Abstract
OBJECTIVE
To define the utility of using routine diagnostic methods to detect influenza in older, hospitalized adults.
DESIGN
Descriptive study.
SETTING
One academic hospital and 1 community hospital during the 2006–2007 and 2007–2008 influenza seasons.
PARTICIPANTS
Hospitalized adults 50 years of age or older.
METHODS
Adults who were 50 years of age or older and hospitalized with symptoms of respiratory illness were enrolled and tested for influenza by use of reverse-transcriptase polymerase chain reaction (RT-PCR). Using RT-PCR as the gold standard, we assessed the performances of rapid antigen tests and conventional influenza culture and the diagnostic use of the clinical definition of influenza-like illness.
RESULTS
Influenza was detected by use of RT-PCR in 26 (11%) of 228 patients enrolled in our study. The sensitivity of the rapid antigen test performed at bedside by research staff members was 19.2% (95% confidence interval, 8.51%–37.9%); the sensitivity of conventional influenza culture was 34.6% (95% confidence interval, 19.4%–53.8%). The ability to detect influenza with both the rapid antigen test and culture was associated with patients with a higher viral load (P=.002 and P=.001, respectively). The ability to diagnose influenza by use of the clinical definition of influenza-like illness had a higher sensitivity (80.8%) but lacked specificity (40.6%).
CONCLUSION
Because rapid antigen testing and viral culture have poor sensitivity (19.2% and 34.6%, respectively), neither testing method is sufficient to use to determine what type of isolation procedures to implement in a hospital setting.
An estimated 36,000 Americans die each year from seasonal influenza, with the majority of deaths occurring among the very old.1 In fact, estimated rates of influenza-associated hospitalization and death increase at around the age of 50 years and continue to increase with age,2 but timely diagnosis of influenza-associated illness in older adults remains elusive. Results from culture, the previously recognized gold standard for diagnosing influenza, usually take 2–5 days to obtain and have been postulated to be less sensitive in older adults.3, 4 The sensitivity of rapid antigen testing, a more convenient diagnostic method, has been shown to be lower5, 6 in older hospitalized adults than in children7 or younger adults.8
Recently, reverse-transcriptase polymerase chain reaction (RT-PCR) has been used as the gold standard for the detection of influenza in research settings9, 10 and has been introduced into some clinical laboratories. Furthermore, the use of quantitative RT-PCR provides the opportunity to measure viral load. Our study was designed to evaluate several different diagnostic methods (including 2 rapid antigen tests, conventional viral culture, and clinical characteristics) and to compare the results of these methods with the results of quantitative RT-PCR in adults 50 years of age or older who were hospitalized for symptoms of respiratory illness or nonlocalizing fever.
METHODS
Study Design
To identify influenza-associated hospitalizations, we prospectively enrolled adults 50 years of age or older who were hospitalized with symptoms of respiratory illness or nonlocalizing fever during the 2006–2007 and 2007–2008 influenza seasons. The influenza season was defined as the weeks encompassing all identified influenza infections in the clinical and research laboratories at Vanderbilt University Medical Center in Nashville, Tennessee. The hospitals included in our study were an academic hospital (2006–2008) and a community hospital (2006–2007), both in Davidson County (Nashville, Tennessee). Our study was approved by the institutional review boards of both hospitals.
Study Population
All adults 50 years of age or older with symptoms of acute respiratory illness or fever (without other symptoms) admitted to a hospital with a surveillance program were eligible for enrollment in our study. Eligible adults resided in Davidson County, were admitted during a 24-hour surveillance period for each enrollment day (4 days per week), and had 1 or more of the following symptoms at hospital admission: cough, fever, nasal congestion and/or coryza, dyspnea, or wheezing. Only patients with symptoms lasting 14 days or less were included in our study.
Demographic and Clinical Information
Patient questionnaires captured demographic data and data on presenting symptoms. Results of the provider-ordered hospital microbiologic and radiographic tests and data on hospital course, disposition at discharge, and discharge diagnoses were obtained by chart review.
Laboratory Methods
Nasal and throat swab specimens were obtained from patients and placed together in Hanks transport media. Specimens were tested for influenza by use of a real-time RT-PCR assay developed and kindly provided by Steve Lindstrom of the Virus Surveillance and Diagnosis Branch in the Influenza Division at the Centers of Disease Control and Prevention in Atlanta, Georgia.11 All samples were tested for β-actin by use of a real-time RT-PCR assay (Applied Biosystems) to ensure the quality of the specimens. If β-actin was absent during 3 consecutive tests, and if the RT-PCR results were negative, then the results were categorized as indeterminate and not included in the analyses. Specimens that tested positive for influenza were retested once for confirmation and were considered positive for influenza if both results were positive regardless of the presence or absence of β-actin. Quantification was determined by use of RNA runoff transcripts of the target gene. The assay was able to quantify a viral load from 24.5 to 2.45 × 108 copies per specimen.
Conventional Culture
All specimens were inoculated into Rhesus monkey kidney cell culture tubes (Diagnostic Hybrids) and incubated at 35°C for 10 days. Hemadsorption with guinea pig red blood cells was performed at 5 and 10 days. Influenza A or B virus was confirmed by use of specific monoclonal antibodies (Viromed).11
Rapid Antigen Tests
Rapid antigen testing for influenza was performed by use of 2 methods. All enrolled patients had a rapid antigen test performed at bedside by a trained research nurse using Quick-Vue Influenza A + B test (Quidel), which we hereafter refer to as the research rapid antigen test. The results of this test were not shared with the patients or their providers. Some patients also had a rapid antigen test ordered as part of routine care directed by their healthcare provider. For this type of testing, both participating hospitals used the same rapid influenza test (BinaxNow Influenza A & B; Binax), which we hereafter refer to as the hospital rapid antigen test.
Clinical Diagnoses
Influenza-like illness was defined as fever with either cough or sore throat.12 Data on these signs and symptoms were collected during enrollment from either the patient or a caregiver. Discharge diagnoses were categorized by use of the International Classification of Diseases, Ninth Edition, codes.
Statistical Analysis
For all analyses, RT-PCR was considered the gold standard. A descriptive analysis was performed by use of the χ2 test or the Fisher exact test for categorical variables and the Wilcoxon test for the comparisons of viral load by age group or laboratory test result. Exploratory graphical analysis was conducted to evaluate the distribution of viral load by age group by use of a smoothing function. Viral loads were log-trans formed. The sensitivity, specificity, and positive and negative predictive values were calculated for each test from 2-by-2 contingency tables. The 95% confidence intervals were generated by use of the binomial distribution. If the point estimate was 100%, then only the 1-sided 95% confidence interval was calculated. The analyses were performed with R, version 2.7.2, and the contributing packages, including Hmisc and ggplot2.
RESULTS
Enrollment and Patient Characteristics
During a 2-year period, 568 hospitalized adults were eligible to participate in our study, 351 (62%) of whom were enrolled. Of these 351 enrolled patients, 261 (74%) were enrolled while influenza was circulating. Of these 261 patients, 231 (89%) had symptoms for 14 days or less. Of these 231 patients, 228 (99%) had an adequate specimen collected. Reasons for nonenrollment of 217 patients included the following: patient refused to participate (123 patients [57%]), surrogate refused (24 patients [11%]), there was no legal guardian (40 patients [18%]), patient was missed or discharged (24 patients [11%]), or there was no language interpreter available (6 patients [3%]). The demographic characteristics of the study population are shown in Table 1.
TABLE 1.
Demographic Characteristics of Older Adult Patients Hospitalized with Symptoms of Respiratory Illness or Nonlocalizing Fever during the 2006–2007 and 2007–2008 Influenza Seasons
| Characteristic | Adult patients (n=228) |
|---|---|
| Median age, years | 66 |
| Median duration of symptoms, days | 5 |
| Race | |
| White | 140 (61) |
| Black | 88 (39) |
| Age group | |
| 50–64 years | 101 (44) |
| ≥65 years | 127 (56) |
| Insurance | |
| Public | 108 (47) |
| Private | 59 (26) |
| Both public and private | 54 (24) |
| Underlying medical conditions | |
| Congestive heart failure | 121 (53) |
| Diabetes mellitus | 84 (37) |
| COPD and/or asthma | 147 (64) |
| Admission to ICU | 22 (10) |
NOTE. Data are no. (%) of patients, unless otherwise indicated. COPD, chronic obstructive pulmonary disease; ICU, intensive care unit.
RT-PCR
RT-PCR testing identified 26 (11%) of 228 patients whose specimens were positive for influenza virus (19 with influenza A and 7 with influenza B). The median duration of symptoms prior to sample collection was 4.5 days, with a median viral load of 3,563 viral copies per specimen (interquartile range, 940–26,190 viral copies per specimen). Controlling for duration of illness, we found that the viral load was higher for patients 65 years of age or older than for patients 50–64 years of age (P = .02).
Rapid Antigen Testing
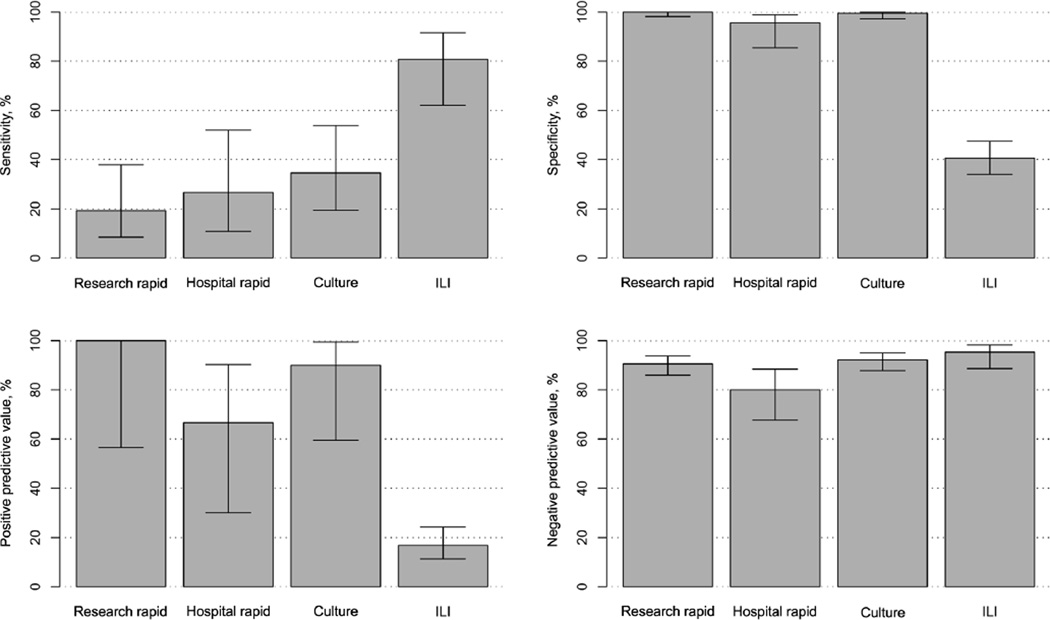
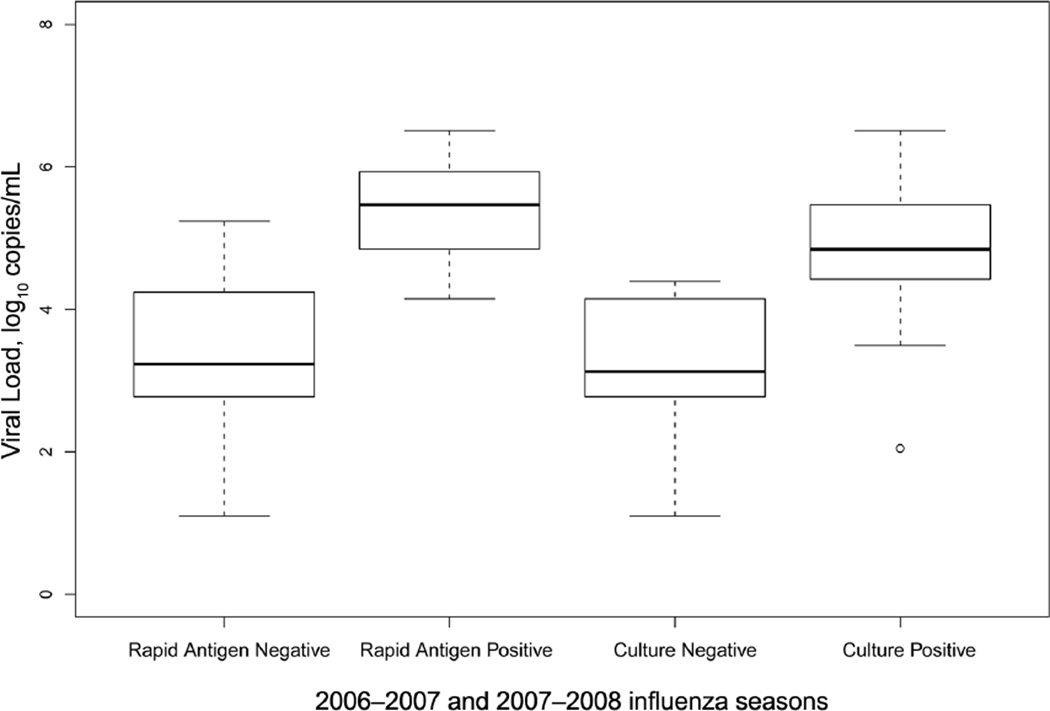
Hospital laboratory rapid antigen testing was performed for 61 (27%) of 228 patients during the 2 respective influenza seasons. Of the 26 patients with RT-PCR–confirmed influenza, 15 (58%) underwent hospital rapid antigen testing for influenza, and only 4 (27%) tested positive. Research rapid antigen testing was performed on all but 1 enrolled patient. Of the 26 patients with influenza identified by use of RT-PCR, all were tested, and only 5 (19%) tested positive. Figure 1 outlines the test characteristics (ie, sensitivity, specificity, positive predictive value, and negative predictive value) of each diagnostic method during both influenza seasons. This figure demonstrates the low sensitivity and adequate specificity of both the hospital and the research rapid antigen testing. Patients who had a positive rapid antigen test result had higher viral loads than those who had negative rapid antigen test result (P = .019) (Figure 2).
FIGURE 1.
Comparison of the test characteristics of each diagnostic method used in our study of older adult patients hospitalized with symptoms of respiratory illness or nonlocalizing fever during the 2006–2007 and 2007–2008 influenza seasons. Error bars represent confidence intervals. Hospital rapid, hospital rapid antigen test; ILI, diagnostic use of the clinical definition of influenza-like illness; Research rapid, research rapid antigen test.
FIGURE 2.
Distribution of viral load for rapid antigen and culture test results during the 2006–2007 and 2007–2008 influenza seasons. The box plots represent a graphic of the viral load for rapid antigen test and culture. The horizontal line in the middle of each box indicates the median, while the top and bottom borders of the box mark the 75th and 25th percentiles, respectively. The error bars represent upper and lower limits of the range of log10 viral loads, except for an extreme outlier, which is indicated by an open circle.
Cultures
Influenza cultures were ordered by treating physicians for only 12 (5%) of 228 patients. However, all samples were cultured in the research laboratory. The specificity, positive predictive value, and negative predictive value (99.5%, 90.0%, and 92.2% respectively) were all high for culture, but the sensitivity (34.6%) was low (Figure 1). Mean viral loads were significantly higher for culture-positive specimens than for culture-negative specimens (4.85 vs 3.13 log10 viral copies per specimen; P = .001) (Figure 2).
Clinical Diagnoses
A diagnosis of influenza-like illness, which is defined as fever and either cough or sore throat, was the most sensitive (80.8%) of all testing methods, but it lacked specificity (40.6%) and positive predictive value (16.8%) (Figure 1). Only 4 (15%) of 26 patients with RT-PCR–confirmed influenza were diagnosed with influenza at hospital discharge. All 4 of these patients had symptoms that met the definition of influenza-like illness; they also had a rapid influenza test ordered by the treating physician that was positive for influenza.
DISCUSSION
Our study highlights the difficulty of diagnosing influenza in hospitalized adults 50 years of age or older by using routine hospital laboratory studies (rapid antigen testing and viral culture) and clinical syndrome–based definitions. In older hospitalized adults, the sensitivity of the research rapid antigen test was 19.2%, and the sensitivity of viral culture was 34.6%. Thus, most adults with influenza were not identified by use of these methods. The specificity of the clinical presentation of influenza-like illness was 40.6%, yielding positive predictive values that were too low for optimal clinical decision making.
It has been reported that older adults infected with influenza are less likely to have the virus recovered from culture, even in the setting of an epidemic, possibly because of decreased shedding or delay in presentation.3, 4 Because there is an interval of 2–5 days before a diagnosis of influenza by culture can be made, this method is not very useful for determining initial treatment or for making decisions about isolating hospitalized adults. New culture techniques, such as the shell-vial technique, decrease the time to diagnosis but have also shown a sensitivity similar to that of conventional techniques.13
Although direct fluorescent antibody assays are used in some hospitals, they are not used routinely in our clinical laboratory, and thus we did not evaluate their performance. Other studies have demonstrated direct fluorescent antibody testing to be more sensitive than rapid antigen testing but less sensitive than viral culture or RT-PCR.14 Because of the results of earlier studies, it is likely that the sensitivity of direct fluorescent antibody testing would be suboptimal in older adults.
Currently, many clinicians rely on rapid antigen testing to diagnose influenza infection and to make decisions about isolating patients. However, we have demonstrated the poor sensitivity (19.2%) of this method in hospitalized older adults. Although our reported sensitivity for rapid antigen testing is somewhat lower than that reported by Walsh et al15 (43%), it is very similar to the sensitivities reported by Steininger et al6 (8%–22% for adults 80 years of age or older).
Physician diagnosis of influenza is another method used to identify influenza. Diagnosis of influenza-like illness has better sensitivity for identifying influenza than does rapid antigen testing or culture, but it lacks specificity because the symptoms of influenza are similar to those of other viral respiratory illnesses and because of the fact that fever may not be a reliable symptom in older adults. In a prospective study by Neuzil et al,16 fever had a sensitivity of only 26% in older adults. Similarly, a study in the Netherlands found fever in only 34% of adults 60 years of age or older.17 In addition, Babcock et al18 reported a poor sensitivity (43%) of influenza-like illness symptoms in adults (approximately half of whom were 65 years of age or older) when obtained by chart review rather than when reported by patient.
None of the diagnostic tests discussed here (except RT-PCR) can be used to quickly determine the exact influenza A subtype. This determination has become increasingly important, because the Centers for Disease Control and Prevention has issued warnings about antiviral resistance based on influenza A subtype. In the fall of 2008, there were 24 of 25 H1N1 isolates that were found to be resistant to oseltamivir, whereas all 5 H3N2 isolates were resistant to adamantanes.19 This determination is even more important in the context of the novel H1N1 2009 influenza virus. To date, the novel H1N1 strain is generally susceptible to oseltamivir.
In our study, quantification of the viral load introduces a novel aspect to earlier studies of influenza diagnosis in older adults and allowed us to explore differences between diagnostic tests. Recently, Lee et al20 showed that higher viral loads were associated with more severe disease. Our study was not powered to evaluate severity based on viral load but was able to show that lower viral loads were associated with negative rapid antigen test results and negative culture results. Although it is unknown whether influenza virus detected by use of RT-PCR remains viable and transmissible or whether the viral load influences transmission, our studies do have important infection control ramifications. It is recommended practice to place patients with suspected or known influenza under droplet precautions to prevent healthcare-associated transmission of influenza virus; however, a negative rapid antigen test result is often used as an indicator that the patient can be removed from isolation. The data presented in the present study suggest that, in older adults, a negative antigen test result does not provide assurance that a patient is uninfected with influenza.
Our study shows the limitations of currently used tools for diagnosing influenza in hospitalized older adults. However, with the use of RT-PCR, influenza and its complications can be more easily identified. The introduction of RT-PCR into hospital clinical laboratories would increase the recognition of disease due to influenza in older adults, aid in appropriate isolation methods, and assure the appropriate use of antiviral therapy.
ACKNOWLEDGMENTS
We thank Paul Harris, PhD, and Carlos Orozco, who designed and implemented the data-entry and management system; Ann Clay, RN, and Dayna Wyatt, RN, who were the nurses and coordinators in our study; Paul McNabb, MD, who was the Davidson County coinvestigator; Amy Podsiad, who is a laboratory staff member at Vanderbilt; and Jody Peters, MS, who performed the RT-PCR and cultures. We also thank all the adults who generously participated in our study.
Financial support. H.K.T. reports that she has received salary support and career development from the National Institutes of Health National Center for Research Resources (grant 5 K12 RR017697-05 to Dr Nancy Brown, principal investigator of the Vanderbilt Mentored Clinical Research Scholar Program) and from the National Institute of Allergy and Infectious Diseases (grant 1K23AI074863-01). K.A.P. reports that she has received salary support and career development from the National Institute of Allergy and Infectious Diseases (grant K23AI65805). K.M.E. reports that she has received funding from the National Institutes of Health Vaccine and Treatment Evaluation Unit (grant N01 AI25462) and from the Centers for Disease Control and Prevention to evaluate the impact of influenza vaccines and to study new influenza vaccines; she also reports that she has received funding from PATH (through the Bill and Melinda Gates Foundation) to evaluate potential new influenza vaccines. The General Clinical Research Center also provided support (grant M01 RR-00095).
H.K.T. reports that she has received research funds from Protein Sciences, VaxInnate, and Wyeth. J.V.W. reports that he has served as a consultant for MedImmune and Alnylam. M.R.G. reports that she has received research funds from MedImmune.
Footnotes
Potential conflicts of interest. All other authors report no conflicts of interest relevant to this article.
Presented in part: 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Annual Meeting of the Infectious Diseases Society of American; Washington, DC; October 25–28, 2008 (Abstract V-922).
References
- 1.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 2.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292(11):1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 3.Barker WH, Menegus MA, Hall CB, et al. Communitywide laboratory-based influenza surveillance focused on older persons, 1989–1992. Am J Prev Med. 1995;11(3):149–155. [PubMed] [Google Scholar]
- 4.Flamaing J, Engelmann I, Joosten E, Van Ranst M, Verhaegen J, Peetermans WE. Viral lower respiratory tract infection in the elderly: a prospective in-hospital study. Eur J Clin Microbiol Infect Dis. 2003;22(12):720–725. doi: 10.1007/s10096-003-1042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falsey AR, Murata Y, Walsh EE. Impact of rapid diagnosis on management of adults hospitalized with influenza. Arch Intern Med. 2007;167(4):354–360. doi: 10.1001/archinte.167.4.ioi60207. [DOI] [PubMed] [Google Scholar]
- 6.Steininger C, Redlberger M, Graninger W, Kundi M, Popow-Kraupp T. Near-patient assays for diagnosis of influenza virus infection in adult patients. Clin Microbiol Infect. 2009;15(3):267–273. doi: 10.1111/j.1469-0691.2008.02674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poehling KA, Griffin MR, Dittus RS, et al. Bedside diagnosis of influenzavirus infections in hospitalized children. Pediatrics. 2002;110(1 Pt 1):83–88. doi: 10.1542/peds.110.1.83. [DOI] [PubMed] [Google Scholar]
- 8.Rashid H, Shafi S, Haworth E, et al. Value of rapid testing for influenza among Hajj pilgrims. Travel Med Infect Dis. 2007;5(5):310–313. doi: 10.1016/j.tmaid.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 9.van Elden LJ, Nijhuis M, Schipper P, Schuurman R, van Loon AM. Simultaneous detection of influenza viruses A and B using real-time quantitative PCR. J Clin Microbiol. 2001;39(1):196–200. doi: 10.1128/JCM.39.1.196-200.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward CL, Dempsey MH, Ring CJ, et al. Design and performance testing of quantitative real time PCR assays for influenza A and B viral load measurement. J Clin Virol. 2004;29(3):179–188. doi: 10.1016/S1386-6532(03)00122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinberg GA, Erdman DD, Edwards KM, et al. Superiority of reverse-transcription polymerase chain reaction to conventional viral culture in the diagnosis of acute respiratory tract infections in children. J Infect Dis. 2004;189(4):706–710. doi: 10.1086/381456. [DOI] [PubMed] [Google Scholar]
- 12.Brammer TL, Murray EL, Fukuda K, Hall HE, Klimov A, Cox NJ. Surveillance for influenza—United States, 1997–98, 1998–99, and 1999–00 seasons. MMWR Surveill Summ. 2002;51(7):1–10. [PubMed] [Google Scholar]
- 13.Matthey S, Nicholson D, Ruhs S, et al. Rapid detection of respiratory viruses by shell vial culture and direct staining by using pooled and individual monoclonal antibodies. J Clin Microbiol. 1992;30(3):540–544. doi: 10.1128/jcm.30.3.540-544.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahman M, Kieke BA, Vandermause MF, Mitchell PD, Greenlee RT, Belongia EA. Performance of Directigen flu A+B enzyme immunoassay and direct fluorescent assay for detection of influenza infection during the 2004–2005 season. Diagn Microbiol Infect Dis. 2007;58(4):413–418. doi: 10.1016/j.diagmicrobio.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Walsh EE, Cox C, Falsey AR. Clinical features of influenza A virus infection in older hospitalized persons. J Am Geriatr Soc. 2002;50(9):1498–1503. doi: 10.1046/j.1532-5415.2002.50404.x. [DOI] [PubMed] [Google Scholar]
- 16.Neuzil KM, O’Connor TZ, Gorse GJ, Nichol KL. Recognizing influenza in older patients with chronic obstructive pulmonary disease who have received influenza vaccine. Clin Infect Dis. 2003;36(2):169–174. doi: 10.1086/345668. [DOI] [PubMed] [Google Scholar]
- 17.Govaert TM, Dinant GJ, Aretz K, Knottnerus JA. The predictive value of influenza symptomatology in elderly people. Fam Pract. 1998;15(1):16–22. doi: 10.1093/fampra/15.1.16. [DOI] [PubMed] [Google Scholar]
- 18.Babcock HM, Merz LR, Dubberke ER, Fraser VJ. Case-control study of clinical features of influenza in hospitalized patients. Infect Control Hosp Epidemiol. 2008;29(10):921–926. doi: 10.1086/590663. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Update: influenza activity—United States, September 28–November 29, 2008. MMWR Morb Mortal Wkly Rep. 2008;57(49):1329–1332. [PubMed] [Google Scholar]
- 20.Lee N, Chan PK, Hui DS, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200(4):492–500. doi: 10.1086/600383. [DOI] [PMC free article] [PubMed] [Google Scholar]