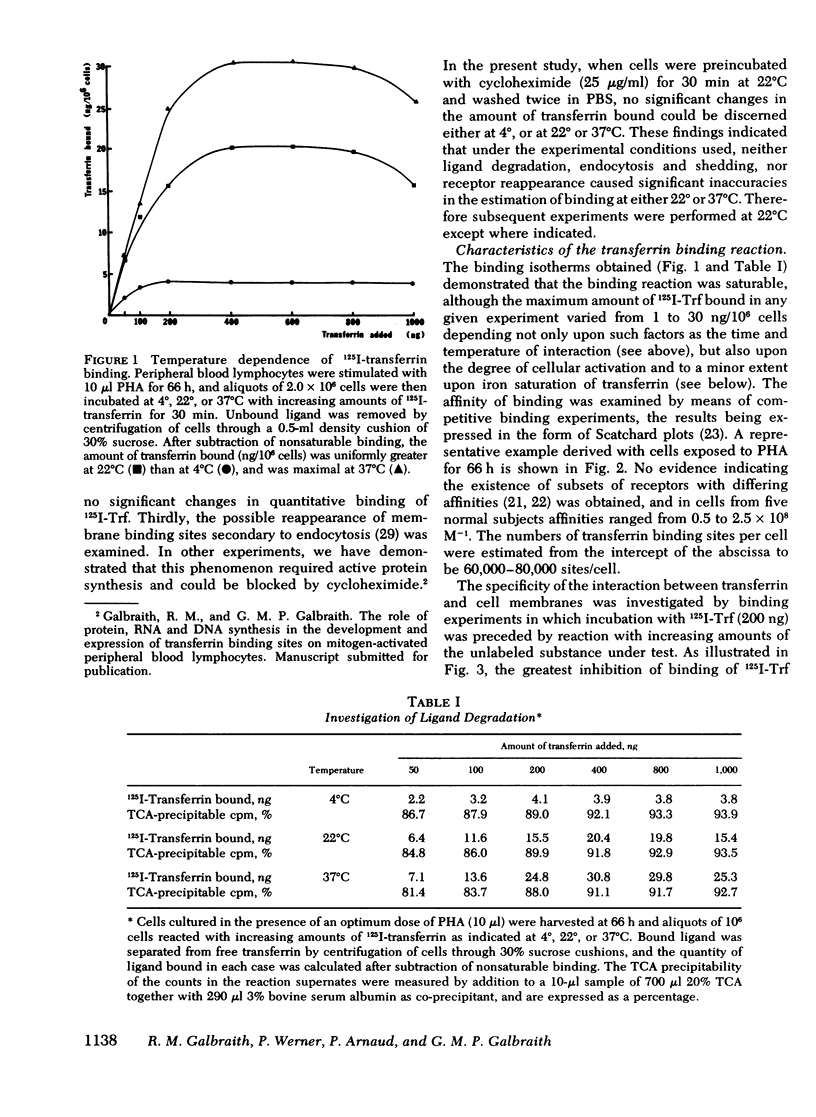
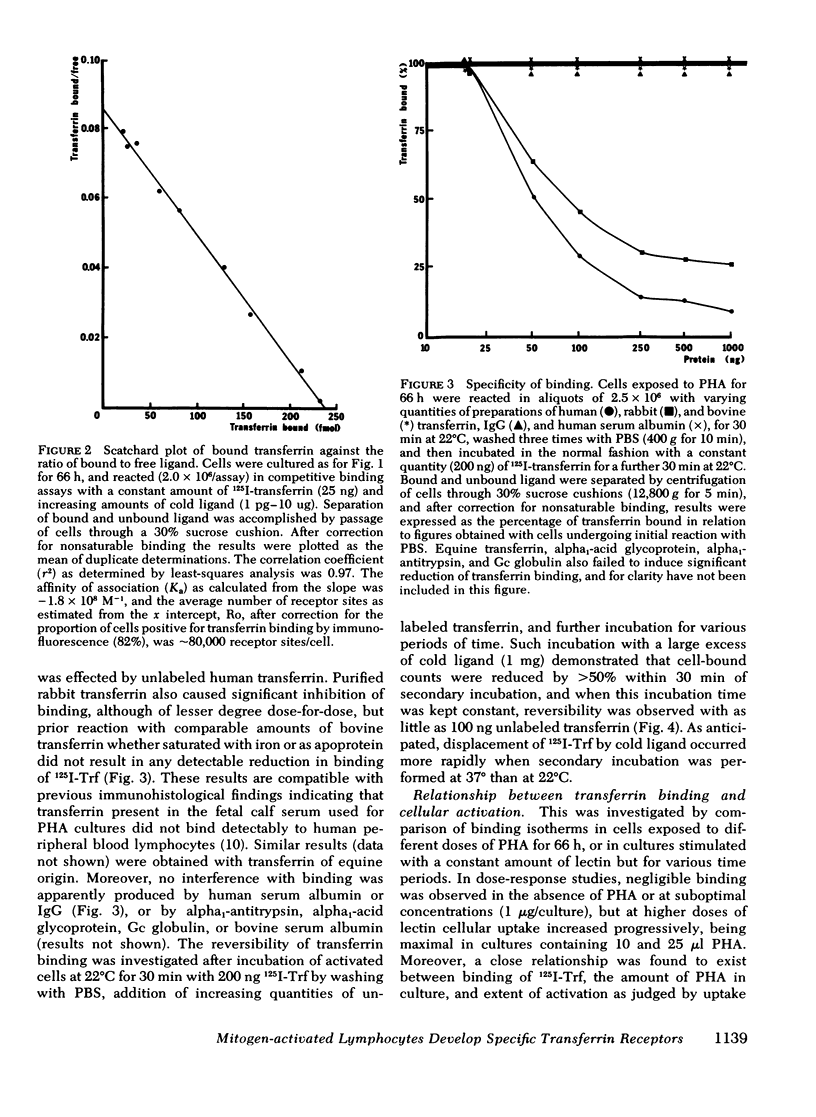
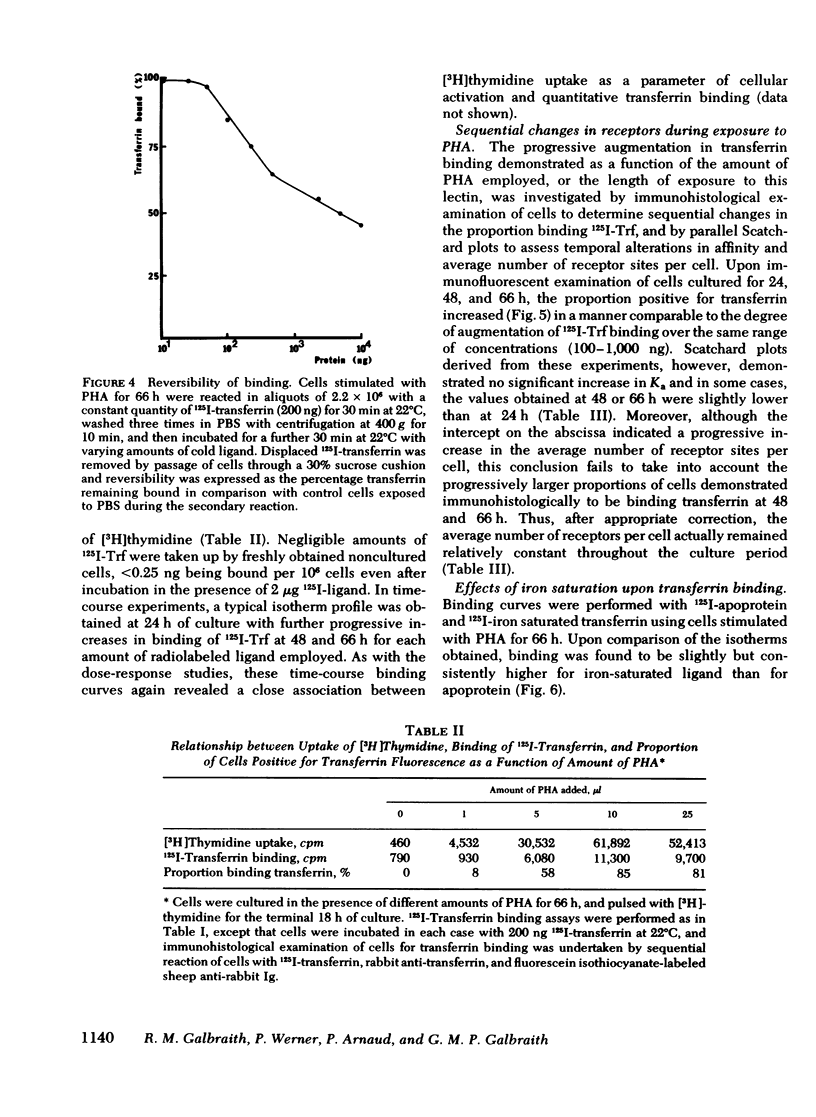
Abstract
Immunohistological studies have indicated that membrane sites binding transferrin are present upon activated human peripheral blood lymphocytes. In this study, we have investigated transferrin uptake in human lymphocytes exposed to phytohemagglutinin (PHA), by quantitative radiobinding and immunofluorescence in parallel. In stimulated lymphocytes, binding was maximal after a 30-min incubation, being greatest at 37°C, and greater at 22°C than at 4°C. Although some shedding and endocytosis of transferrin occurred at 22° and 37°C, these factors, and resulting synthesis of new sites, did not affect measurement of binding which was found to be saturable, reversible, and specific for transferrin (Ka 0.5-2.5 × 108 M−1). Binding was greater after a 48-h exposure to PHA than after 24 h, and was maximal at 66 h. Sequential Scatchard analysis revealed no significant elevation in affinity of interaction. However, although the total number of receptors increased, the proportion of cells in which binding of ligand was detected immunohistologically increased in parallel, and after appropriate correction, the cellular density of receptors remained relatively constant throughout (60,000-80,000 sites/cell). Increments in binding during the culture period were thus due predominantly to expansion of a population of cells bearing receptors. Similar differences in binding were apparent upon comparison of cells cultured in different doses of PHA, and in unstimulated cells binding was negligible. Transferrin receptors appear, therefore, to be readily detectable only upon lymphocytes that have been activated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaud P., Galbraith R. M., Galbraith G. M., Allen R. C., Fudenberg H. H. A new allele of human alpha1-antitrypsin: PiNhampton. Am J Hum Genet. 1978 Nov;30(6):653–659. [PMC free article] [PubMed] [Google Scholar]
- Arnaud P., Wilson G. B., Koistinen J., Fudenberg H. H. Immunofixation after electrofocusing: improved method for specific detection of serum proteins with determination of isoelectric points. I. Immunofixation print technique for detection of alpha-1-protease inhibitor. J Immunol Methods. 1977;16(3):221–231. doi: 10.1016/0022-1759(77)90200-9. [DOI] [PubMed] [Google Scholar]
- Baker E., Morgan E. H. The kinetics of the interaction between rabbit transferrin and reticulocytes. Biochemistry. 1969 Mar;8(3):1133–1141. doi: 10.1021/bi00831a046. [DOI] [PubMed] [Google Scholar]
- Baker E., Morgan E. H. The role of iron in the reaction between rabbit transferrin and reticulocytes. Biochemistry. 1969 Jul;8(7):2954–2958. doi: 10.1021/bi00835a040. [DOI] [PubMed] [Google Scholar]
- Brown C. P., Galbraith R. M., Galbraith G. M. Recent progress in immunohistology. Ann Clin Lab Sci. 1980 Jan-Feb;10(1):1–8. [PubMed] [Google Scholar]
- Cambiaso C. L., Goffinet A., Vaerman J. P., Heremans J. F. Glutaraldehyde-activated aminohexyl- derivative of Sepharose 4B as a new verstile immunoabsorbent. Immunochemistry. 1975 Apr;12(4):273–278. doi: 10.1016/0019-2791(75)90175-5. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Hollenberg M. D., Chang K. J., Bennett V. Hormone receptor complexes and their modulation of membrane function. Recent Prog Horm Res. 1975;31:37–94. doi: 10.1016/b978-0-12-571131-9.50006-2. [DOI] [PubMed] [Google Scholar]
- Galbraith G. M., Galbraith R. M., Faulk W. P. Immunological studies of transferrin and transferrin receptors of human placental trophoblast. Placenta. 1980 Jan-Mar;1(1):33–46. doi: 10.1016/s0143-4004(80)80014-2. [DOI] [PubMed] [Google Scholar]
- Galbraith G. M., Galbraith R. M., Faulk W. P. Transferrin binding by human lymphoblastoid cell lines and other transformed cells. Cell Immunol. 1980 Jan;49(1):215–222. doi: 10.1016/0008-8749(80)90072-6. [DOI] [PubMed] [Google Scholar]
- Galbraith G. M., Galbraith R. M., Temple A., Faulk W. P. Demonstration of transferrin receptors on human placental trophoblast. Blood. 1980 Feb;55(2):240–242. [PubMed] [Google Scholar]
- Galbraith G. M., Goust J. M., Mercurio S. M., Galbraith R. M. Transferrin binding by mitogen-activated human peripheral blood lymphocytes. Clin Immunol Immunopathol. 1980 Aug;16(4):387–395. doi: 10.1016/0090-1229(80)90180-4. [DOI] [PubMed] [Google Scholar]
- Galbraith R. A., Wise C., Buse M. G. Insulin binding and response in erythroblastic leukemic cells. Diabetes. 1980 Jul;29(7):571–578. doi: 10.2337/diab.29.7.571. [DOI] [PubMed] [Google Scholar]
- Hamilton T. A., Wada H. G., Sussman H. H. Identification of transferrin receptors on the surface of human cultured cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6406–6410. doi: 10.1073/pnas.76.12.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANDL J. H., KATZ J. H. The plasma-to-cell cycle of transferrin. J Clin Invest. 1963 Mar;42:314–326. doi: 10.1172/JCI104718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karonen S. L., Mörsky P., Siren M., Seuderling U. An enzymatic solid-phase method for trace iodination of proteins and peptides with 125-iodine. Anal Biochem. 1975 Jul;67(1):1–10. doi: 10.1016/0003-2697(75)90266-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larrick J. W., Cresswell P. Transferrin receptors on human B and T lymphoblastoid cell lines. Biochim Biophys Acta. 1979 Apr 3;583(4):483–490. doi: 10.1016/0304-4165(79)90065-5. [DOI] [PubMed] [Google Scholar]
- Leibman A., Aisen P. Distribution of iron between the binding sites of transferrin in serum: methods and results in normal human subjects. Blood. 1979 Jun;53(6):1058–1065. [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métais P., Schirardin H., Warter J. Mesures de la capacité d'inhibition trypsique du sérum humain. Clin Chim Acta. 1966 May;13(5):602–610. doi: 10.1016/0009-8981(66)90164-1. [DOI] [PubMed] [Google Scholar]
- Phillips J. L., Azari P. Effect of iron transferrin on nucleic acid synthesis in phytohemagglutinin-stimulated human lymphocytes. Cell Immunol. 1975 Jan;15(1):94–99. doi: 10.1016/0008-8749(75)90167-7. [DOI] [PubMed] [Google Scholar]
- Phillips J. L. Specific binding of zinc transferrin to human lymphocytes. Biochem Biophys Res Commun. 1976 Sep 20;72(2):634–639. doi: 10.1016/s0006-291x(76)80087-3. [DOI] [PubMed] [Google Scholar]
- Roth J., Kahn C. R., Lesniak M. A., Gorden P., De Meyts P., Megyesi K., Neville D. M., Jr, Gavin J. R., 3rd, Soll A. H., Freychet P. Receptors for insulin, NSILA-s, and growth hormone: applications to disease states in man. Recent Prog Horm Res. 1975;31:95–139. doi: 10.1016/b978-0-12-571131-9.50007-4. [DOI] [PubMed] [Google Scholar]
- Saravis C. A., Zamcheck N. Isoelectric focusing in agarose. J Immunol Methods. 1979;29(1):91–96. doi: 10.1016/0022-1759(79)90130-3. [DOI] [PubMed] [Google Scholar]
- Seligman P. A., Schleicher R. B., Allen R. H. Isolation and characterization of the transferrin receptor from human placenta. J Biol Chem. 1979 Oct 25;254(20):9943–9946. [PubMed] [Google Scholar]
- Svendsen P. J. Fused rocket immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:69–70. doi: 10.1111/j.1365-3083.1973.tb03781.x. [DOI] [PubMed] [Google Scholar]
- Tormey D. C., Mueller G. C. Biological effects of transferrin on human lymphocytes in vitro. Exp Cell Res. 1972 Sep;74(1):220–226. doi: 10.1016/0014-4827(72)90500-9. [DOI] [PubMed] [Google Scholar]
- Wada H. G., Hass P. E., Sussman H. H. Transferrin receptor in human placental brush border membranes. Studies on the binding of transferrin to placental membrane vesicles and the identification of a placental brush border glycoprotein with high affinity for transferrin. J Biol Chem. 1979 Dec 25;254(24):12629–12635. [PubMed] [Google Scholar]
- van der Heul C., Kroos M. J., van Eijk H. G. Binding sites of iron transferrin on rat reticulocytes. Inhibition by specific antibodies. Biochim Biophys Acta. 1978 Aug 17;511(3):430–441. doi: 10.1016/0005-2736(78)90279-1. [DOI] [PubMed] [Google Scholar]