JV, Brandt SA. Effects of GABA(A) and GABA(B) agonists on interhemispheric inhibition in man. Clin. Neurophysiol. 2007;118:308–316. doi: 10.1016/j.clinph.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in human motor function. Neurology. 2002;58:630–635. doi: 10.1212/wnl.58.4.630. [DOI] [PubMed] [Google Scholar]
- Naccarato M, Calautti C, Jones PS, Day DJ, Carpenter TA, Baron JC. Does healthy aging affect the hemispheric activation balance during paced index-to-thumb opposition task? An fMRI study. Neuroimage. 2006;32:1250–1256. doi: 10.1016/j.neuroimage.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oliviero A, Profice P, Tonali PA, Pilato F, Saturno E, Dileone M, Ranieri F, Di Lazzaro V. Effects of aging on motor cortex excitability. Neurosci. Res. 2006;55:74–77. doi: 10.1016/j.neures.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Pitcher JB, Ogston KM, Miles TS. Age and sex differences in human motor cortex input–output characteristics. J. Physiol. 2003;546:605–613. doi: 10.1113/jphysiol.2002.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecker A, Groschel K, Ackermann H, Steinbrink C, Witte O, Kastrup A. Functional significance of age-related differences in motor activation patterns. Neuroimage. 2006;32:1345–1354. doi: 10.1016/j.neuroimage.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Motor and cognitive functions of the ventral premotor cortex. Curr. Opin. Neurobiol. 2002;12:149–154. doi: 10.1016/s0959-4388(02)00308-2. [DOI] [PubMed] [Google Scholar]
- Rothwell JC. Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J. Neurosci. Methods. 1997;74:113–122. doi: 10.1016/s0165-0270(97)02242-5. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Siebner H, Filipovic SR, Cordivari C, Gerschlager W, Rothwell J, Frackowiak R. Aging is associated with contrasting changes in local and distant cortical connectivity in the human motor system. Neuroimage. 2006;32:747–760. doi: 10.1016/j.neuroimage.2006.03.061. [DOI] [PubMed] [Google Scholar]
- Sale MV, Semmler JG. Age-related differences in corticospinal control during functional isometric contractions in left and right hands. J. Appl. Physiol. 2005;99:1483–1493. doi: 10.1152/japplphysiol.00371.2005. [DOI] [PubMed] [Google Scholar]
- Shimazu H, Maier MA, Cerri G, Kirkwood PA, Lemon RN. Macaque ventral premotor cortex exerts powerful facilitation of motor cortex outputs to upper limb motoneurons. J. Neurosci. 2004;24:1200–1211. doi: 10.1523/JNEUROSCI.4731-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournaux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme; Stuttgart: 1998. [Google Scholar]
- Talelli P, Waddingham W, Ewas A, Rothwell JC, Ward NS. The effect of age on task-related modulation of interhemispheric balance. Exp. Brain Res. 2008;186(1):59–66. doi: 10.1007/s00221-007-1205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekes A, Mohamed MA, Browner NM, Calhoun VD, Yousem DM. Effect of age on visuomotor functional MR imaging. Acad. Radiol. 2005;12:739–745. doi: 10.1016/j.acra.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW, Phillips BA, Morris I, Byrnes ML, Sacco P, Mastaglia FL. Differences in functional magnetic resonance imaging of sensorimotor cortex during static and dynamic finger flexion. Exp. Brain Res. 1999;126:431–438. doi: 10.1007/s002210050749. [DOI] [PubMed] [Google Scholar]
- Tomaiuolo F, MacDonald JD, Caramanos Z, Posner G, Chiavaras M, Evans AC, Petrides M. Morphology, morphometry and probability mapping of the pars opercularis of the inferior frontal gyrus: an in vivo MRI analysis. Eur. J. Neurosci. 1999;11:3033–3046. doi: 10.1046/j.1460-9568.1999.00718.x. [DOI] [PubMed] [Google Scholar]
- Ward NS. Compensatory mechanisms in the aging motor system. Aging Res. Rev. 2006;5:239–254. doi: 10.1016/j.arr.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain. 2003;126:1430–1448. doi: 10.1093/brain/awg145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Frackowiak RS. Age-related changes in the neural correlates of motor performance. Brain. 2003;126:873–888. doi: 10.1093/brain/awg071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Arch. Neurol. 2004;61:1844–1848. doi: 10.1001/archneur.61.12.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Swayne OB, Newton JM. Age-dependent changes in the neural correlates of force modulation: An fMRI study. Neurobiol. Aging. 2007 Jun 11; doi: 10.1016/j.neurobiolaging.2007.04.017. in press. Electronic publication ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Newton JM, Swayne OB, Lee L, Frackowiak RS, Thompson AJ, Greenwood RJ, Rothwell JC. The relationship between brain activity and peak grip force is modulated by corticospinal system integrity after subcortical stroke. Eur. J. Neurosci. 2007;25:1865–1873. doi: 10.1111/j.1460-9568.2007.05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Hallett M. The influence of normal human ageing on automatic movements. J. Physiol. 2005;562:605–615. doi: 10.1113/jphysiol.2004.076042. [DOI] [PMC free article] [PubMed] [Google Scholar]