Abstract
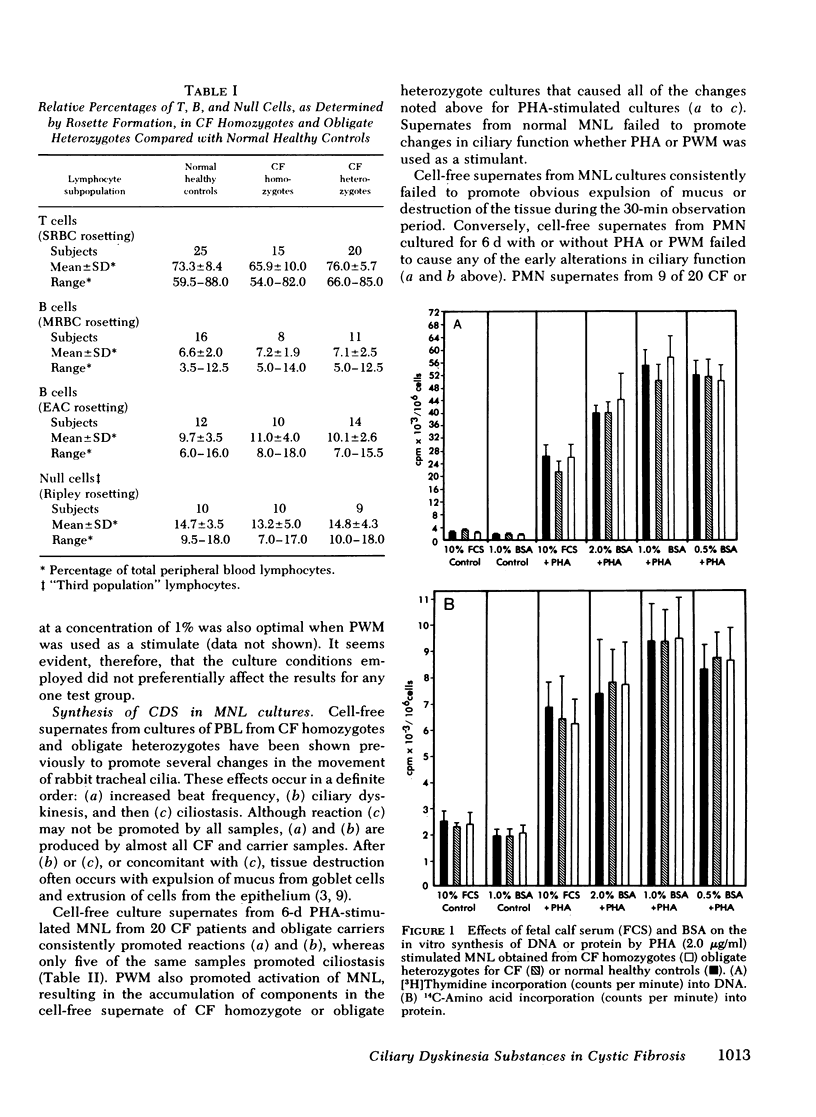
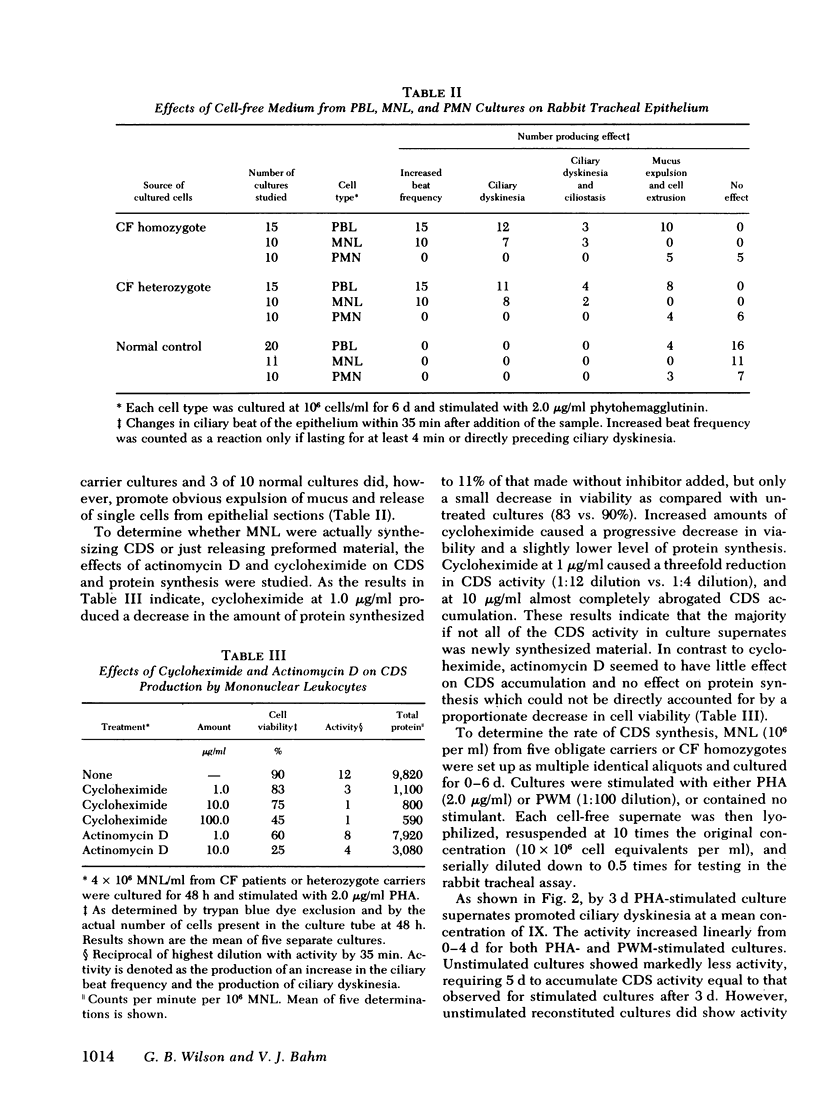
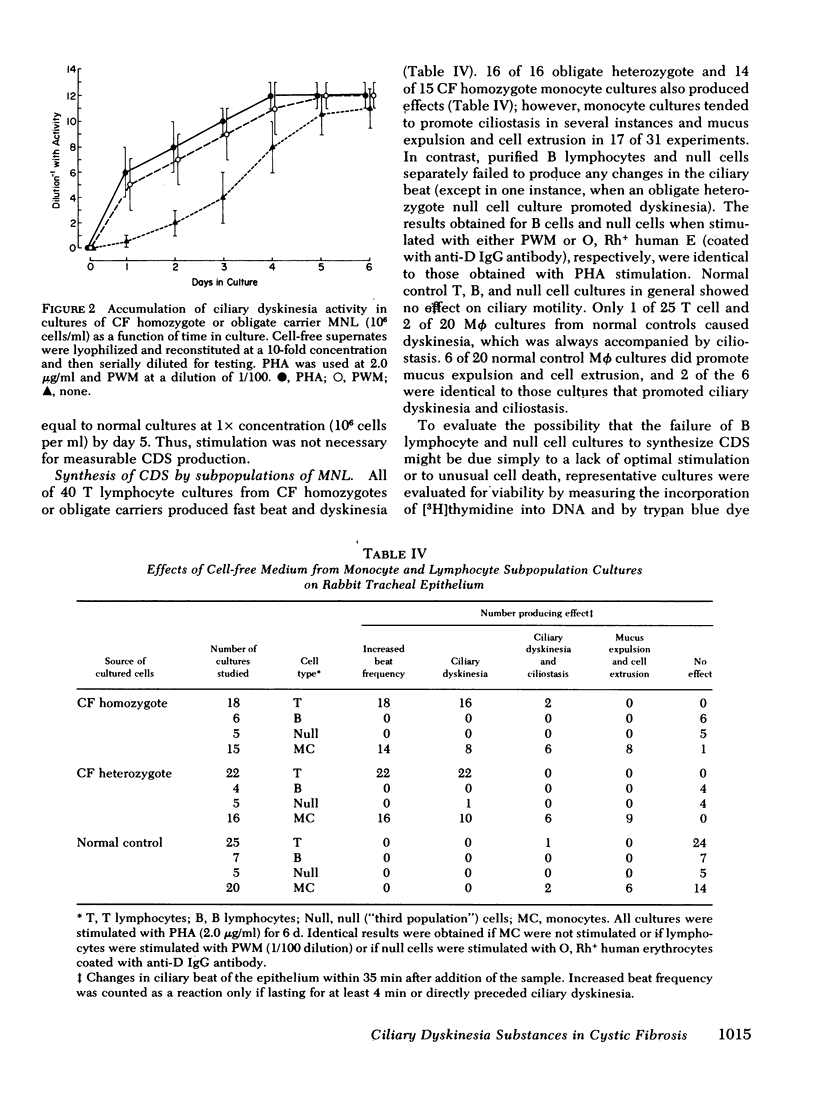
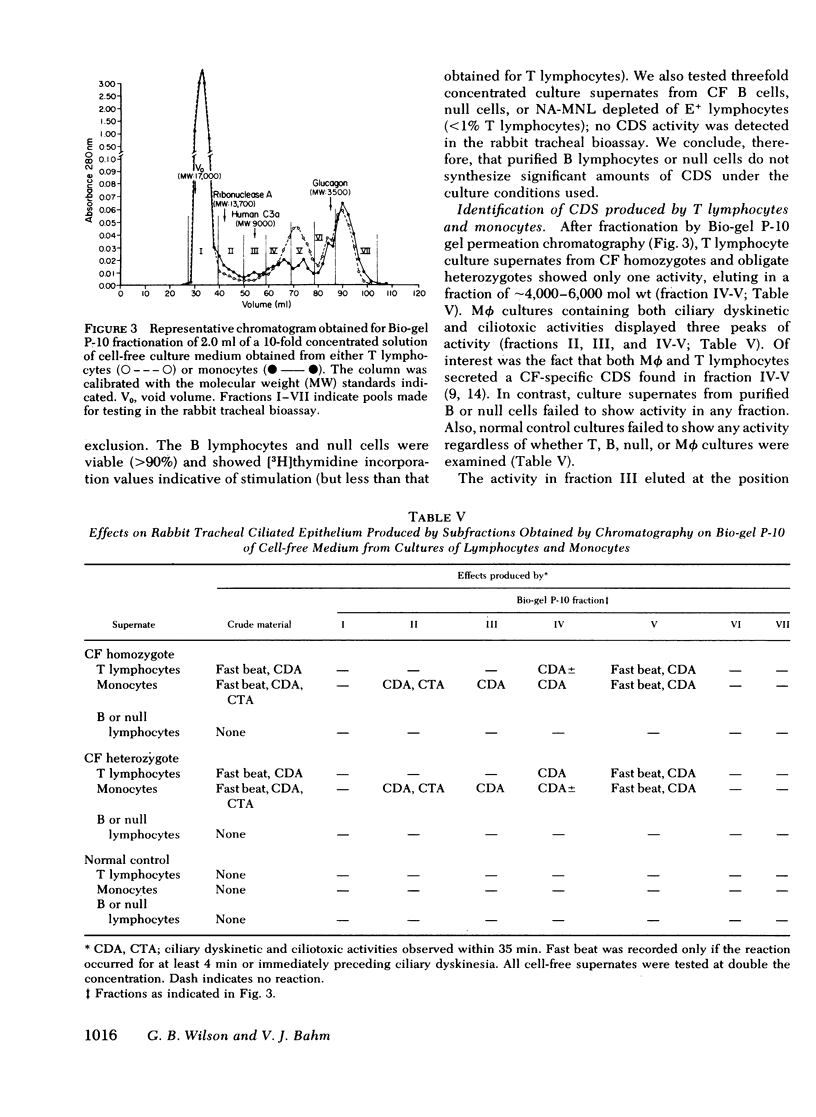
Cultured peripheral blood leukocytes (PBL) from individuals homozygous or heterozygous for the defective gene causing the inherited disease cystic fibrosis (CF) secrete three different ciliary dyskinesia substances (CDS), which can be detected by their activity in vitro in a rabbit mucociliary bioassay. Their PBL also release substances that promote mucus expulsion and destruction of the ciliated epithelium. In the present study the relative numbers of lymphocytes (T, B, and null), monocytes-macrophages (Mφ), and polymorphonuclear neutrophils were found to be normal in subjects with the CF gene, as were the responses of their PBL to phytohemagglutinin and pokeweed mitogen. Using purified subpopulations of leukocytes, we obtained evidence that both monocytes and T lymphocytes can secrete CDS in vitro with no requirement for cooperation with other lymphocyte subsets, whereas B and “null” lymphocytes probably require either differentiation or cellular cooperation for optimal secretion of CDS. Mucus expulsion and tissue destruction were produced by substances released primarily from polymorphonuclear neutrophils and secondarily from Mφ. Using cycloheximide and actinomycin D, we obtained evidence that CDS accumulation requires active protein synthesis and is not dependent on newly synthesized RNA, at least in short-term cultures. Gel filtration chromatography of active culture supernates showed that T lymphocytes synthesized only a CF-specific CDS, whereas Mφ synthesized all three CDS found in PBL cultures. Evidence is presented that one CDS is related structurally to C3a, since it can be removed with rabbit antisera specific for human C3a.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ades E. W., Dougherty P., Shore S. L., Balch C. M. E-rosette receptors induced by phytohemagglutinin on human K cells expressing T-cell surface antigens. Cell Immunol. 1979 Apr;44(1):179–185. doi: 10.1016/0008-8749(79)90038-8. [DOI] [PubMed] [Google Scholar]
- Bennett W. E., Cohn Z. A. The isolation and selected properties of blood monocytes. J Exp Med. 1966 Jan 1;123(1):145–160. doi: 10.1084/jem.123.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beratis N. G., Conover J. H., Conod E. J., Bonforte R. J., Hirschhorn K. Studies on ciliary dyskinesia factor in cystic fibrosis. 3. Skin fibroblasts and cultured amniotic fluid cells. Pediatr Res. 1973 Dec;7(12):958–964. doi: 10.1203/00006450-197312000-00004. [DOI] [PubMed] [Google Scholar]
- Bowman B. H., Barnett D. R., Matalon R., Danes B. S., Bearn A. G. Cystic fibrosis: fractionation of fibroblast media demonstrating ciliary inhibition. Proc Natl Acad Sci U S A. 1973 Feb;70(2):548–551. doi: 10.1073/pnas.70.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess L., MacDermott R. P., Schlossman S. F. Immunologic functions of isolated human lymphocyte subpopulations. I. Quantitative isolation of human T and B cells and response to mitogens. J Immunol. 1974 Oct;113(4):1113–1121. [PubMed] [Google Scholar]
- Conover J. H., Beratis N. G., Conod E. J., Ainbender E., Hirschhorn K. Studies on ciliary dyskinesia factor in cystic fibrosis. II. Short term leukocyte cultures and long term lymphoid lines. Pediatr Res. 1973 Apr;7(4):224–228. doi: 10.1203/00006450-197304000-00029. [DOI] [PubMed] [Google Scholar]
- Conover J. H., Conod E. J., Hirschhorn K. Studies on ciliary dyskinesia factor in cystic fibrosis. IV. Its possible identification as anaphylatoxin (C3a)-IgG complex. Life Sci. 1974 Jan 16;14(2):253–266. doi: 10.1016/0024-3205(74)90055-1. [DOI] [PubMed] [Google Scholar]
- Conover J. H., Conod E. J. The influence of cystic fibrosis serum and calcium on secretion in the rabbit tracheal mucociliary apparatus. Biochem Biophys Res Commun. 1978 Aug 29;83(4):1595–1601. doi: 10.1016/0006-291x(78)91404-3. [DOI] [PubMed] [Google Scholar]
- Czegledy-Nagy E., Sturgess J. M. Cystic fibrosis: effects of serum factors on mucus secretion. Lab Invest. 1976 Dec;35(6):588–595. [PubMed] [Google Scholar]
- Danes B. S. Expression of the cystic fibrosis genotype in cultured somatic cells. Tex Rep Biol Med. 1976;34(1):135–150. [PubMed] [Google Scholar]
- Davies P., Bonney R. J. Secretory products of mononuclear phagocytes: a brief review. J Reticuloendothel Soc. 1979 Jul;26(1):37–47. [PubMed] [Google Scholar]
- Di Sant'Agnese P. A., Davis P. B. Research in cystic fibrosis (first of three parts). N Engl J Med. 1976 Aug 26;295(9):481–485. doi: 10.1056/NEJM197608262950905. [DOI] [PubMed] [Google Scholar]
- GOLDBERG I. H., REICH E. ACTINOMYCIN INHIBITION OF RNA SYNTHESIS DIRECTED BY DNA. Fed Proc. 1964 Sep-Oct;23:958–964. [PubMed] [Google Scholar]
- Glade P. R., Hirschhorn K. Products of lymphoid cells in continuous culture. Am J Pathol. 1970 Sep;60(3):483–494. [PMC free article] [PubMed] [Google Scholar]
- Glassman A. B., Bennett C. E. B and T lymphocytes: methodology and normal ranges. Ann Clin Lab Sci. 1977 Nov-Dec;7(6):519–523. [PubMed] [Google Scholar]
- Gupta S., Good R. A., Siegal F. P. Rosette-formation with mouse erythrocytes. II. A marker for human B and non-T lymphocytes. Clin Exp Immunol. 1976 Aug;25(2):319–327. [PMC free article] [PubMed] [Google Scholar]
- Hoiby N., Mathiesen L. Pseudomonas aeruginosa infection in cystic fibrosis. Distribution of B and T lymphocytes in relation to the humoral immune response. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Aug;82(4):559–566. [PubMed] [Google Scholar]
- Janossy G., Greaves M. F., Doenhoff M. J., Snajdr J. Lymphocyte activation. V. Quantitation of the proliferative responses to mitogens using defined T and B cell populations. Clin Exp Immunol. 1973 Aug;14(4):581–596. [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman J., Kaneshiro W. Abnormal response of cultured lymphocytes to phytohemagglutinin and autologous serum in cystic fibrosis. Am Rev Respir Dis. 1977 Dec;116(6):1047–1055. doi: 10.1164/arrd.1977.116.6.1047. [DOI] [PubMed] [Google Scholar]
- Mac Dermott R. P., Chess L., Schlossman S. F. Immunologic functions of isolated human lymphocyte subpopulations. V. Isolation and functional analysis of a surface Ig negative, E rosette negative subset. Clin Immunol Immunopathol. 1975 Sep;4(3):415–424. doi: 10.1016/0090-1229(75)90010-0. [DOI] [PubMed] [Google Scholar]
- Marchi A. G., Marchi M. A., Mastella G., Nordio S. Abnormal beta-glucuronidase activity in cystic fibrosis. Helv Paediatr Acta. 1973 Nov;28(5):427–435. [PubMed] [Google Scholar]
- Natvig J. B., Frøland S. S. Detection of a third lymphocyte-like cell type by rosette formation with erythrocytes sensitized by various anti-Rh antibodies. Scand J Immunol. 1976 Jun;Suppl 5:83–89. doi: 10.1111/j.1365-3083.1976.tb03859.x. [DOI] [PubMed] [Google Scholar]
- Polley M. J., Bearn A. G. Cystic fibrosis: current concepts. J Med Genet. 1974 Sep;11(3):249–252. doi: 10.1136/jmg.11.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. D., Rabellino E. M., Polley M. J., Grey H. M. Combined studies of complement receptor and surface immunoglobulin-bearing cells and sheep erythrocyte rosette-forming cells in normal and leukemic human lymphocytes. J Clin Invest. 1973 Feb;52(2):377–385. doi: 10.1172/JCI107194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen R. U., Stern R. C., Polmar S. H. Cellular immunity to bacteria: impairment of in vitro lymphocyte responses to Pseudomonas aeruginosa in cystic fibrosis patients. Infect Immun. 1977 Dec;18(3):735–740. doi: 10.1128/iai.18.3.735-740.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdugo P., Hinds T. R., Vincenzi F. F. Laser light-scattering spectroscopy: preliminary results on bioassay of cystic fibrosis factor(s). Pediatr Res. 1979 Feb;13(2):131–135. doi: 10.1203/00006450-197902000-00009. [DOI] [PubMed] [Google Scholar]
- Wallwork J. C., Brenchley P., McCarthy J., Allan J. D., Moss D., Ward A. M., Holzel A., Williams R. F., McFarlane H. Some aspects of immunity in patients with cystic fibrosis. Clin Exp Immunol. 1974 Nov;18(3):303–320. [PMC free article] [PubMed] [Google Scholar]
- Ward P. A. A plasmin-split fragment of C'3 as a new chemotactic factor. J Exp Med. 1967 Aug 1;126(2):189–206. doi: 10.1084/jem.126.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. B. Cystic fibrosis protein, a confirmed diagnostic marker for detecting heterozygote carriers: significance in relation to future screening and to a proposed primary defect in alpha 2-macroglobulin. Pediatr Res. 1979 Sep;13(9):1079–1081. doi: 10.1203/00006450-197909000-00028. [DOI] [PubMed] [Google Scholar]
- Wilson G. B., Fudenberg H. H. Separation of ciliary dyskinesia substances found in serum and secreted by cystic fibrosis leukocytes and lymphoid cell lines, using protein A--Sepharose CL-4B. J Lab Clin Med. 1978 Sep;92(3):463–482. [PubMed] [Google Scholar]
- Wilson G. B., Fudenberg H. H. Studies on cystic fibrosis using isoelectric focusing. IV. Distinction between ciliary dyskinesia activity in cystic fibrosis and asthmatic sera and association of cystic fibrosis protein with the activity in cystic fibrosis serum. Pediatr Res. 1977 Apr;11(4):317–324. doi: 10.1203/00006450-197704000-00011. [DOI] [PubMed] [Google Scholar]
- Wilson G. B., Monsher M. T., Fudenberg H. H. Studies on cystic fibrosis using isoelectric focusing. III. Correlation between cystic fibrosis protein and ciliary dyskinesia activity in serum shown by a modified rabbit tracheal bioassay. Pediatr Res. 1977 Feb;11(2):143–146. doi: 10.1203/00006450-197702000-00016. [DOI] [PubMed] [Google Scholar]
- Wilson G. B., Walker J. H., Jr, Watkins J. H., Jr, Wolgroch D. Determination of subpopulations of leukocytes involved in the synthesis of alpha 1-antitrypsin in vitro. Proc Soc Exp Biol Med. 1980 May;164(1):105–114. doi: 10.3181/00379727-164-40832. [DOI] [PubMed] [Google Scholar]
- Wood R. E., Boat T. F., Doershuk C. F. Cystic fibrosis. Am Rev Respir Dis. 1976 Jun;113(6):833–878. doi: 10.1164/arrd.1976.113.6.833. [DOI] [PubMed] [Google Scholar]


