Abstract
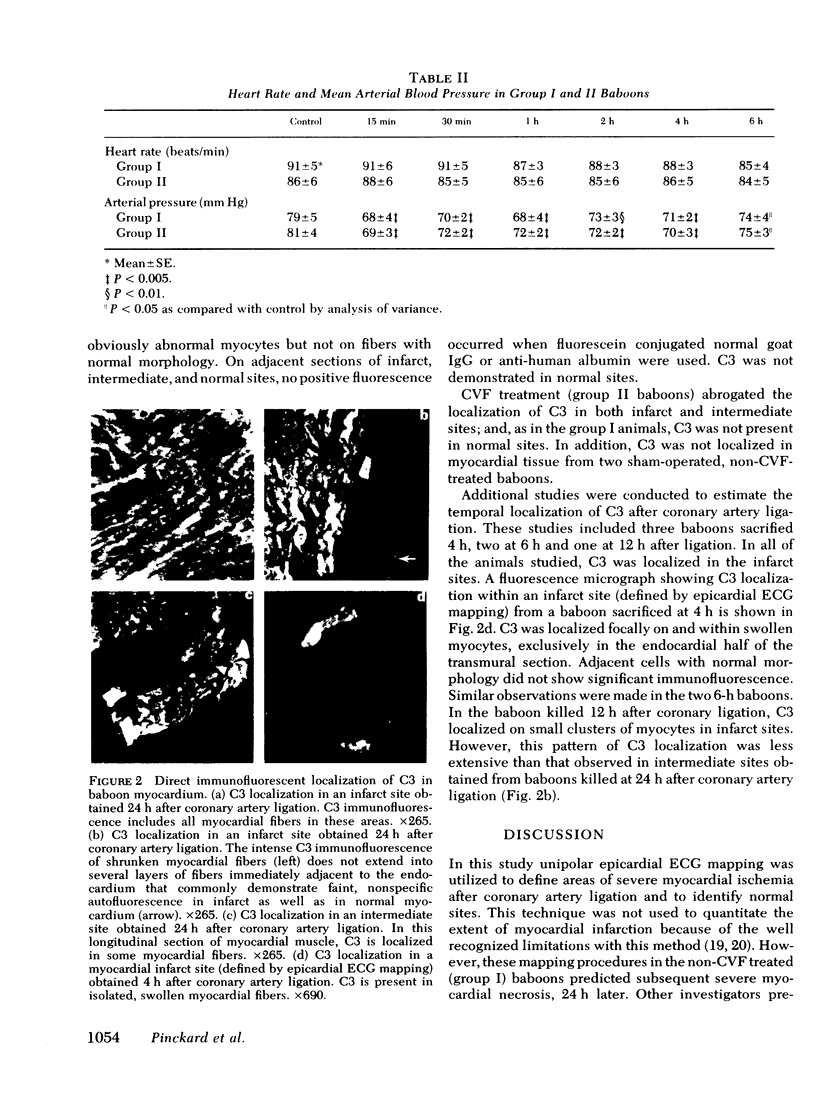
We sought to determine whether the third component of complement (C3) is localized in ischemic baboon myocardium after coronary artery ligation. Furthermore, we assessed the effects of prior C3 depletion on myocardial necrosis. We studied seven control baboons (group I) and seven C3-depleted (group II) baboons that were killed 24 h after ligation of the anterior descending coronary artery. Multiple tissue samples were obtained from infarct, intermediate, and normal myocardial sites as defined by serial unipolar epicardial ECG mapping. In group I baboons, myocardial creatine kinase content from infarct sites was reduced as compared with normal sites (12.6±0.92 [SE] vs. 24.4±0.75 IU/mg protein, P < 0.001). The intermediate sites from group I contained more creatine kinase (19.0±1.25 IU/mg protein) than infarct sites (P < 0.001), but less (P < 0.025) than normal sites. In group II, intermediate sites showed no significant reduction in creatine kinase from normal sites and there was significantly less creatine kinase depletion in infarct sites when compared with group I animals (33.7±4.6 and 51.4±1.8% depletion, respectively, P < 0.001). In all seven group I baboons, uniform C3 localization was observed in infarct sites by direct immunofluorescence but appeared in mosaic patterns in intermediate sites. C3 was not demonstrated in any normal sites, nor in any site from group II baboons. Additional studies on baboons killed at earlier times after ligation indicated that C3 was localized focally on swollen myocytes in infarct sites as early as 4 h after coronary ligation. These results strongly implicate the active participation of the complement system of inflammatory proteins in the pathogenesis of myocardial tissue injury following coronary occlusion.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruyneel K. J. Use of moving epicardial electrodes in defining ST-segment changes after acute coronary occlusion in the baboon. Relation to primary ventricular fibrillation. Am Heart J. 1975 Jun;89(6):731–741. doi: 10.1016/0002-8703(75)90188-x. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G., Müller-Eberhard H. J., Aikin B. S. Depletion of plasma complement in vivo by a protein of cobra venom: its effect on various immunologic reactions. J Immunol. 1970 Jul;105(1):55–69. [PubMed] [Google Scholar]
- Fozzard H. A., DasGupta D. S. ST-segment potentials and mapping. Theory and experiments. Circulation. 1976 Oct;54(4):533–537. doi: 10.1161/01.cir.54.4.533. [DOI] [PubMed] [Google Scholar]
- Giclas P. C., Pinckard R. N., Olson M. S. In vitro activation of complement by isolated human heart subcellular membranes. J Immunol. 1979 Jan;122(1):146–151. [PubMed] [Google Scholar]
- Hill J. H., Ward P. A. The phlogistic role of C3 leukotactic fragments in myocardial infarcts of rats. J Exp Med. 1971 Apr 1;133(4):885–900. doi: 10.1084/jem.133.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis L. D., Askenazi J., Braunwald E., Radvany P., Muller J. E., Fishbein M. C., Maroko P. R. Use of changes in the epicardial QRS complex to assess interventions which modify the extent of myocardial necrosis following coronary artery occlusion. Circulation. 1976 Oct;54(4):591–598. doi: 10.1161/01.cir.54.4.591. [DOI] [PubMed] [Google Scholar]
- Holland R. P., Brooks H. TQ-ST segment mapping: critical review and analysis of current concepts. Am J Cardiol. 1977 Jul;40(1):110–129. doi: 10.1016/0002-9149(77)90109-6. [DOI] [PubMed] [Google Scholar]
- Maclean D., Fishbein M. C., Braunwald E., Maroko P. R. Long-term preservation of ischemic myocardium after experimental coronary artery occlusion. J Clin Invest. 1978 Mar;61(3):541–551. doi: 10.1172/JCI108965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroko P. R., Braunwald E. Modification of myocardial infarction size after coronary occlusion. Ann Intern Med. 1973 Nov;79(5):720–733. doi: 10.7326/0003-4819-79-5-720. [DOI] [PubMed] [Google Scholar]
- Maroko P. R., Carpenter C. B., Chiariello M., Fishbein M. C., Radvany P., Knostman J. D., Hale S. L. Reduction by cobra venom factor of myocardial necrosis after coronary artery occlusion. J Clin Invest. 1978 Mar;61(3):661–670. doi: 10.1172/JCI108978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroko P. R., Kjekshus J. K., Sobel B. E., Watanabe T., Covell J. W., Ross J., Jr, Braunwald E. Factors influencing infarct size following experimental coronary artery occlusions. Circulation. 1971 Jan;43(1):67–82. doi: 10.1161/01.cir.43.1.67. [DOI] [PubMed] [Google Scholar]
- Maroko P. R., Libby P., Sobel B. E., Bloor C. M., Sybers H. D., Shell W. E., Covell J. W., Braunwald E. Effect of glucose-insulin-potassium infusion on myocardial infarction following experimental coronary artery occlusion. Circulation. 1972 Jun;45(6):1160–1175. doi: 10.1161/01.cir.45.6.1160. [DOI] [PubMed] [Google Scholar]
- Page D. L., Caulfield J. B., Kastor J. A., DeSanctis R. W., Sanders C. A. Myocardial changes associated with cardiogenic shock. N Engl J Med. 1971 Jul 15;285(3):133–137. doi: 10.1056/NEJM197107152850301. [DOI] [PubMed] [Google Scholar]
- Pinckard R. N., Olson M. S., Giclas P. C., Terry R., Boyer J. T., O'Rourke R. A. Consumption of classical complement components by heart subcellular membranes in vitro and in patients after acute myocardial infarction. J Clin Invest. 1975 Sep;56(3):740–750. doi: 10.1172/JCI108145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. F., Deems R. A., Mincey T. C., Dennis E. A. Chemical modification of the histidine residue in phospholipase A2 (Naja naja naja). A case of half-site reactivity. J Biol Chem. 1977 Apr 10;252(7):2405–2411. [PubMed] [Google Scholar]
- Rosalki S. B. An improved procedure for serum creatine phosphokinase determination. J Lab Clin Med. 1967 Apr;69(4):696–705. [PubMed] [Google Scholar]



