Abstract
Introduction:
Identifying successful smoking treatment interventions and methods of delivery is critical given the smoking rates among HIV-positive populations and the medical implications of smoking in this population. This study compared the efficacy of 3 smoking cessation interventions provided in HIV clinical treatment settings.
Methods:
Following a baseline assessment, 209 HIV-positive smokers were randomly assigned to 1 of 3 conditions in a parallel group design. Treatment conditions were individual counseling plus nicotine replacement treatment (NRT), a computer-based Internet smoking treatment plus NRT, and self-help plus NRT. Smoking status was determined at follow-up assessments completed at 12, 24, 36, and 52 weeks following treatment initiation.
Results:
Cessation rates ranged from 15% to 29%; however, no statistically significant differences in abstinence were found among the treatment conditions over time. Those employed, those who reported a greater desire to quit, or those with lower mood disturbance scores were more likely to achieve abstinence (p < .01). The number of cigarettes participants reported smoking in the 24hr prior to each assessment significantly declined over time (p < .001).
Conclusions:
Although we found no differences in abstinence rates across groups, the results indicate that integration of smoking cessation interventions is feasible in HIV clinical treatment settings, and cessation results are promising. The overall abstinence rates we report are comparable to those found in similar treatment studies across multiple populations. Further research is warranted.
INTRODUCTION
Identifying successful smoking cessation treatment and effective methods of treatment delivery is critical given the smoking rates among HIV-positive (HIV+) populations and the medical implications of smoking in this population. Although national estimates of smoking indicate that approximately 21% of the adult population smokes (Center for Disease Control, 2007), higher rates, up to 57%, have been reported in numerous HIV+ cohorts (Burns et al., 1991; Collins et al., 2001; Gritz, Vidrine, Lazev, Amick, & Arduino, 2004; Mamary, Bahrs, & Martinez, 2002). Research suggest that HIV+ smokers smoke an average of 15–20 cigarettes per day and have moderate levels of nicotine dependence (Burkhalter, Springer, Chhabra, Ostroff, & Rapkin, 2005; Gritz et al., 2004; Mamary et al., 2002; Vidrine, Arduino, Lazev, & Gritz, 2006).
Smoking has been found to predict an increased likelihood of a variety of HIV-related medical complications including bacterial pneumonia, HIV-related pulmonary emphysema, hairy leukoplakia, oral candidiasis, and AIDS dementia (Boulter et al., 1996; Burns et al., 1996; Conley et al., 1996; Diaz et al., 2000; Greenspan, Barr, Sciubba, & Winkler, 1992; Hirschtick et al., 1995; Palacio, Hilton, Canchola, & Greenspan, 1997; Reardon, Kim, Wagner, Koziel, & Kornfeld, 1996). In addition, daily tobacco use seemed to attenuate by 40% the immune and virological response to antiretroviral therapies. Cigarette smoking has also been found to have a negative impact on the health-related quality of life in persons with HIV (Turner et al., 2001).
Research on smoking cessation following HIV diagnosis is limited. The available data indicate that cessation rates are low (Burkhalter et al., 2005; Collins et al., 2001; Gritz et al., 2004) with the majority of smokers continuing tobacco use after HIV diagnosis. Unique factors that may influence cessation among HIV+ smokers may include perceived competence to manage one’s health and psychological adjustment to a HIV disease diagnosis. Individuals with HIV face multiple treatment demands including complex medication regimens, regular medical monitoring visits, and frequent visits with allied health professionals. Perceived competence, or self-efficacy, has consistently been associated with adherence to HIV treatment (Arnsten et al., 2007; Barclay et al., 2007; Johnson et al., 2007). Perceived competence to manage health issues has also been associated with high levels of HIV quality of life (Wallston, Osborn, Wagner, & Hilker, 2011). Overall perceived ability to manage multiple health demands may be particularly relevant to smoking cessation in this population. Related to this, psychological adjustment to an illness can be an important dimension of treatment engagement (Ross, Hunter, Condon, Collins, & Begley, 1994). Psychological responses to HIV such as hopelessness or minimization of the disease may inhibit health behavior changes, whereas responses such as active coping or having a “fighting spirit” may promote health behavior change (Kelley et al., 2000).
To date, three clinical trials evaluating smoking treatment for HIV+ smokers have been reported. Ingersoll, Cropsey, and Heckman (2009) conducted a pilot study with 40 HIV+ smokers testing two treatments, motivational interviewing plus nicotine patch versus self-guided reading. They found no differences in cessation rates at a 3-month follow-up. Vidrine, Marks, Arduino, and Gritz (2012) compared a standard care intervention to a cell-phone-delivered proactive counseling intervention in a sample of 474 HIV+ smokers. They found a significant difference between groups at 3 months with 11.9% abstinence among smokers in the cell-phone-delivered intervention compared with 3.4% of smokers in the usual-care condition. Six- and twelve-month follow-up data have not been reported. Lloyd-Richardson et al. (2009) compared a two-session behavioral intervention plus nicotine patch to a four-session motivational enhancement plus patch intervention. They found no significant differences in cessation rates between groups with overall quit rates of about 10% at 6 months.
Integrating smoking treatment into clinical treatment settings may be particularly effective for individuals with chronic medical conditions, which often require frequent medical visits over an extended period of time. Traditionally, behavioral treatments are delivered via face-to-face counseling. Although effective, there are multiple barriers to providing counseling in a clinical care setting because of lack of space, lack of time, and lack of training. A valuable resource for providing health-related information and dissemination of behavior change treatments is computer technology and the Internet (Civljak, Sheikh, Stead, & Car, 2010). If efficacious, computer-based smoking treatments may be easier to integrate into clinical settings than more traditional treatments.
The primary aim of this study was to determine the efficacy of targeted smoking treatments that incorporate behavioral interventions to address the unique needs of the HIV+ smoker. Two targeted smoking treatments were developed. One treatment was delivered in a more “traditional” manner, by face-to-face individual counseling (IC). The other treatment was delivered in a nontraditional manner, by computer technology and the Internet. Both treatments were compared with a self-help (SH) condition. All treatments included nicotine replacement treatment (NRT) and were delivered in HIV clinical care settings.
Our primary hypothesis was that the counseling and Internet-based treatment conditions would result in higher point prevalence abstinence rates than the SH condition. We had two secondary hypotheses. First, levels of perceived health competence would predict outcome. That is, participants with higher levels of competence would be more likely to quit smoking than participants with lower levels of competence. Second, psychological adjustment to HIV would predict outcome. That is, participants with higher levels of psychological adjustment to HIV will be more likely to quit smoking than participants with low levels of psychological adjustment to HIV.
METHODS
Study Design
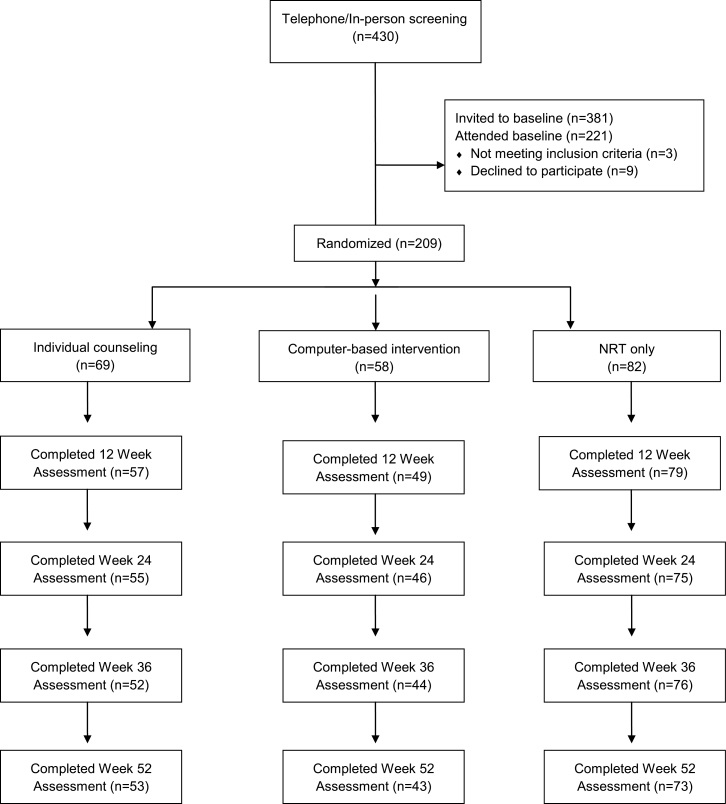
Following a baseline assessment, 209 HIV+ smokers were stratified based on number of cigarettes per day (greater than 20 cigarettes per day vs. not), depressed or not based on Composite International Diagnostic Interview (CIDI) diagnosis, and gender. Then, within those strata, they were randomized via computer algorithm to one of three conditions in 1:1:1 fashion into a parallel group design. Treatment conditions included a traditional IC intervention targeted to HIV+ smokers plus NRT, a computer-based Internet smoking treatment (CBI) targeted to HIV+ smokers plus NRT, and a SH plus NRT condition. Smoking status was determined at follow-up assessments completed at 12, 24, 36, and 52 weeks following treatment initiation. The study was conducted from June 2006 through February 2010. Participant flow can be seen in Figure 1.
Figure 1.
Participant flow. The uneven sample sizes per condition were a function of two factors. The primary one was that we did not reach the targeted N so that, at the time enrollment ceased, the randomization had not returned to balance. The other was that we were able to recruit fewer participants in one of our strata than expected, those who smoked greater than 20 cigarettes per day. This may have exaggerated the unbalancing.
Participants
HIV+ smokers were recruited from three clinics serving HIV+ persons in San Francisco, CA. Eighty-one individuals were patients at the University of California, San Francisco AIDS Health Project, an organization that provides HIV testing and counseling, substance abuse treatment, and mental health services. One hundred twenty-four participants were patients at the Positive Health Practice at San Francisco General Hospital (SFGH), a public health care facility. Four additional participants were recruited from Tenderloin Health, a community-based program providing case-management service to people with HIV many of whom receive medical care at SFGH. To be eligible, participants must be 18 years or older, smoke most days of the month, and be registered patients at one of the facilities. Individuals were excluded if they were already enrolled in other smoking cessation treatment, or were experiencing significant or severe cognitive impairment or dementia, which would negatively impact treatment.
The sample size was based on effect sizes found in studies using conditions similar to the SH and CBI treatment conditions as well as our own work with NRT plus group treatments similar to the IC intervention. Based on finding from studies evaluating SH interventions plus NRT (Daughton et al., 1998; Joseph & Antonnucio, 1999; Solomon, Scharoun, Flynn, Secker-Walker, & Sepinwall, 2000; Stapleton et al., 1995), we expected that 12-, 24-, 36-, and 52-week abstinence rates in the SH condition would be about 20%, 14%, 12%, and 11%, respectively. Given the lack of previous research on computer-based interventions in a clinical setting, we estimated abstinence rates for the CBI intervention on results from another technology-based intervention, telephone counseling plus NRT (Lando et al., 1997; Reid, Pipe, & Dafoe, 1999; Solomon et al., 2000). Thus, we expected that the 12-, 24-, 36-, and 52-week abstinence rates in the CBI condition would be 38%, 25%, 23%, and 22%, respectively. Based on our work with similar counseling interventions plus NRT, we expected that the 12-, 24-, 36-, and 52-week abstinence rates in the IC condition will be 56%, 30%, 28%, and 28%, respectively (Hall et al., 2002; Humfleet et al., 2002).
Recruitment
Participants were recruited from all three sites using direct provider referral and display of post cards and flyers at the clinics. Recruitment letters were sent to home addresses of patients, who had previously consented to be contacted for research purposes. Potential participants were provided an overview of the study and screened briefly for exclusion criteria via telephone. If interested and eligible, they were scheduled for a baseline assessment where study procedures were reviewed and informed consent was obtained.
Baseline Assessment
Interview Data
During the baseline interview, participants completed the CIDI schedule, a structured, computerized interview that provides DSM-IV diagnoses (World Health Organization, 1997); modules measuring nicotine and alcohol abuse and dependence, as well as depression and bipolar disorder, were administered. The Addiction Severity Index (McLellan et al., 1992) was used to assess current and past alcohol and other drug use, as well as psychiatric history and treatment.
Self-reported Information
Demographic information, smoking history, and current use patterns were obtained using self-report questionnaires developed by our group and used across multiple studies. Participants also completed several smoking-related measures including the Fagerström Test for Nicotine Dependence, a six-item instrument measuring smoking behaviors indicative of physical dependence (Payne, Smith, McCracken, McSherry, & Antony, 1994); the Thoughts About Abstinence Scale, a four-item measure that assesses the desire to quit, anticipation about successfully quitting, anticipated difficulty with remaining abstinent, and an abstinence-related goal (Hall, Havassy, & Wasserman, 1990); and the Stages of Change measure, a five-item scale assessing the readiness to quit smoking (DiClemente et al., 1991). Participants also completed several psychosocial measures including the Profiles of Mood States (POMS), a 60-item measure of mood disturbance (McNair, Lorr, & Droppleman, 1992); the Perceived Health Competence Scale, which assesses competence to manage health conditions (Smith, Wallston, & Smith, 1995); the Perceived Stress Scale, a general stress measure (Cohen, Kamarck, & Mermelstein, 1983); the HIV Risk Behavior Scale, assessing sexual and drug use risks related to HIV (Darke, Hall, Heather, Ward, & Wodak, 1991); and the Adjustment to HIV Scale (Ross et al., 1994).
Following the baseline assessment, eligible smokers were stratified based on gender (male/female), history of major depressive disorder (MDD+/MDD-), and number of cigarettes per day (20 or more/less than 20), and then, the eligible smokers were randomized to one of the following three arms: IC, computer-based intervention, or the SH arm.
Pharmacological Treatment
NRT was available to all participants who smoked five or more cigarettes per day. We chose five or more cigarettes per day to ensure a level of dependence that warrants NRT. Participants could obtain a 10-week course of either nicotine patch or nicotine gum, both over-the-counter nicotine replacement medications.
At the Week 1 visit, all participants received a written overview of the NRT medications including a chart comparing the NRTs on ease of use, flexibility of use, and primary side effects. Participants were given written instructions specific to the form of NRT they chose and an initial 4-week supply. Additional NRT was scheduled for distribution at Weeks 5 and 9. Participants who experienced significant side effects or difficulties using one form of NRT had the option to switch to another form of NRT. NRT dose was based on the written instructions provided by the manufacturer.
Behavioral Treatments
All treatments were provided at the clinical sites. No incentives were provided for completing treatment sessions.
Individual Counseling
The IC condition included six IC sessions. The intervention was based on a cognitive behavioral treatment model used in previous work by our group (Hall et al., 2009; Hall, Humfleet, Reus, Munoz, & Cullen, 2004) and was targeted to the needs of HIV+ smokers. Targeting was based on research with HIV+ individuals indicating the importance of the negative impact of smoking on HIV-related health conditions, high levels of stress, high levels of depression, and low levels of social support. Thus, the intervention included specific information on HIV-related health issues and smoking, a stress management component, a mood management component, and a social support component. The intervention was also individually tailored through the development of a written “Personal Quit Plan.” Through discussion, exercises, and homework assignments, individuals were encouraged to consider how each topic may apply to their lives, develop specific cessation strategies based on this discussion and then incorporate these strategies into their quit plan. Counseling sessions were held during Weeks 1, 2, 3, 5, 8, and 12. Each session was 40–60min in length. During Session 1, the smoker’s reasons for quitting were identified, and information on smoking and HIV-related health conditions was discussed. A quit date was scheduled for the day of Session 2, thus strategies for preparing to quit were reviewed. The counselor reviewed the available NRT medications. An initial supply of NRT was provided, and directions for use were reviewed. Session 2, and all remaining sessions, began with a “check in” including a report of smoking status and review of withdrawal symptoms, cravings, difficulties, and successes experienced by the participant. Abstinence was positively reinforced. Those who relapsed discussed alternative strategies and were encouraged to set a new quit date. New content was introduced at each subsequent session and a homework exercise based on the new content was assigned. The new content for Sessions 2–5 was mood management, social support, maintaining motivation, and stress management. All content areas were reviewed during Session 6 and long-term nonsmoking goals were discussed.
Counselors were clinicians with a master’s or doctoral degree in social work or psychology and had previous experience in smoking cessation treatment. Prior to treating study participants, counselors were trained on the study protocol through didactic sessions, role playing of each session and observation with pilot participants. Counselors were trained and supervised by the lead author.
Computer-Based Internet Treatment
Participants randomized into the CBI condition were offered access to a Web site intervention modeled on the counseling intervention content.
The intervention content was provided at sixth-grade reading level. The Web site home page included an overview of the treatment and directions for using the Web site. Each treatment component was structured into a “step” roughly corresponding to the first five sessions of the counseling intervention. Session 6 of the counseling intervention was a review of the previous five sessions and was not included in the Web site. Each step was interactive. Specific information on the topic was introduced, and then individuals were directed to complete self-assessment exercises and homework assignments. Individuals were encouraged to develop cessation strategies based on the reading and feedback from the exercises and incorporate these strategies into their online “Personal Quit Plan.” Pilot work in the development of this intervention indicated that the steps took 30–45min to complete on average. The Web site contained the following five steps: Step 1, education and preparation; Step 2, managing your mood while quitting; Step 3, social support for quitting; Step 4, stress management; and Step 5, increasing and maintaining motivation. Although we recommended following the steps in sequence, participants could access any webpage at any time. To mirror real-world applications, participants could access the Web site for 12 months. We did not suggest a specific schedule for Web site visits.
The Web site included a message board where participants could communicate with other study participants in this treatment condition. The bulletin board also included an “Ask the Expert” component to allow smokers to obtain information and feedback from project staff. Sixteen percent of the participants assigned to CBI used the message board.
A CBI orientation meeting was held during Week 1. In this meeting, the research staff determined the participant’s personal access to the Internet, established a username and password for the Web site, and provided an overview of the Web site. Usernames and passwords were required to limit access to the Web site to only those participants assigned to the CBI condition. During the overview, the staff guided the participant through a self-assessment exercise in Step 1 demonstrating how to complete the exercise, consider appropriate strategies for quitting and incorporating those strategies into the Personal Quit Plan. The staff discussed a quit date with the smoker and entered this date into their plan. Participants could access the Web site at computer stations located in the clinic, public computers in the community, or at home. Individuals with limited access to the Internet were given a list of alternative free access locations. Vouchers were also provided to participants who did not have Internet access at home and were interested in accessing the Internet at business locations, such as cafes, near their home. The participants were given a packet of materials including a written guide on accessing and navigating the Web site, maps identifying locations for free and voucher-based Internet access, and copies of all printable forms available on the Web site. Participants were provided contact information if they experienced difficulties in accessing the Web site. No additional cessation guidance was provided. The orientation meeting was 45–60min.
SH Plus NRT
Participants randomized into the SH condition met briefly with the research staff. Participants received How to Quit Smoking (FastMark, 1998), a laminated quick reference guide. Study staff briefly reviewed the guide and recommended establishing a quit date during Week 2. The staff member reviewed the NRT medications available, provided directions for using the NRT, and dispensed an initial supply of NRT. Participants were scheduled to pick up subsequent NRT supplies. No further cessation guidance was provided.
Follow-Up Assessments
Follow-up assessments were completed at 12, 24, 36, and 52 weeks following treatment initiation. Retention rates were 89% at 12 weeks, 84% at 24 weeks, 82% at 36 weeks, and 81% at 52 weeks.
Cigarette Use
Biochemically verified 7-day abstinence from cigarettes was the primary outcome variable. Smoking abstinence included both self-reported 7-day point prevalence abstinence and sustained abstinence obtained at each follow-up assessment. Point prevalence abstinence was verified by CO level. Participants were coded as abstinent only if they reported no cigarette use, not even a puff, in the last 7 days and if their CO level was ≤10 ppm. For participants who reported abstinence but were unable or unwilling to come into the clinic to provide a breath sample, we accepted a confirmatory statement from a significant other or someone with whom they have weekly contact in lieu of the breath sample. A proxy report was implemented for one participant.
Second, we measured sustained abstinence based on recent recommendations in the smoking cessation literature (Hughes et al., 2003). Smoking was defined as any cigarette on 7 or more consecutive days since the previous assessment. Thus, participants who report smoking, but for less than seven consecutive days, were coded positive for sustained abstinence. This definition requires repeated use of tobacco to be considered relapsed and allows individuals who experience isolated “slips” to be considered abstinent. We chose not to supplement CO measurement with other forms of biological confirmation based on recent recommendations by an expert panel (SRNT Subcommittee on Biochemical Verification, 2002).
Data Analysis
As models, which use generalized estimating equations, assume missing data are missing completely at random and violation of that assumption can lead to biased estimates (Schneider, Hedeker, Bailey, Cook, & Spring, 2010), we tested to see if there was evidence that the outcome was related to missingness by including an index of whether they completed all assessments. As results indicated that the missingness was not completely at random (i.e., completers were more likely to be abstinent), we used a mixed-effects nonlinear regression model via Proc NlMixed in SAS version 9.2 to compare the verified point prevalence abstinence rates across postbaseline assessments. Prior to the final analysis, we used a multivariate model to test a set of candidate covariates for their relationship to the outcome and retained variables with a p-value < .10 for inclusion in the final model. Candidate variables were the variables listed in Tables 1 and 2. Terms in the final model were the treatment condition (IC, CBI, or SH), week of the assessment (12, 24, 36, and 52), their interaction, and the baseline measures of whether employed, desire to quit, POMS total mood disturbance, and number of cigarettes usually smoked in 24hr. The model estimation allowed full use of all observed data. As 76% of participants completed all assessments, completer status (completed all assessments vs. not) was included in the models instead of the number of assessments completed.
Table 1.
Demographic Characteristics (n = 209)
| Category | Data |
|---|---|
| Mean agea (SD) | 45 (8.0) |
| Sexual identification | |
| Gay/lesbian | 61.5% |
| Straight | 24.3% |
| Bisexual | 7.4% |
| Gender | |
| Male | 81.7% |
| Female | 15.1% |
| Transgender | 3.2% |
| Ethnicity | |
| Hispanic | 13.5% |
| Race | |
| African American | 26.6% |
| White | 52.7% |
| American Indian | 2.4% |
| Multiple/other | 18.3% |
| Education | |
| <High school | 20.6% |
| High school/general equivalency degree | 44.5% |
| Associate’s degree/vocational | 15.0% |
| Bachelor’s degree | 15.9% |
| Graduate degree | 3.7% |
| Marital status | |
| Married | 4.8% |
| Never married | 73.4% |
| Separated/divorced/widowed | 21.3% |
| Living situation | |
| Own/rent | 51.2% |
| Halfway house/therapeutic community | 13.1% |
| Single-room occupancy hotel | 16.9% |
| Homeless | 5.8% |
| Other | 13.4% |
| Employment | |
| Employed | 14.0% |
| Unemployed | 58.0% |
| Retired | 10.6% |
| Other | 17.5% |
| Income | |
| <$10,000 | 42.2% |
| $10,000–$20,999 | 40.8% |
| $21,000–$30,999 | 8.7% |
| >$31,000 | 8.9% |
Note. aSignificant differences between treatment conditions. Smokers in self-help treatment were older than smokers in the other two treatments (p < .01).
Table 2.
Baseline Psychiatric, Drug Use, and Treatment Variables (n = 209)
| Percentage | |||
|---|---|---|---|
| Lifetime psychiatric diagnosesa | |||
| Major depressive episode | 41 | ||
| Bipolar disorder | 22 | ||
| Alcohol dependence | 55 | ||
| Drug use past 30 days | |||
| Marijuana | 37 | ||
| Cocaine | 8 | ||
| Amphetamines | 8 | ||
| Heroin | 1 | ||
| Mean | Standard deviation | ||
| Tobacco use | |||
| Daily cigarettes | 19.8 | 10.5 | |
| Age smoking regularly | 17.2 | 6.2 | |
| Prior quit attempts | 4.1 | 6.5 | |
| Fagerström test for nicotine dependence score | 4.9 | 2.4 | |
| Percentage | |||
| Smoking treatment goal | |||
| No clear goal | 5.1 | ||
| Controlled smoking | 3.2 | ||
| Abstain for some time | 5.5 | ||
| Smoke occasionally | 2.8 | ||
| Quit—might slip | 26.6 | ||
| Quit forever | 44.0 | ||
| Other | 12.8 | ||
Note. aSignificant differences between treatment conditions. Smokers in the self-help condition were more likely to report a history of a major depressive episode than smokers in the other two conditions (p < .05). Smokers in the computer-based intervention were more likely to report a history of bipolar disorder than smokers in the other two conditions (p < .05).
In secondary analyses, we tested for treatment effects on the number of cigarettes reportedly smoked in the prior 24hr using a mixed-effects model by Proc NlMixed in SAS. Finally, as data were collected at three sites, effects for site were also tested.
RESULTS
Participant Characteristics
Detailed participant characteristics can be found in Tables 1 and 2. Retention rates by treatment condition are shown in Table 3. The study sample was predominantly male, ethnically/racially diverse, and of low socioeconomic status. Less than a fifth of the sample was employed, and a large proportion was homeless or living in transitional housing. Rates of lifetime psychiatric disorders and illicit drug use in the past month were high. Significant differences between treatment groups were found on three variables. Smokers in the SH condition were older (p < .01) and more likely to have a history of a major depressive episode (p < .05) than participants in the other two conditions. Smokers in the CBI condition were more likely to have met criteria for bipolar disorder than the other two conditions (p < .05).
Table 3.
Percent Abstinent (a) and Mean Number of Cigarettes Smoked (b) by Treatment Condition and Assessment Week
| Week | Brief | Counseling | CBI |
|---|---|---|---|
| a. | |||
| 12 | 24.36 | 25.93 | 29.17 |
| 24 | 15.07 | 15.09 | 26.67 |
| 36 | 18.92 | 21.28 | 20.93 |
| 52 | 19.72 | 20.41 | 25.58 |
| b. | |||
| Baseline | 17.5 | 18.4 | 17.2 |
| 12 | 6.1 | 7.1 | 6.4 |
| 24 | 7.4 | 7.7 | 11.0 |
| 36 | 8.7 | 7.5 | 10.5 |
| 52 | 8.4 | 8.4 | 10.5 |
Note. CBI = computer-based Internet smoking treatment.
The number of cigarettes smoked per day and the Fagerström dependence score indicate moderate levels of nicotine dependence. Less than half of the sample (44%) endorsed a smoking treatment goal of total cigarette abstinence. These baseline characteristics are consistent with clinic census data indicating that the sample is representative of the clinic populations.
Smoking Outcome
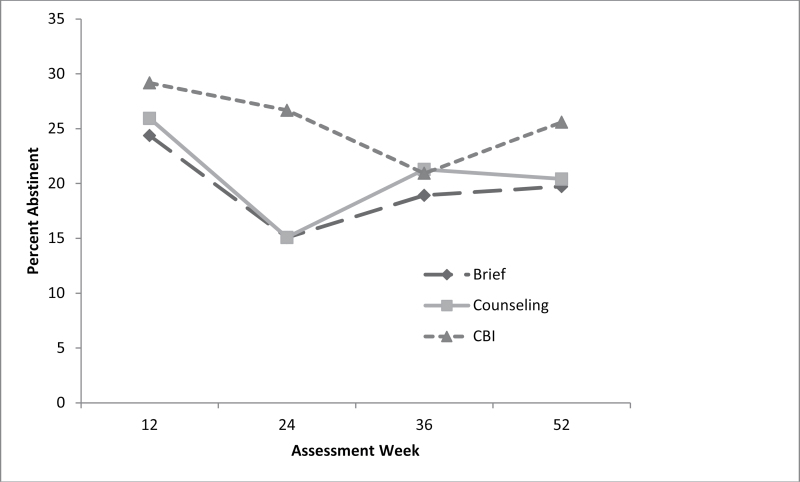
Percentage smoking abstinent ranged from 15% to 29% with no statistically significant difference among the treatment conditions (Figure 2 and Table 3). Estimates from the mixed-effects regression model are summarized in Table 4. Those employed, those who reported a greater desire to quit, or those with lower mood disturbance scores were more likely to achieve abstinence.
Figure 2.
Analyses of point prevalence abstinence rates by treatment condition and time indicate no significant overall differences between treatments across time.
Table 4.
Estimates and Tests of Model Parameters
| Variable | Estimate | Odds ratio | Lower (95% confidence interval) | Upper (95% confidence interval) |
|---|---|---|---|---|
| CBI | 1.21 | 3.37 | 0.46 | 24.64 |
| Counseling | 0.20 | 1.23 | 0.17 | 8.73 |
| Assessment | -0.01 | 0.99 | 0.96 | 1.02 |
| Completer | 2.58 | 13.19 | 1.28 | 136.2 |
| Employed | -0.39 | 0.68* | 0.49 | 0.93 |
| Desire to quit | 0.54 | 1.72* | 1.11 | 2.68 |
| POMS mood disturbance | -0.02 | 0.98* | 0.96 | 1.00 |
| Usual cigarettes per day | 0.025 | 1.03 | 0.97 | 1.09 |
Note. CBI = computer-based Internet smoking treatment.
*p < .01
No differences in sustained abstinence were found nor were there effects based on recruitment site. However, the number of cigarettes participants reported smoking in the 24hr prior to each assessment significantly declined over time (p < .001) with means at each assessment of 17.8, 8.7, 10.5, 11.1, and 11.5. The treatment conditions did not differ in this decline (see Table 3), and a similar effect was found when participants were asked about the number of cigarettes in the prior week.
NRT Use
Self-report of NRT use was not collected. NRT distribution was recorded as a surrogate of NRT compliance. There were no significant differences in NRT distribution as a function of treatment condition. Mean number of patches distributed was 51.5, 44.7, and 47.0 for participants in the SH, CBI, and IC conditions, respectively (p = .172). Mean pieces of gum distributed was 530.9, 467.5, and 471.4 in the SH, CBI, and IC conditions, respectively (p = .757).
Adjustment to HIV and Perceived Health Control
There was no difference in outcome as a function of perceived health control. However, individuals with higher levels of active coping/positive outlook regarding HIV were more likely to quit smoking (p < .05).
CONCLUSIONS
The lack of incremental efficacy for the behavioral treatments is surprising given combined behavioral and pharmacological treatments usually result in higher quit rates than either behavioral or pharmacological treatment alone (Fiore et al., 2008). The lack of differences does not appear to be related to minimal use of the behavioral treatments. The mean number of counseling sessions attended was 3.6 out of 6 (or 60%), and the mean number of visits to the CBI Web site was 3.2. As can been seen in Figure 2 and Table 4, the CBI condition demonstrated higher initial quit rates and a promising odds ratio. Investigation of strategies to further optimize an Internet-based intervention, such as telephone text messaging, may prove useful.
The lack of intervention differences may also be associated with the relatively high quit rates reported by participants in the SH condition. Previous studies using NRT with HIV+ smokers found much lower success rates (Ingersoll et al., 2009; Vidrine et al., 2012). Based on previous research, one would expect about a 10%–12% one-year quit rate for smokers treated with NRT alone, whereas our sample nearly doubled that rate. The participants in the SH condition used slightly more NRT than participants in the other interventions; however, the difference was not significant. In this study, participants obtained NRT in the HIV clinic setting and were not required to go to a distant location (e.g., pharmacy) to obtain medication. It is possible that convenient access to the NRT resulted in increased use, particularly for participants in the control condition. An average of 7 weeks of patches was distributed to this treatment group, which would suggest about 70% adherence. This seems to be a fairly good level of adherence. However, adherence to NRT is rarely reported in the literature, so a reliable reference group is unavailable (Ferguson, Shiffman, & Gitchell, 2011). Further research is certainly warranted.
Regardless of treatment condition, we found that those employed, those who reported a greater desire to quit, or those with lower mood disturbance scores were more likely to achieve abstinence. Strategies to enhance desire to quit (motivational interventions) and address mood disturbances (pharmacological and behavioral treatments) as a part of smoking treatment should be considered. Employment may be associated with several variables supportive of behavior change, for example, self-efficacy, reduced stress, financial/housing stability, and so on. In HIV+ populations, employment may also be an indicator of health status. If so, healthier HIV-infected smokers may be more motivated to stay quit and more optimistic about the benefits of quitting. We do not have the data to examine this possibility in this study. Future research should explore the contribution of employment and associated variables.
We found some support for our secondary hypotheses. Although perceived health control was not related to abstinence, we found that adjustment to HIV was related to successful quitting. Individuals with higher levels of active coping/positive outlook regarding HIV were more likely to quit smoking than those with lower levels. It may be that individuals with a more positive outlook are more likely to consider smoking a significant health issue that needs to be addressed. Use of active coping skills in dealing with HIV may easily transfer or generalize to smoking cessation strategies.
Our study is not without limitations. We did not have HIV-related health data that may impact smoking cessation motivation and outcome. Our sample was recruited from public health settings, so the findings may not generalize to individuals of higher socioeconomic status or those with insurance. Also, the study was not designed to examine differential efficacy of the treatments as a function of targeted content. Additional research in these areas is recommended.
Our findings regarding the characteristics of this sample are consistent with other reports of HIV+ smokers, describing a complex medical group. HIV+ smokers face a variety of psychological, environmental, and economic challenges that are associated with high risks for smoking and lower levels of treatment success. A large proportion of this sample was unemployed, had extremely low incomes, and had unstable living situations. In addition, a significant proportion of the sample report current alcohol and illicit drug use, which have been associated with smoking treatment failure (Humfleet, Munoz, Sees, Reus, & Hall, 1999). Participants also had high rates of lifetime major depressive episodes, bipolar disorders, and alcohol dependence, all associated with tobacco use and smoking treatment failure. Future research should consider these variables when developing strategies to assist this group of smokers.
Although we did not find significant differences in outcome between our experimental treatment conditions, the overall abstinence rates are comparable to those found in studies of NRT plus behavioral interventions across multiple populations (Fiore et al., 2008). This differs from other studies of HIV+ smokers finding lower cessation rates with NRT. At a minimum, the results indicate that smoking cessation treatment is feasible and potentially efficacious in HIV clinical care settings. Further research is critical to our understanding of smoking cessation in this unique population.
FUNDING
This work was supported by NIDA grants (P50-DA09253, R01-DA15791, and R01-DA02538) and California TRDRP grant (15RT-0165).
DECLARATION OF INTERESTS
There are no competing interests to declare for any of the authors.
ACKNOWLEDGMENTS
The authors would like to acknowledge George Harrison, M.D., Meg Newman, M.D., Kevin Kelley, M.A., Barb Adler, M.A., Jeannie Little, M.S.W. as well as the staffs of the AIDS Health Project, the San Francisco General Positive Health Program, and the Tenderloin Health Center for their assistance and support in successfully completing this project.
REFERENCES
- Arnsten J. H., Li X., Mizuno Y., Knowlton A. R., Gourevitch M. N., Handley K, … for the INSPIRE Study Team (2007). Factors associated with antiretroviral therapy adherence and medication errors among HIV-infected injection drug users. Journal of Acquired Immune Deficiency Syndromes, 46(2), S64–S71.10.1097/QAI.0b013e31815767d6 [DOI] [PubMed] [Google Scholar]
- Barclay T. R., Hinkin C. H., Castellon S. A., Mason K. I., Reinhard M. J., Marion S. D, … Durvasula R. S. (2007). Age-associated predictors of medication adherence in HIV-positive adults: Health beliefs, self-efficacy, and neurocognitive status. Health Psychology, 26, 40–49.10.1037/0278-6133.26.1.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter A. W., Soltanpoor N., Swan A. V., Birnbaum W., Johnson N. W., Teo C. G. (1996). Risk factors associated with Epstein-Barr virus replication in oral epithelial cells of HIV-infected individuals. AIDS, 10(9), 935–940.10.1097/00002030-199610090-00002 [DOI] [PubMed] [Google Scholar]
- Burkhalter J. E., Springer C. M., Chhabra R., Ostroff J. S., Rapkin B. D. (2005). Tobacco use and readiness to quit smoking in low-income HIV-infected persons. Nicotine & Tobacco Research, 7(4), 511–522.10.1080/14622200500186064 [DOI] [PubMed] [Google Scholar]
- Burns D. N., Hillman D., Neaton J. D., Sherer R., Mitchell T., Capps L, … Gordin F. M. (1996). Cigarette smoking, bacterial pneumonia, and other clinical outcomes in HIV-1 infection. Terry Beirn Community Programs for Clinical Research on AIDS. Journal of Acquired Immune Deficiency Syndrome and Human Retrovirology, 13(4), 374–383. 10.1097/ 00042560-199612010-00012 [DOI] [PubMed] [Google Scholar]
- Burns D. N., Kramer A., Yellin F., Fuchs D., Wachter H., DiGioia R. A, … Biggar R. J. (1991). Cigarette smoking: A modifier of human immunodeficiency virus type 1 infection? Journal of Acquired Immune Deficiency Syndromes, 4(1), 76–83. 10.1097/00126334-199101000-00011 [PubMed] [Google Scholar]
- Center for Disease Control (2007). Cigarette smoking among adults - United States, 2006. MMWR, 56(44), 1157–1161 [PubMed] [Google Scholar]
- Civljak M., Sheikh A., Stead L. F., Car J. (2010). Internet-based interventions for smoking cessation. Cochrane Database of Systematic Reviews, (9), CD007078.10.1002/14651858.CD007078.pub3 [DOI] [PubMed] [Google Scholar]
- Cohen S., Kamarck T., Mermelstein R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24, 385–396.10.2307/2136404 [PubMed] [Google Scholar]
- Collins R. L., Kanouse D. E., Gifford A. L., Senterfitt J. W., Schuster M. A., McCaffrey D. F, … Wenger N. S. (2001). Changes in health-promoting behavior following diagnosis with HIV: Prevalence and correlates in a national probability sample. Health Psychology, 20(5), 351–360.10.1037/0278-6133.20.5.351 [PubMed] [Google Scholar]
- Conley L. J., Bush T. J., Buchbinder S. P., Penley K. A., Judson F. N., Holmberg S. D. (1996). The association between cigarette smoking and selected HIV-related medical conditions. AIDS, 10(10), 1121–1126.10.1037/0278-6133.20.5.351 [PubMed] [Google Scholar]
- Darke S., Hall W., Heather N., Ward J., Wodak A. (1991). The reliability and validity of a scale to measure HIV risk-taking behaviour among intravenous drug users. AIDS, 5(2), 181–185.10.1097/00002030-199102000-00008 [DOI] [PubMed] [Google Scholar]
- Daughton D., Susman J., Sitorius M., Belenky S., Millatmal T., Nowak R, … Rennard S. (1998). Transdermal nicotine therapy and primary care: Importance of counseling, demographic, and participant selection factors on 1-year quit rates. Archives of Family Medicine, 7, 425–430. 10.1001/archfami.7.5.425 [DOI] [PubMed] [Google Scholar]
- Diaz P. T., King M. A., Pacht E. R., Wewers M. D., Gadek J. E., Nagaraja H. N, … Clanton T. L. (2000). Increased susceptibility to pulmonary emphysema among HIV-seropositive smokers. Annals of Internal Medicine, 132(5), 369–372. 10.1378/chest.117.5_suppl_1.285S [DOI] [PubMed] [Google Scholar]
- DiClemente C. C., Prochaska J. O., Fairhurst S. K., Velicer W. F., Velasquez M. M., Rossi J. S. (1991). The process of smoking cessation: An analysis of precontemplation, contemplation, and preparation stages of change. Journal of Consulting and Clinical Psychology, 59(2), 295–304.10.1037//0022-006X.59.2.295 [DOI] [PubMed] [Google Scholar]
- FastMark (1998). How to quit smoking (1st ed.). Palo Alto, CA: FastMark; [Google Scholar]
- Ferguson S. G., Shiffman S., Gitchell J. G. (2011). Nicotine replacement therapies: Patient safety and persistence. Patient Related Outcome Measures, 2, 111–117. 10.2147/PROM.S11545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore M. C., Jaen C. R., Baker T. B., Bailey W. C., Benowitz N. L., Curry S. J., Dorfman S. F. (2008). Treating tobacco use and dependence: 2008 Update. Clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services; [Google Scholar]
- Greenspan J. S., Barr C. E., Sciubba J. J., Winkler J. R. (1992). Oral manifestations of HIV infection. Definitions, diagnostic criteria, and principles of therapy. The U.S.A. Oral AIDS Collaborative Group. Oral Surgery, Oral Medicine, and Oral Pathology, 73(2), 142–144.10.1016/0030-4220(92)90185-S [DOI] [PubMed] [Google Scholar]
- Gritz E. R., Vidrine D. J., Lazev A. B., Amick B. C., Arduino R. C. (2004). Smoking behavior in a low-income multiethnic HIV/AIDS population. Nicotine & Tobacco Research, 6(1), 71–77.10.1080/14622200310001656885 [DOI] [PubMed] [Google Scholar]
- Hall S. M., Havassy B. E., Wasserman D. A. (1990). Commitment to abstinence and acute stress in relapse to alcohol, opiates, and nicotine. Journal of Consulting and Clinical Psychology, 58(2), 175–181.10.1037//0022-006X.58.2.175 [DOI] [PubMed] [Google Scholar]
- Hall S. M., Humfleet G. L., Muñoz R. F., Reus V. I., Robbins J. A., Prochaska J. J. (2009). Extended treatment of older cigarette smokers. Addiction, 104(6), 1043–1052.10.1111/j.1360-0443.2009.02548.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S. M., Humfleet G. L., Reus V. I., Muñoz R. F., Cullen J. (2004). Extended nortriptyline and psychological treatment for cigarette smoking. American Journal of Psychiatry, 161(11), 2100–2107.10.1176/appi.ajp. 161.11.2100 [DOI] [PubMed] [Google Scholar]
- Hall S. M., Humfleet G. L., Reus V. I., Munoz R. F., Hartz D. T., Maude-Griffin R. (2002). Psychological intervention and antidepressant treatment in smoking cessation. Archives of General Psychiatry, 59(10), 930–936. 10.1001/archpsyc.59.10.930 [DOI] [PubMed] [Google Scholar]
- Hirschtick R. E., Glassroth J., Jordan M. C., Wilcosky T. C., Wallace J. M., Kvale P. A, … Hopewell P. C. (1995). Bacterial pneumonia in persons infected with the human immunodeficiency virus. Pulmonary complications of HIV Infection Study Group. New England Journal of Medicine, 333(13), 845–851.10.1056/NEJM199509283331305 [DOI] [PubMed] [Google Scholar]
- Hughes J. R., Keely J. P., Niaura R. S., Ossip-Klein D. J., Richmond R. L., Swan G. E. (2003). Measures of abstinence in clinical trials: Issues and recommendations. Nicotine & Tobacco Research, 5(1), 13–25.10.1080/14622200307270 [PubMed] [Google Scholar]
- Humfleet G., Hall S., Sees K. L., Reus V., Munoz R., Hartz D. (2002). Brief versus extended smoking treatment: Preliminary findings. Quebec City, QC, Canada: College on Problems in Drug Dependence; [Google Scholar]
- Humfleet G., Muñoz R., Sees K., Reus V., Hall S. (1999). History of alcohol or drug problems, current use of alcohol or marijuana, and success in quitting smoking. Addictive Behaviors, 24(1), 149–154.10.1016/S0306-4603(98)00057-4 [DOI] [PubMed] [Google Scholar]
- Ingersoll K. S., Cropsey K. L., Heckman C. J. (2009). A test of motivational plus nicotine replacement interventions for HIV positive smokers. AIDS and Behavior, 13(3), 545–554.10.1007/s10461-007-9334-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. O., Neilands T. B., Dilworth S. E., Morin S. F., Remien R. H., Chesney M. A. (2007). The role of self-efficacy in HIV treatment adherence: Validation of the HIV Treatment Adherence Self-Efficacy Scale (HIV-ASES). Journal of Behavioral Medicine, 30(5), 359–370.10.1007/s10865-007-9118-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph A. M., Antonnucio D. O. (1999). Lack of efficacy of transdermal nicotine in smoking cessation. New England Journal of Medicine, 335, 1157–1158. 10.1056/NEJM199910073411514 [DOI] [PubMed] [Google Scholar]
- Kelley B., Raphael B., Burrows G., Judd F., Kernutt G., Burnett P, … Dunne M. (2000). Measuring psychological adjustment to HIV infection. International Journal of Psychiatry, 30, 41–59. 10.2190%2FFK4E-B9VJ-K4UG-0H0R [DOI] [PubMed] [Google Scholar]
- Lando H. A., Rolnick S., Klevan D., Roski J., Cherney L., Lauger G. (1997). Telephone support as an adjunct to transdermal nicotine in smoking cessation. American Journal of Public Health, 87(10), 1670–1674. 10.2105/AJPH.87.10.1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Richardson E. E., Stanton C. A., Papandonatos G. D., Shadel W. G., Stein M., Tashima K, … Niaura R. (2009). Motivation and patch treatment for HIV+ smokers: A randomized controlled trial. Addiction, 104(11), 1891–1900.10.1111/j.1360-0443.2009.02623.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamary E. M., Bahrs D., Martinez S. (2002). Cigarette smoking and the desire to quit among individuals living with HIV. AIDS Patient Care and STDs, 16(1), 39–42.10.1089/108729102753429389 [DOI] [PubMed] [Google Scholar]
- McLellan A. T., Kushner H., Metzger D., Peters R., Smith I., Grissom G, … Argeriou M. (1992). The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment, 9(3), 199–213 [DOI] [PubMed] [Google Scholar]
- McNair D. M., Lorr M., Droppleman L. F. (1992). Manual: Profile of Mood States (POMS)–Revised. San Diego, CA: Educational and Instructional Testing Service; [Google Scholar]
- Palacio H., Hilton J. F., Canchola A. J., Greenspan D. (1997). Effect of cigarette smoking on HIV-related oral lesions. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology, 14(4), 338–342.10.1097/00042560-199704010-00005 [DOI] [PubMed] [Google Scholar]
- Payne T. J., Smith P. O., McCracken L. M., McSherry W. C., Antony M. M. (1994). Assessing nicotine dependence: A comparison of the Fagerström Tolerance Questionnaire (FTQ) with the Fagerström Test for Nicotine Dependence (FTND) in a clinical sample. Addictive Behaviors, 19(3), 307–317.10.1016/0306-4603(94)90032–9 [DOI] [PubMed] [Google Scholar]
- Reardon C. C., Kim S. J., Wagner R. P., Koziel H., Kornfeld H. (1996). Phagocytosis and growth inhibition of Cryptococcus neoformans by human alveolar macrophages: Effects of HIV-1 infection. AIDS, 10(6), 613–618.10.1097/00002030-199606000-00006 [DOI] [PubMed] [Google Scholar]
- Reid R. D., Pipe A., Dafoe W. A. (1999). Is telephone counselling a useful addition to physician advice and nicotine replacement therapy in helping patients to stop smoking? A randomized controlled trial. Canadian Medical Association Journal, 160, 1577–1581 [PMC free article] [PubMed] [Google Scholar]
- Ross M. W., Hunter C. E., Condon J., Collins P., Begley K. (1994). The mental adjustment to HIV scale: Measurement and dimensions of response to AIDS/HIV disease. AIDS Care, 6(4), 407–411.10.1080/09540129408258655 [DOI] [PubMed] [Google Scholar]
- Schneider K. L., Hedeker D., Bailey K. C., Cook J. W., Spring B. (2010). A comment on analyzing addictive behaviors over time. Nicotine & Tobacco Research, 12(4), 445–448.10.1093/ntr/ntq060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. S., Wallston K. A., Smith C. A. (1995). The development and validation of the Perceived Health Competence Scale. Health Education Research, 10(1), 51–64.10.1093/her/10.1.51 [DOI] [PubMed] [Google Scholar]
- Solomon L. J., Scharoun G. M., Flynn B. S., Secker-Walker R. H., Sepinwall D. (2000). Free nicotine patches plus proactive telephone peer support to help low-income women stop smoking. Preventive Medicine, 31, 68–74. 10.1006/pmed.2000.0683 [DOI] [PubMed] [Google Scholar]
- SRNT Subcommittee on Biochemical Verification (2002). Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research, 4(2), 149–159.10.1080/ 14622200210123581 [DOI] [PubMed] [Google Scholar]
- Stapleton J. A., Russell M. A. H., Feyerabend C., Wiseman S. M., Gustavsson G., Sawe U., Wiseman D. (1995). Dose effects and predictors of outcome in a randomized trial of transdermal nicotine patches in general practice. Addiction, 90, 31–42. 10.1111/j.1360-0443.1995.tb01007.x [DOI] [PubMed] [Google Scholar]
- Turner J., Page-Shafer K., Chin D. P., Osmond D., Mossar M., Markstein L, … Pulmonary Complications of HIV Infection Study Group (2001). Adverse impact of cigarette smoking on dimensions of health-related quality of life in persons with HIV infection. AIDS Patient Care and STDs, 15(12), 615–624.10.1089/108729101753354617 [DOI] [PubMed] [Google Scholar]
- Vidrine D. J., Arduino R. C., Lazev A. B., Gritz E. R. (2006). A randomized trial of a proactive cellular telephone intervention for smokers living with HIV/AIDS. AIDS, 20(2), 253–260.10.1097/01.aids.0000198094. 23691.58 [DOI] [PubMed] [Google Scholar]
- Vidrine D. J., Marks R. M., Arduino R. C., Gritz E. R. (2012). Efficacy of cell phone-delivered smoking cessation counseling for persons living with HIV/AIDS: 3-month outcomes. Nicotine & Tobacco Research, 14, 106–110.10.1093/ntr/ntr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallston K. A., Osborn C. Y., Wagner L. J., Hilker K. A. (2011). The Perceived Medical Condition Self-Management Scale applied to persons with HIV/AIDS. Journal of Health Psychology, 16, 109–115.10.1177/1359105310367832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (1997). Composite International Diagnostic Interview (Version 2.1). Geneva: : World Health Organization; [Google Scholar]