Abstract
While the general understanding of muscle regenerative capacity is that it declines with increasing age due to impairments in the number of muscle progenitor cells and interaction with their niche, studies vary in their model of choice, indices of myogenic repair, muscle of interest and duration of studies. We focused on the net outcome of regeneration, functional architecture, compared across three models of acute muscle injury to test the hypothesis that satellite cells maintain their capacity for effective myogenic regeneration with age. Muscle regeneration in extensor digitorum longus muscle (EDL) of young (3 mo-old), old (22 mo-old) and senescent female mice (28 mo-old) was evaluated for architectural features, fiber number and central nucleation, weight, collagen and fat deposition. The 3 injury paradigms were: a myotoxin (notexin) which leaves the blood vessels and nerves intact, freezing (FI) that damages local muscle, nerve and blood vessels and denervation-devascularization (DD) which dissociates the nerves and blood vessels from the whole muscle. Histological analyses revealed successful architectural regeneration following notexin injury with negligible fibrosis and fully restored function, regardless of age. In comparison, the regenerative response to injuries that damaged the neurovascular supply (FI and DD) was less effective, but similar across the ages. The focus on net regenerative outcome demonstrated that old and senescent muscle has a robust capacity to regenerate functional architecture.
Keywords: skeletal muscle, aging, progenitor cells, fiber branching, contractility
Introduction
The function of skeletal muscle is directly determined by its architecture; the numbers and size of muscle fibers and relative contribution of fibrotic tissue and fat. Regeneration is a key process in skeletal muscle in which competition between these different cell types to populate the regenerated muscle significantly impacts muscle function. The regenerative process in skeletal muscle is understood to be altered during aging with some reports concluding that it is impaired1-4 and reports that it is delayed, but resolves.5,6 The field has debated relative roles of cell-intrinsic and/or cell-extrinsic changes responsible for declining regenerative potential with increasing age and focused on the role(s) of the muscle-resident stem cell, the satellite cell.1,2,4,7,8 Most studies of rodent muscle report a reduction in satellite cell number with increasing age which may underpin impaired regeneration.9-11 Numerous reports have employed a wide range of assays of muscle regeneration to investigate the time-course of the process, regenerative capacity in health and disease and the temporal and spatial relationship of myogenesis to inflammation and re-innervation. However, significant variations across those studies, related to injury protocol, animal strain, sex and age, use of parabiotic pairs, and the preservation of the neurovascular supply continue to obscure a clear understanding of whether and how aging per se, affects regeneration capacity in muscle.
In the present study, we used three muscle acute injury paradigms, denervation-devascularization (DD), freeze (FI) and notexin, to evaluate the differential impact on the outcome of regeneration of functional architecture from neurovascular damage at different ages. We employed DD injury which requires complete re-establishment of vascular perfusion and re-innervation of the muscle for successful regeneration.12-14 By comparison, freeze injury causes localized muscle damage and destroys only local nerves and vasculature at the site of injury15 and results in partial neurovascular damage and peri-regional fibrosis.2 In contrast to such “physically inclusive” injuries that impact multiple aspects of the muscle architecture, myotoxins such as notexin cause fiber damage through depolarization and sarcolemmal degradation and importantly leave intact architectural scaffolds including the basal lamina and associated nerves, neuromuscular junctions, intramuscular vessels and satellite cells.16,17 These highly effective scaffolds ensure coordination of debris removal by macrophages and regeneration by satellite cells to form muscle with near-normal architecture.18 Analyses of these three muscle injury paradigms allowed us to address contrasting reports of impaired vs. delayed regeneration in aging muscle.
To investigate age-related changes in regenerative capacity of muscle, we focused on the net outcome of regeneration rather than the process. That outcome, restoration of normal muscle structure (and in one case, function) was examined comparatively using injury paradigms that maintain neurovascular supply (notexin) or damage to the neurovascular supply (FI or DD). The study employed young, old and senescent mice at 3, 22 and 28 mo of age, respectively, in a strain that demonstrates atrophy and sarcopenia by 18 mo of age.19,20 At all three ages, muscles displayed full architectural regeneration after notexin injury, as revealed by histomorphometry and contractility experiments. Freeze injury produced regional damage and commensurate regeneration. DD, in contrast, showed delayed regeneration and collagen deposition. Results indicate that old and even senescent muscles maintain robust intrinsic regenerative responses to injury that are comparable to young muscle.
Results
Muscle histology in aging
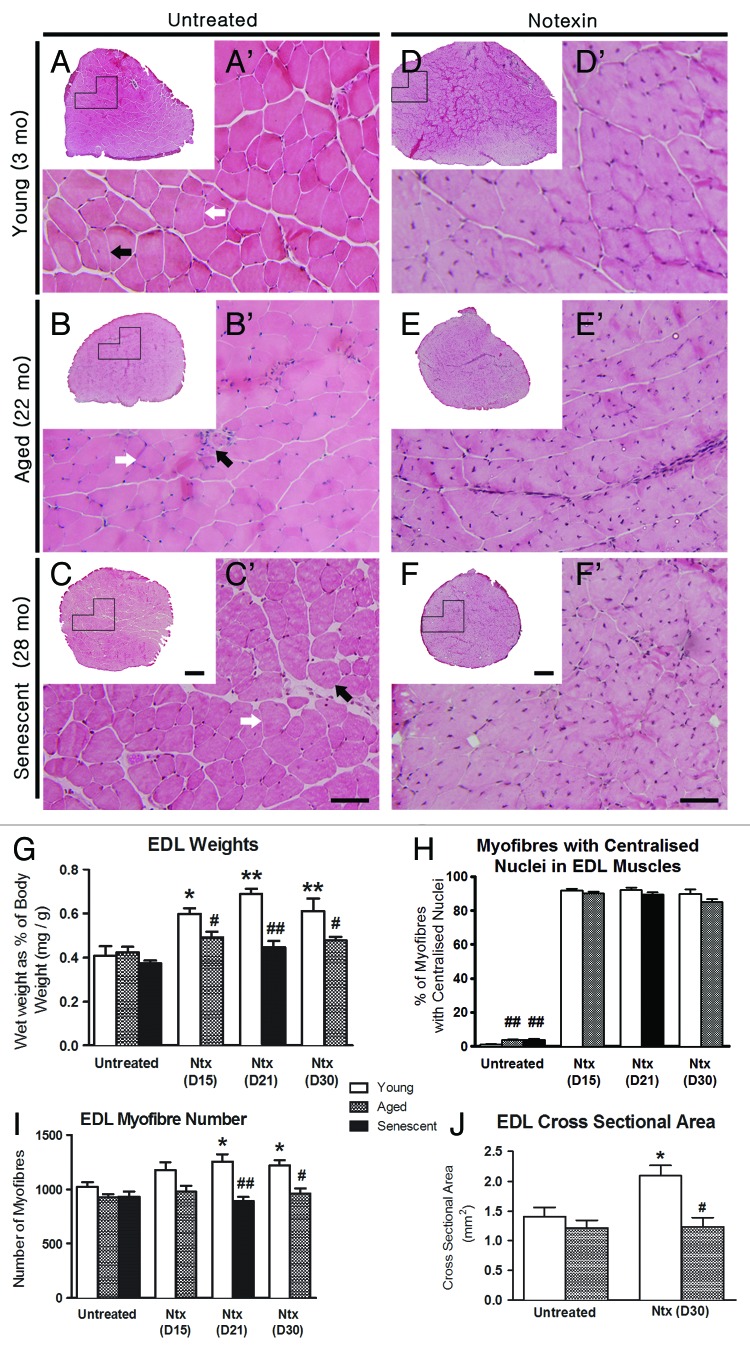
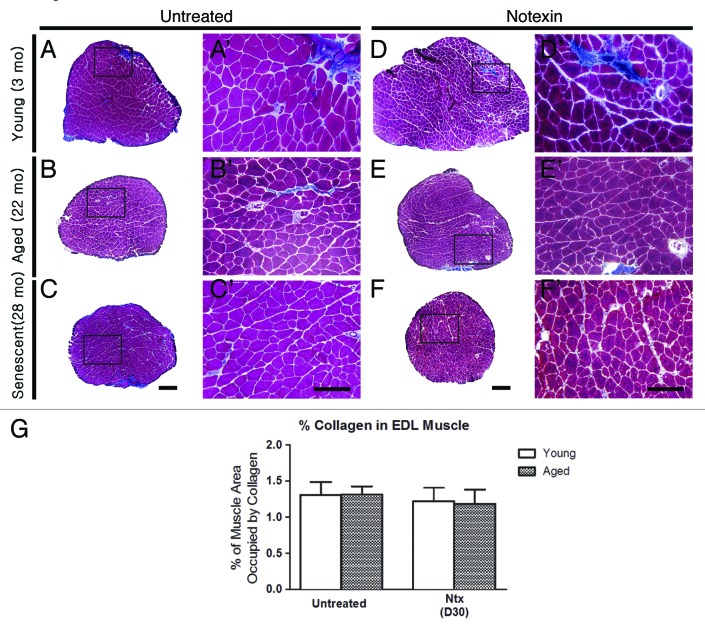
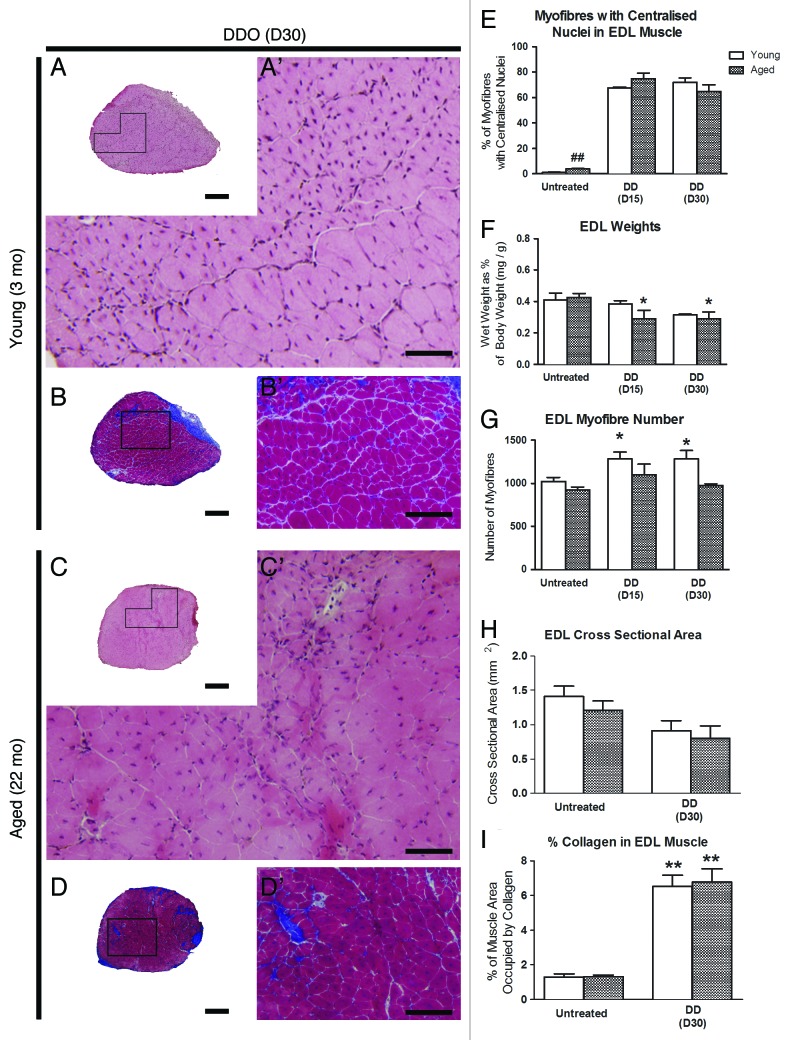
Control uninjured muscles displayed typical histological features (Fig. 1 and 2) of polygonal fibers within fascicles outlined with thinly deposited collagen (Fig. 2), the percent area of which did not change significantly with age (Fig. 2G). Muscle weight relative to BW were similar at all ages (Fig. 1G), likely since muscle weight peaks at about 15 mo (6; Leiter and Anderson, unpublished) and then declines such that muscles in the old and senescent mice are sarcopenic.19,20 As well, EDLs from 22 and 28 mo-old mice showed a small increase in the proportion of fibers with central nuclei from 0.5% at 3 mo to 4–5% in aged and senescent mice (p < 0.01, Fig. 1H). This may be due to regeneration caused by general “wear-and-tear”14,21 or alternatively, as a result of a process involving partial myofiber denervation, motor unit remodelling and maladaptive axonal sprouting associated with satellite cell proliferation and fusion that occurs in aged muscle.22-24
Figure 1. Regenerative response to notexin-induced injury does not differ by age. Upper panel shows representative H&E-stained sections of EDL from mice at 3 (top), 22 (middle) and 28 mo of age (bottom) mice. The left panel is untreated controls (A-C); the right panel is notexin-treated EDLs (D-F) 30 d (3 mo, 22 mo) or 21 d after notexin injury (28 mo). Images A’-F’ are higher magnifications of the boxed regions in A-F, respectively. In untreated EDLs, myofibers primarily show peripheral nuclei (white arrows) with occasional central nuclei (black arrows) at all ages. In treated EDLs, approximately 90% of myofibers show central nuclei. Data on nuclear location were collected at a higher magnification (400X) than represented here, for clear observation of fiber membranes. Scale bars: (A-F) = 200 μm; (A’- F’) = 50 μm. (G) EDL weight relative to body weight for untreated muscles and for regenerated EDL at day 15 (D15), day 21 (D21) and day 30 (D30) after notexin (Ntx) injury. (H) The percentage (%) of myofibers with central nuclei). (I) Total myofiber number. (J) Cross-sectional area of EDLs. Statistical differences are indicated as follows: * p < 0.05, ** p < 0.01 compared with untreated age-matched control group; # p < 0.05, ## p < 0.01 compared with young muscle after the same treatment.
Figure 2. Collagen deposition after notexin-induced regeneration does not differ by age. Representative images of Gomori trichrome-stained EDL sections showing blue-stained collagen in untreated EDLs (A-C, A’-C’) and EDLs after notexin (Ntx) (D-F, D’-F’) at day 30 for young (3 mo) and old mice (22 mo) mice and day 21 for senescent mice (28 mo). A’-F’ are higher magnifications of the boxed regions in A-F, respectively. Scale bars: A-F = 200 μm; A’-F’ = 100 μm. (G) Graph showing %muscle area occupied by collagen.
Effective notexin-induced regeneration at all ages
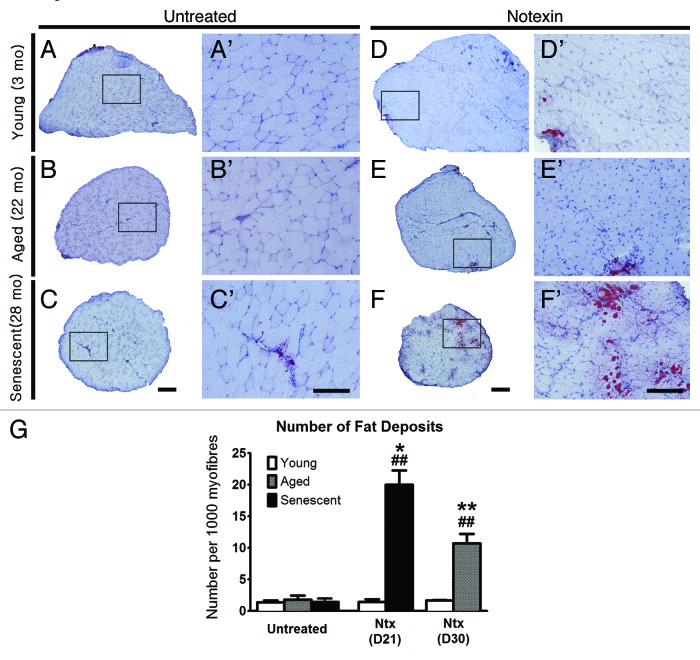
Following notexin injury (Fig. 1 and 2), myofiber regeneration was completed in all age groups by 2–3 weeks post-injury, with nearly normal muscle architecture (Fig. 1D’-F’ c.f. Fig. 1A’-C’) except that the regenerated EDL in young mice was 30–40% larger than in uninjured controls (p < 0.01) (Fig. 1A c.f. 1D; and see Fig. 1G and 1J), possibly due to ongoing growth at this age. EDL fiber number in 3 mo-old mice also increased after regeneration from notexin compared with control EDLs (p < 0.05). Approximately 90% of myofibers contained central nuclei, regardless of age and the small amount of superficial collagen observed by trichrome staining did not change with age (Fig. 2G). Overall morphology, muscle weight, central nucleation and fiber number were not different between post-injury days 15 and 30 for 3 and 22 mo-old mice, indicating that structural regeneration was complete by day 15 post-injury. The number of fat deposits in EDLs after notexin injury was higher than in age-matched controls in 22 and 28 mo-old mice; this change was not observed in young mice (Fig. 3).
Figure 3. Fat deposition is increased in regenerated EDLs from old and senescent mice after notexin treatment. Representative images of EDLs stained for fat with Oil Red O, from at day 30 post-notexin in young (3 mo) and old mice (22 mo) and at day 21 for senescent mice (28 mo). Images A’-F’ are higher magnifications of the boxed regions in A-F, respectively. Scale bars: A-f = 200 μm; A’- F’ = 100 μm. (G) Graph of the number of inter-myofiber fat deposits per EDL. Statistical differences are indicated as follows: * p < 0.05, ** p < 0.01 compared with untreated age-matched group; ## p < 0.01 compared with young muscle after the same treatment.
Functional recovery following notexin-induced injury in senescent mice
Contractility experiments were used to determine any age-related differences in the functional effectiveness of EDL regeneration between 3 and 28 mo-old mice, 21 d after notexin-induced damage, in comparison to untreated age-matched control EDLs (Table 2). Specific force was unchanged in regenerated muscles compared with controls. The time-to-peak-twitch force (TTP) was also unchanged. Half-relaxation time in untreated muscles was longer in 3 mo-old than 28 mo-old mice and decreased following regeneration in the younger mice (p < 0.01). The frequency-force relationship, Hill coefficient and the stimulation frequency required to produce half-maximal force (EC50), all indicators of Ca2+ sensitivity, were unchanged after regeneration from notexin compared with untreated muscles. Active stiffness was also unchanged after regeneration compared with controls in 3 or 28 mo-old mice, while passive stiffness increased after regeneration in both 3 and 28 mo-old mice. The recovery of contractile properties in 28 mo-old regenerated EDLs, 21 d post-notexin, approached the properties of young control EDLs, demonstrating that the EDL in senescent, 28 mo-old mice undergoes robust and functionally effective regeneration of muscle architecture after notexin-induced injury.
Table 2. In vitro contractility measurements of isolated EDL muscles from young and senescent mice.
| Untreated Young | Untreated Senescent | Ntx-treated Young | Ntx-treated Senescent | |
|---|---|---|---|---|
| Muscle Weight (mg) |
9.04 ± 0.16 |
9.84 ± 0.24 |
13.53 ± 0.54b |
11.90 ± 0.80a |
| Absolute Force (mN) |
134.4 ± 7.8 |
144.8 ± 8.2 |
143.7 ± 10.0 |
168.8 ± 8.1 |
| Specific Force (mN/mm2) |
205.3 ± 13.5 |
226.8 ± 16.3 |
159.6 ± 14.7 |
236.4 ± 17.1 |
| TTP (ms) |
23.67 ± 0.78 |
20.78 ± 1.00 |
21.88 ± 0.30 |
23.22 ± 0.52 |
| HRT (ms) |
27.74 ± 3.06 |
20.78 ± 0.86 |
22.67 ± 1.79a |
18.17 ± 1.82 |
| Hill Slope |
4.67 ± 0.38 |
4.71 ± 0.38 |
5.15 ± 0.48 |
5.17 ± 0.29 |
| EC50 (Hz) |
45.5 ± 0.9 |
44.2 ± 1.0 |
42.6 ± 1.1 |
42.3 ± 0.6 |
| Active Stiffness (N/m) |
421 ± 33 |
489 ± 27 |
451 ± 28 |
479 ± 38 |
| Passive Stiffness (N/m) |
39.8 ± 2.3 | 29.5 ± 3.3 | 55.9 ± 2.4a | 57.5 ± 2.4a |
Values are mean ± SEM for n = 9−10/group. TTP, time-to-peak force; HRT, half-relaxation time. Statistical differences (one-way ANOVA) from untreated muscles of the same age, 21 d post-notexin injury, are indicated by superscripts as follows: a indicates p < 0.05, b indicates p < 0.01.
Regeneration from freeze injury in senescent mice occurs without significant fibrosis
To determine whether regeneration after freezing is impaired in senescent muscles, we performed a standardized FI to EDL in 3 and 28 mo-old mice. After FI at both ages, there were distinct areas of regenerated, centrally nucleated fibers 30 d after FI (Fig. 4) occupying approximately 50−60% of the muscle cross-section, regardless of age. At the same time post-FI, in trichrome-stained sections, the border between areas of regenerated fibers and fibers unaffected by FI was delineated by collagen in muscles injured at 3 and 28 mo of age. However, the % area of collagen and other morphological features did not differ at either age from age-matched untreated EDL, demonstrating effective structural regeneration by muscles by 30 d after FI in young and senescent mice.
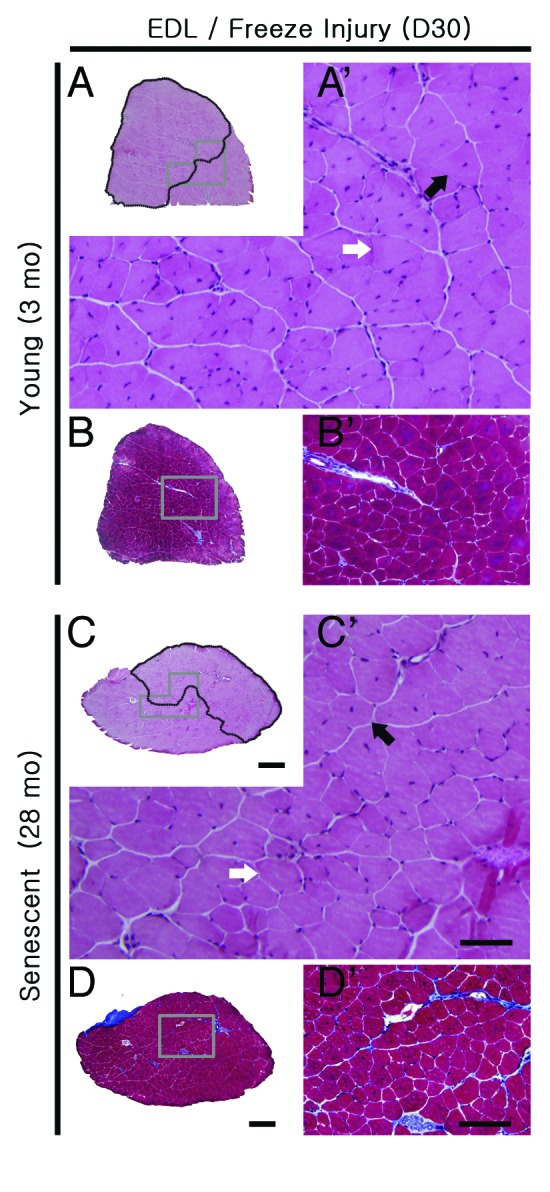
Figure 4. Regenerative response of EDLs to freeze-induced injury (FI) does not differ by age. Representative H&E (A, C) and Gomori’s trichrome stained (B, D) sections of EDLs from young (3 mo) and senescent (28 mo) mice 30 d following freeze injury. Images A’-D’ are higher magnifications of the boxed regions in A-D, respectively. Areas damaged by FI (containing fibers with central nuclei) are outlined in black in (A) and (C). Collagen deposits (blue) are evident at the margins of the area affected by FI (B, D). Myofibers with central nuclei (black arrows) and peripheral nuclei (white arrows) are present (A, C). Scale bars: A-D = 500 μm; A’, C’ = 50 μm; B’, D’ = 100 μm.
Structural regeneration from denervation-devascularization is fibrotic and delayed
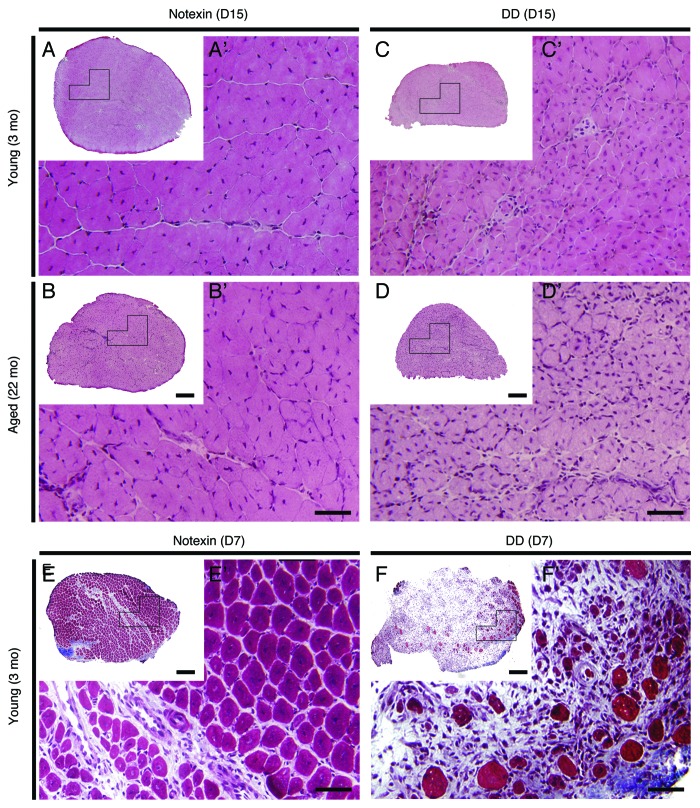
Similar to notexin-induced injury, DD results in more extensive damage to muscle fibers than FI. In contrast to notexin, DD additionally affects motor and sensory nerves and blood vessels.9 Regardless of age or the recovery time after DD, regenerated EDLs contained smaller, centrally nucleated myofibers compared with those after notexin (Fig. 5 and 6). Regenerated EDLs tended to be smaller in cross-sectional area compared with control EDL in age-matched mice 30 d post-DD (p < 0.08, Fig. 6H). DD spared roughly one quarter of the muscle, since only 70–75% of fibers were centrally nucleated after 15 or 30 d of regeneration (Fig. 6E) compared with > 90% of fibers at the comparable intervals after notexin injury (Fig. 1H). The regenerative response in old, 22 mo-old mice was delayed compared with notexin-induced regeneration; 30 d after DD EDLs weighed less than untreated age-matched control EDLs, and a similar trend was observed at 3 mo of age (p < 0.07) (Fig. 6F). Fiber number, however, was increased 15 and 30 d after DD in 3 mo-old mice (Fig. 6G). The % area of collagen deposition was increased 3 to 4-fold, in and around EDLs regenerated for 30 d from DD injury in both 3 and 22 mo-old mice (Fig. 6B-B’, 6D-D’, 6I). The number of fat deposits did not increase by 30 d after DD injury (Fig. 7).
Figure 5. Muscle regenerative response following notexin- and DD-induced injury in young and old mice. Representative H&E stained sections of EDL muscles of young (3 mo) and aged (22 mo) mice 15 d (D15) following notexin (A, A’, B, B’) and DD (C, C’, D, D’) injury. Images A’-F’ are higher magnifications of the boxed regions in A-F, respectively. Representative images of transverse sections of EDL muscles stained with Gomori’s Trichrome (blue = collagen) of young (3 mo) mice 7 d (D7) following notexin (E, E’) or DD (F, F’) injury. Scale bars: A- F = 200 μm; A’- F’ = 50 μm.
Figure 6. Muscle regeneration is incomplete 30 d following denervation-devascularization (DD) injury in both young and aged mice. Representative images of transverse sections of EDL muscles from young (3 mo) and aged (22 mo) mice, 30 d (D30) following DD injury stained with H&E (A, A’, C, C’) and Gomori’s Trichrome (blue = collagen) (B, B’, D, D’). Images A’-D’ are higher magnifications of the boxed regions in A-D, respectively. Scale Bars: A-D = 200 μm; A’, C’ = 50 μm; B’, D’ = 100 μm. (E) Percentage of myofibers with central nuclei. (F) EDL muscle weight as percentage of body weight. (G) total number of myofibers. (H) Cross-sectional area of the EDL muscle. (I) Percentage of muscle area occupied by collagen. Statistical differences are indicated as follows: * p < 0.05, ** p < 0.01 compared with untreated age-matched group; ## p < 0.01 compared with young muscle of the same treatment group.
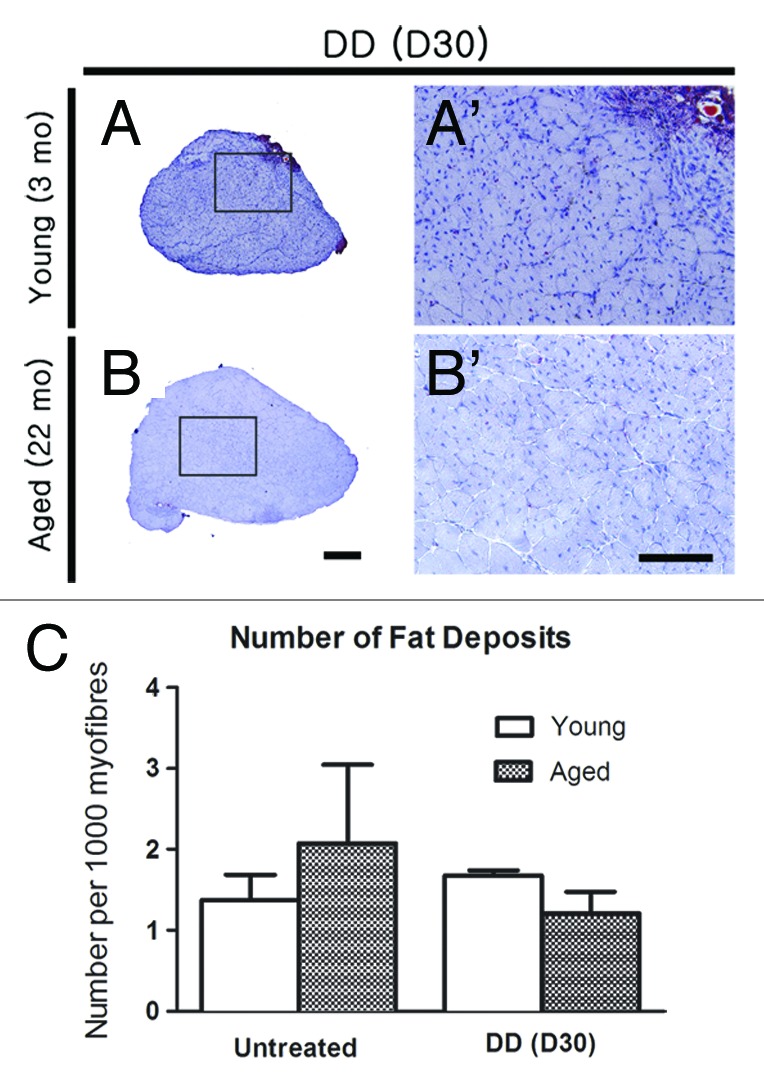
Figure 7. Fat deposition is increased in regenerated aged and senescent muscle after denervation-devascularization (DD) injury. (A-B, A’-B’) Representative images of Oil Red O-stained sections of EDLs from young (3 mo) and old (22 mo) mice, showing fat (red) deposits 30 d (D30) after DD. Images A’-B’ are higher magnifications of the boxed regions in (A-B), respectively. Scale bars: A-B = 200 μm; A’-B’ = 100 μm. (C) Number of inter-myofiber fat deposits per EDL. Statistical differences are indicated as follows: * p < 0.05, ** p < 0.01 compared with untreated age-matched group; ## p < 0.01 compared with young muscle of the same treatment group.
Discussion
The novelty of this report is its emphasis on the net outcome of muscle regeneration with a focus on architecture at different ages, after three distinct injuries that span injury paradigms used in previous studies that reported contradictory findings.1–4vs5,6 For notexin injury, architecture, regeneration and function were compared in young, old and senescent/sarcopenic mice. Muscle architecture and regeneration were also compared between young and senescent mice after freeze injury (FI) and between young and old mice after denervation-devascularization injury (DD). Notably, all mice were healthy, without overt comorbidity in the senescent group. Regeneration by 30 d post-injury, that is regeneration at its net outcome, was as effective in senescent and old mice as in young animals and most effective when neurovascular supply was spared from injury.
The effectiveness or outcome of architectural regeneration in the EDL showed remarkably little age-dependent variation in the three muscle-injury protocols used in this study, notexin, FI and DD. Notexin injury produced the most uniform and extensive damage to myofibers compared with FI and DD injury. Structural regeneration after notexin was also the most complete compared with that after FI and DD and included increased muscle weight without fibrosis by 30 d following muscle injury to young and old mice. Restoration of contractile function by 21 d post-injury, further illustrates the exquisite level of notexin-induced EDL regeneration in both senescent and young mice. The only age-dependent differences in regeneration from notexin injury were an increased fiber number in young 3 mo-old mice at 21 d post-injury that was not seen in 22 or 28 mo-old mice and increased fat in the EDL after regeneration in 22 and 28 mo-old mice at the same interval after injury. At all ages (3, 22 and 28 mo of age), muscle architecture was restored similar to that of untreated age-matched EDL. Freezing affected about 50% of the muscle in our protocol, and FI-induced regeneration did not affect muscle weight, fiber number or fat and collagen deposition (except that collagen delineated the margin of regenerated tissue). In contrast, while the extent of DD injury was intermediate between notexin and FI, affecting ~70% of the muscle fibers, the outcome of muscle tissue regeneration was worse than after FI and especially compared with notexin injury. Although there was no fat deposited in DD-regenerated muscles, there was increased collagen at 30 d post-DD at both ages, muscle weight was lower in 22 mo-old regenerated vs. age-matched control EDL and fiber number was increased after DD-induced regeneration in 3 mo-old mice. These findings together demonstrate that the nature of a tissue injury and in particular whether the neurovascular supply is retained (notexin) or damaged (FI, DD), is a greater determinant of the outcome of skeletal muscle regeneration than age, per se and illustrates the remarkable capacity of skeletal muscle to regenerate, even in senescent mice.
Two prominent short-term studies reported an age-dependent difference in the effectiveness of regeneration following FI in mice at 5 d post injury that was rescued by parabiosis between young and old mice.7,8 In contrast, long-term studies including this one and Shavlakadze et al.6 that used an injury paradigm similar to DD, whole muscle graft, report excellent regenerative capacity in muscle of old mice. In addition, two recent studies using human muscle cells do not support the conclusions from the parabiosis studies that there are inherent defects in aged muscle progenitor cells. One examined potential influences of serum constituents to sustain the proliferation and differentiation of human myoblasts in culture and found serum from older and young humans equally support the two processes inherent in myogenic regeneration.25 The second noted that the phenotypic characteristics of myoblasts isolated from young and older human donors have similar patterns of proliferation, differentiation and senescence under tissue culture conditions.26 The present study clearly identified that the net outcome of regeneration did not differ between young and old or even senescent mice, using FI or notexin injury. These contrasting reports from different studies may be due to protocol differences that affect the extent of FI between laboratories. These include differences in mouse strain (C57Bl/Ka-Ly5.2 backcrossed to C57BL/Ka-Thy1.1 mice and parabiosed to old C57BL/6 mice vs. C57BL/6J in this study), animal age, the range of ages included in different treatment groups and the size of the muscles damaged by freezing (e.g., larger tibialis anterior muscles vs. EDLs in the present study). Other contributing factors could be the use of a parabiosis system vs. use of a single-mouse protocol, and most significantly, a focus on earlier time-points vs. the emphasis in our study and others5,6,25 on the longer-term outcome of regeneration.
Our study and that of Shavlakadze et al.6 clearly demonstrate that muscle progenitor cells in senescent mouse skeletal muscle have a regenerative capacity that is not significantly different from those in young muscle. The implications of these findings, vs. those focused on short-term outcomes, have practical implications. Restorative procedures employing surgical grafting approaches (of various features of muscle including vessels, motor and/or nerves, fibers or whole-muscle flaps), transplantation of myoblasts, muscle stem cells or induced/transformed stem cells or treatment with platelet enriched plasma, assess regeneration of muscle contractile function within a particular window of elapsed time during rehabilitation. Our findings indicate that short-term assessment may be misleading in older patients and that delays in some aspects of the regeneration of old muscle do not rule out net functional regeneration, per se.
The neurovascular system plays an essential role in the normal inflammatory response during muscle regeneration.5,6,12,27-30 The production of chemokines and their respective receptors by injured muscle regulates the migration of macrophages and other immune cells such as neutrophils, which in turn regulate the proliferation and differentiation of myogenic precursors.31,32 Indeed, without access to the injury site through functionally patent vasculature, macrophages resident in muscle tissue would mount the primary response before neutrophils and stimulate the revascularization of an avascular tissue, as demonstrated in studies of transplanted whole muscle grafts.5,6 As well, activated satellite cells in regenerating muscle in vivo secrete a neuro-repellant factor, Semaphorin3A, proposed to coordinate the timing of re-innervation until later stages of myogenic differentiation.33,34 However, the establishment and growth of new myofibers per se, is essentially nerve-independent until the late phase of regeneration, when fiber-type phenotype is re-established.35 The focus in this study, on the long-term net outcome of regeneration, was able to separate the impact of a delay needed to re-establish vascular perfusion and/or re-innervation after DD and FI, respectively, from complete functional recovery.
Only female mice were used in this study and therefore, it is important to consider the results in light of possible sex differences in vascular supply, muscle growth and regenerative capacity. Many studies in male animals have examined age-related changes in muscle metabolism, satellite cell populations or responses to exercise.36-38 One detailed study of isolated fibers found no sex-related differences in the number of satellite cells per fiber following endurance exercise in vivo.39 However, there are reported differences in the satellite cell response to activation, specifically its refractoriness in 8 mo-old females40 or responsiveness in 10 mo-old males.36 Another report did not find sex-specific differences in the decline of muscle mass with age, the drop in levels of sex steroids and heat-shock proteins or the rise in cytokines with age.41 Of direct relevance to present findings, a study by Peng and colleagues42 showed muscle in female mice recovers vascular flow after hindlimb ischemia less effectively and with greater use impairment than in male mice. The differential was attributed to lower levels of vascular endothelial growth factor and endothelial NOS in muscle from females seven days after ischemia that accompanied a smaller angiogenic response compared with males. The possibility that females have less effective architectural remodeling of collateral vessels after muscle injury and no difference in capillary density in the absence of injury underscores the present results in female mice and the importance of the vascular system in muscle regeneration. Additionally interesting in this regard are many other aspects relevant to tissue regeneration that show important differences between males and females, such as inflammatory responses, T cells and immune status (particularly in females) in relation to inducible nitric oxide synthase43 and exercise,44,45 phagocytosis in necrotic muscle tissue,46 and muscle function in males and females related to levels of estrogen receptor β.47
Functional regeneration following notexin injury, even at 28 mo of age, achieved a near match with age-matched controls, with the exception of increased passive stiffness. Increased passive stiffness was not due to an increase in collagen deposition. This latter finding suggests that more subtle changes to the structure of the collagen fibrils that interweave around myofibers48,49 and/or post-translational glycation and cross-linking of collagen may contribute to increase the passive stiffness of EDL after notexin-induced regeneration. The shortened half-relaxation time after such effective regeneration from notexin injury likely relates to the initial establishment of fast-twitch fibers in the regenerated EDL35; HRT would be expected to lengthen (toward HRT in controls) after adaptive remodeling of the regenerated muscle. The additional influence of an age-related sensitivity of elastic fibers to modulations would come into play in considering changes in physiology after regeneration by young vs. old muscle. These are directly related to the changes in tissue architecture in skeletal muscle with age, including elastic properties of skeletal muscle itself as well as muscle tendons and have implications for treatments of surgical and traumatic wounds to muscle tissues, which interestingly also show sex differences.50-52 The effectiveness of anti-fibrotic treatments such as suramin and halofuginone relates directly to the capability to reduce architectural stiffness in some pathological muscle conditions and improve function.53,54
Fiber number increased following regeneration in young mice after both notexin and DD injuries, likely due to fiber branching.55-57 Fiber branching in aged mice is therefore, not due to delayed regeneration. Myocyte fusion to form new myofibers in regeneration is partly mediated by nitric oxide (NO) released from myogenic cells.58,59 The level and distribution of NO synthase-1 changes with aging19,60 and would affect NO levels in the direct vicinity of myocytes during regeneration and contribute to an inverse age-dependent effect on the rate of regeneration.
Fat deposition in notexin-induced regeneration in old and senescent, but not young mice is consistent with changes in regulation of fibro-adipogenic cells during aging in vivo.61-64 This deposition of fat cells was not observed after DD or FI. Two types of fat-cell progenitors either promote65 or inhibit myogenesis in vitro66 suggesting there is interesting “cross-talk” among cell-type lineages during regeneration. However, fat deposition did not impair functional regeneration after notexin injury. This intriguing feature of regeneration recalls the infiltration of adipose tissue in human muscle after injury or disease such as muscular dystrophy and likely reflects age-related metabolic changes in muscle and/or shifts in the “fate” of precursor cells that respond to injury. The mechanisms regulating fat deposition in particular injury-repair scenarios is not known.
Conclusions
We make two independent, but related conclusions. First, the experiments reported in the current study show that old myogenic stem cells would be useful for transplantation to repair muscle in disease or after traumatic injury, without expectation of a sub-optimal outcome as predicted by previous studies. Our data fail to detect a difference between the intrinsic capacity of young, old or senescent muscle to regenerate the functional architecture of a muscle, but do indicate some potential age-related differences in the regenerative capacity of the vasculature and nerve supply.
Arguably more important, given the serious impact of muscle atrophy in the aging population, results of this study directly challenge the widely held notion that therapy with muscle stem cells offers promise for treatment to promote the growth of aging human muscle. This was a comprehensive study of muscle architecture, including histological and physiological indices of overall regeneration; it reports that old muscle can regenerate without intervention. These findings directly refute the idea that the myogenic capacity of muscle satellite cells in aged muscle is decreased and indeed suggest that the conditions of the niche within which myogenic cells respond, is the more relevant focus of therapeutics. The findings of this report are therefore, directly relevant to the need to promote effective muscle regeneration through improved treatments, enabled by new understanding of repair capacity. The experiments are timely in addressing the strong interest in this topical field and of particularly critical to the increasing interest to the aging global human population where a positive functional outcome of any treatment intervention is of primary importance.
Materials and Methods
Mice
Female C57BL/6J mice were obtained from Animal Resources Centre (Canning Vale, WA, Australia) at 2 mo of age and housed until 3 (young), 22 (old) or 28 (senescent) months of age. The mice were housed 5−6 to a cage in an environment-controlled room with a 12 h light-dark cycle and had access to food and water ad libitum. Only healthy mice were used for the experiments and tissue collection. Notably, the parent C57BL/6 strain is known to have significant sarcopenia (fiber atrophy and weak forelimb grip strength) by 18 mo of age.19,20
Muscle injury procedures
All procedures were approved by the UNSW Animal Ethics Committee and performed in accordance with the guidelines of the Australian Code of practice for the Care and Use of Animals for Scientific Purposes.
Mice were anaesthetized with ketamine [0.1 mg/g body weight (BW); Purnell Laboratories) and xylazine (20 µg/g BW: Troy Laboratories]. A 1-cm incision on the lateral side of both hind limbs exposed the extensor digitorum longus (EDL) muscles, in preparation for one of the three muscle-injury approaches: (1) notexin (Ntx) (Latoxan),67,68 (2) devascularization-denervation (DD)12 and (3) freeze injury (FI).2,7,8 The latter two methods induce degeneration by a physical insult while notexin is myotoxic. Notexin (0.2 µg) was injected into the EDL muscle using a 50 µl Hamilton syringe with a 33-gauge needle (Hamilton Company). DD-induced degeneration of the EDL muscle was performed by exposing the distal tendon of the EDL and severing the associated blood vessels and nerves with a proximal-distal movement of a 5–0 nylon loop (Ethicon) surrounding the muscle. Proximal and distal tendons were also crushed with forceps (room temperature) for 10 sec to ensure complete devascularization of the muscle. FI injury was performed by applying an ultra-cold (cooled on liquid nitrogen) 3 mm2 metal probe directly to the exposed surface of EDL for two seconds. Care was taken to freeze the same portion of EDL in each mouse, at the mid-point of the proximal-distal muscle axis. Following muscle injury, the skin was sutured closed.
Use of the three injury paradigms across a wide age-range of mice (3, 22 and 28 mo of age) produced a differential severity of trauma inflicted by the injuries. As well, senescent mice often developed abdominal tumors (5/16 mice in this study).69 For these reasons, tissues were collected at slightly different times post-injury, to minimize exclusion of animals due to morbidity or poor mobility, and to gather data at comparable phases of regeneration, when previous studies using the three methods have reported it as complete. Notexin, for example, causes degeneration of almost all myofibers in a mouse EDL within 24–48 h68 and complete regeneration of the muscle within 15 d in young mice, 2−3 mo-old. Comparisons among different types of injury were made using additional recovery times (Table 1); group size was 3−10/group for each type of injury at each time-point in recovery from injury. Therefore, all data were collected from tissue samples from healthy mice that, at autopsy, had no sign of disease.
Table 1. Summary of the injury paradigms, notexin, freezing and denervation-devascularization (Den-Devasc) and the time-points in regeneration examined in young (3 mo), old (22 mo) and senescent mice (28 mo), including use of special staining methods to detect deposition of fat (Oil Red O) and collagen (Gomori’s trichrome) in regenerated EDLs.
| Injury | Days recovery | Young (3 mo) |
Old (22 mo) |
Senescent (28 mo) |
Special stains for: |
|---|---|---|---|---|---|
|
Notexin |
7 |
x |
|
|
|
| 15 |
x |
x |
|
|
|
| 21 |
x (F) |
|
x (F) |
fat |
|
| 30 |
x |
x |
|
fat, collagen |
|
|
Freeze |
30 |
x |
|
x |
|
| Den-Devasc | 7 |
x |
|
|
|
| 15 |
x |
x |
|
|
|
| 30 | x | x | fat, collagen |
indicates contractility studies of muscle function were performed
Sample preparation
EDL muscles were removed, freed from any visible fat and blood, embedded in Tissue-Tek O.C.T. compound (Sakura Finetek, ProSciTech) and rapidly frozen in liquid nitrogen-cooled isopentane. To ensure that the same region of FI muscle was examined systematically, the muscle was cut transversally at the mid-point of the injury, embedded in O.C.T. and sections made at the site of injury. Transverse sections were cut (1 0µm thick) using a cryostat (Leica CM 1950 or Microm HM 500M) and processed to assess routine histology, fat deposition and fibrosis, as follows.
Histological analysis
Sections were air-dried, fixed with 4% paraformaldehyde (ProSciTech) for 10 min and stained with Harris hematoxylin (Australian Biostain) and 1% alcoholic eosin (Fronine Laboratory Supplies) to visualize overall morphology and nuclear location. The degree of fibrosis was visualized using Gomori’s trichrome staining protocol (Blue Collagen Staining Kit; Richard Allen Scientific, Thermo Scientific) which stains collagen/connective tissue blue and muscle fibers red. The Oil Red O staining procedure was used to analyze fat deposition in the EDL muscles.70
Image capture and analysis
Sections were examined using an Olympus BX51 microscope; images were captured with a DP70 digital camera (Olympus) and analyzed using Image J software (NIH). The degree of muscle regeneration was measured by determining the proportion of EDL fibers with centralized nuclei, viewed and photographed at 400 × magnification. The degree of fibrosis was assessed by measuring the proportion of the whole EDL section occupied by blue-stained collagen after Gomori’s trichrome staining. The number of inter-fiber fat deposits ≥ 10µm in diameter per 1000 fibers in each muscle was counted in EDL sections stained with Oil Red O. Observations were made on coded sections to minimize observer bias.
Muscle contractility measurements
Ex vivo contractility studies were performed on isolated EDLs, as previously reported.71,72 Muscles were stimulated with a supra-maximal 1-ms electrical pulse and the resulting twitch recorded. Twitch data were smoothed by averaging the raw data over 2.5 ms intervals; time-to-peak twitch (TTP) and half-relaxation time were obtained from smoothed data. Maximum force was measured at increasing frequencies (2−125 Hz), fitting tracings to a force-frequency curve (Version 5.00, GraphPad Software) also as described and specific force (force per cross sectional area), maximum tetanic force and Hill Coefficient (h) were calculated. Passive and active stiffness were measured by stimulating muscle in a relaxed state or during a maximal isometric contraction at 100 Hz, respectively. Under these testing conditions, tendon properties have little influence on the mechanical, contractile properties of the muscle as it is attached to the testing equipment at the muscle-tendon junction.
Statistical analysis
Data are presented as mean ± SEM. Analyses were conducted using analysis of variance and post hoc means tests, using standard statistical software (GraphPad Prism Version 5.00).
Acknowledgments
Authors would like to thank the staff at the Biological Resources Services at the University of New South Wales for care of animals in this study. This work was generously supported by the National Health and Medical Research Council (Grant 630474) and the Kid’s Cancer Project, Australia. AJK is a Fellow of the Kid’s Cancer Project, Childhood Cancer Cytoskeletal Consortium (C4). PWG was a Principal Research Fellow of the National Health Medical Research Council (Grant 163626). JEA was an Endeavor Executive Award Visiting Fellow to the School of Medical Sciences, University of New South Wales (3299–2012) and received research support from the Muscular Dystrophy Association (USA) during the work.
Glossary
Abbreviations:
- DD
denervation-devascularization
- EDL
extensor digitorum longus
- FI
freeze injury
- HRT
half-relaxation time
- Ntx
notexin
- TA
tibialis anterior
- TTP
time-to-peak twitch
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/BioArchitecture/article/24966
References
- 1.Brack AS, Rando TA. Intrinsic changes and extrinsic influences of myogenic stem cell function during aging. Stem Cell Rev. 2007;3:226–37. doi: 10.1007/s12015-007-9000-2. [DOI] [PubMed] [Google Scholar]
- 2.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–10. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 3.Carosio S, Berardinelli MG, Aucello M, Musarò A. Impact of ageing on muscle cell regeneration. Ageing Res Rev. 2011;10:35–42. doi: 10.1016/j.arr.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature. 2012;490:355–60. doi: 10.1038/nature11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smythe GM, Shavlakadze T, Roberts P, Davies MJ, McGeachie JK, Grounds MD. Age influences the early events of skeletal muscle regeneration: studies of whole muscle grafts transplanted between young (8 weeks) and old (13-21 months) mice. Exp Gerontol. 2008;43:550–62. doi: 10.1016/j.exger.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Shavlakadze T, McGeachie J, Grounds MD. Delayed but excellent myogenic stem cell response of regenerating geriatric skeletal muscles in mice. Biogerontology. 2010;11:363–76. doi: 10.1007/s10522-009-9260-0. [DOI] [PubMed] [Google Scholar]
- 7.Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–7. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- 8.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–4. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 9.Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 2007;25:885–94. doi: 10.1634/stemcells.2006-0372. [DOI] [PubMed] [Google Scholar]
- 10.Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z. Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol. 2006;294:50–66. doi: 10.1016/j.ydbio.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brack AS, Bildsoe H, Hughes SM. Evidence that satellite cell decrement contributes to preferential decline in nuclear number from large fibres during murine age-related muscle atrophy. J Cell Sci. 2005;118:4813–21. doi: 10.1242/jcs.02602. [DOI] [PubMed] [Google Scholar]
- 12.Anderson JE. Dystrophic changes in mdx muscle regenerating from denervation and devascularization. Muscle Nerve. 1991;14:268–79. doi: 10.1002/mus.880140311. [DOI] [PubMed] [Google Scholar]
- 13.Bodine-Fowler S. Skeletal muscle regeneration after injury: an overview. J Voice. 1994;8:53–62. doi: 10.1016/S0892-1997(05)80319-4. [DOI] [PubMed] [Google Scholar]
- 14.Pastoret C, Sebille A. Age-related differences in regeneration of dystrophic (mdx) and normal muscle in the mouse. Muscle Nerve. 1995;18:1147–54. doi: 10.1002/mus.880181011. [DOI] [PubMed] [Google Scholar]
- 15.Price HM, Howes EL, Jr., Blumberg JM. Ultrastructural alterations in skeletal muscle fibers injured by cold. II. Cells on the sarcolemmal tube: observations on “discontinuous” regeneration and myofibril formation. Lab Invest. 1964;13:1279–302. [PubMed] [Google Scholar]
- 16.Gutiérrez JM, Núñez J, Díaz C, Cintra AC, Homsi-Brandeburgo MI, Giglio JR. Skeletal muscle degeneration and regeneration after injection of bothropstoxin-II, a phospholipase A2 isolated from the venom of the snake Bothrops jararacussu. Exp Mol Pathol. 1991;55:217–29. doi: 10.1016/0014-4800(91)90002-F. [DOI] [PubMed] [Google Scholar]
- 17.Harris JB. Myotoxic phospholipases A2 and the regeneration of skeletal muscles. Toxicon. 2003;42:933–45. doi: 10.1016/j.toxicon.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Sanes JR. The basement membrane/basal lamina of skeletal muscle. J Biol Chem. 2003;278:12601–4. doi: 10.1074/jbc.R200027200. [DOI] [PubMed] [Google Scholar]
- 19.Leiter JR, Upadhaya R, Anderson JE. Nitric oxide and voluntary exercise together promote quadriceps hypertrophy and increase vascular density in female 18-mo-old mice. Am J Physiol Cell Physiol. 2012;302:C1306–15. doi: 10.1152/ajpcell.00305.2011. [DOI] [PubMed] [Google Scholar]
- 20.Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol. 1988;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson JE, Garrett K, Moor A, McIntosh L, Penner K. Dystrophy and myogenesis in mdx diaphragm muscle. Muscle Nerve. 1998;21:1153–65. doi: 10.1002/(SICI)1097-4598(199809)21:9<1153::AID-MUS6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 22.Brooks SV, Faulkner JA. Skeletal muscle weakness in old age: underlying mechanisms. Med Sci Sports Exerc. 1994;26:432–9. doi: 10.1249/00005768-199404000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Dedkov EI, Kostrominova TY, Borisov AB, Carlson BM. Reparative myogenesis in long-term denervated skeletal muscles of adult rats results in a reduction of the satellite cell population. Anat Rec. 2001;263:139–54. doi: 10.1002/ar.1087. [DOI] [PubMed] [Google Scholar]
- 24.Gordon T, Hegedus J, Tam SL. Adaptive and maladaptive motor axonal sprouting in aging and motoneuron disease. Neurol Res. 2004;26:174–85. doi: 10.1179/016164104225013806. [DOI] [PubMed] [Google Scholar]
- 25.Sadeh M. Effects of aging on skeletal muscle regeneration. J Neurol Sci. 1988;87:67–74. doi: 10.1016/0022-510X(88)90055-X. [DOI] [PubMed] [Google Scholar]
- 26.George T, Velloso CP, Alsharidah M, Lazarus NR, Harridge SD. Sera from young and older humans equally sustain proliferation and differentiation of human myoblasts. Exp Gerontol. 2010;45:875–81. doi: 10.1016/j.exger.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Alsharidah M, Lazarus NR, George TE, Agley CC, Velloso CP, Harridge SD. Primary human muscle precursor cells obtained from young and old donors produce similar proliferative, differentiation and senescent profiles in culture. Aging Cell. 2013 doi: 10.1111/acel.12051. [DOI] [PubMed] [Google Scholar]
- 28.Hansen-Smith FM, Carlson BM. Cellular responses to free grafting of the extensor digitorum longus muscle of the rat. J Neurol Sci. 1979;41:149–73. doi: 10.1016/0022-510X(79)90035-2. [DOI] [PubMed] [Google Scholar]
- 29.Hansen-Smith FM, Carlson BM, Irwin KL. Revascularization of the freely grafted extensor digitorum longus muscle in the rat. Am J Anat. 1980;158:65–82. doi: 10.1002/aja.1001580107. [DOI] [PubMed] [Google Scholar]
- 30.Roberts P, McGeachie JK. Endothelial cell activation during angiogenesis in freely transplanted skeletal muscles in mice and its relationship to the onset of myogenesis. J Anat. 1990;169:197–207. [PMC free article] [PubMed] [Google Scholar]
- 31.Griffin CA, Apponi LH, Long KK, Pavlath GK. Chemokine expression and control of muscle cell migration during myogenesis. J Cell Sci. 2010;123:3052–60. doi: 10.1242/jcs.066241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pillon NJ, Bilan PJ, Fink LN, Klip A. Cross-talk between skeletal muscle and immune cells: muscle-derived mediators and metabolic implications. Am J Physiol Endocrinol Metab. 2013;304:E453–65. doi: 10.1152/ajpendo.00553.2012. [DOI] [PubMed] [Google Scholar]
- 33.Tatsumi R, Sankoda Y, Anderson JE, Sato Y, Mizunoya W, Shimizu N, et al. Possible implication of satellite cells in regenerative motoneuritogenesis: HGF upregulates neural chemorepellent Sema3A during myogenic differentiation. Am J Physiol Cell Physiol. 2009;297:C238–52. doi: 10.1152/ajpcell.00161.2009. [DOI] [PubMed] [Google Scholar]
- 34.Do MK, Sato Y, Shimizu N, Suzuki T, Shono J, Mizunoya W, et al. Growth factor regulation of neural chemorepellent Sema3A expression in satellite cell cultures. Am J Physiol Cell Physiol. 2011;301:C1270–9. doi: 10.1152/ajpcell.00257.2011. [DOI] [PubMed] [Google Scholar]
- 35.Launay T, Noirez P, Butler-Browne G, Agbulut O. Expression of slow myosin heavy chain during muscle regeneration is not always dependent on muscle innervation and calcineurin phosphatase activity. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1508–14. doi: 10.1152/ajpregu.00486.2005. [DOI] [PubMed] [Google Scholar]
- 36.Betters JL, Lira VA, Soltow QA, Drenning JA, Criswell DS. Supplemental nitric oxide augments satellite cell activity on cultured myofibers from aged mice. Exp Gerontol. 2008;43:1094–101. doi: 10.1016/j.exger.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Allen DL, Harrison BC, Maass A, Bell ML, Byrnes WC, Leinwand LA. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J Appl Physiol. 2001;90:1900–8. doi: 10.1152/jappl.2001.90.5.1900. [DOI] [PubMed] [Google Scholar]
- 38.Konhilas JP, Widegren U, Allen DL, Paul AC, Cleary A, Leinwand LA. Loaded wheel running and muscle adaptation in the mouse. Am J Physiol Heart Circ Physiol. 2005;289:H455–65. doi: 10.1152/ajpheart.00085.2005. [DOI] [PubMed] [Google Scholar]
- 39.Shefer G, Rauner G, Yablonka-Reuveni Z, Benayahu D. Reduced satellite cell numbers and myogenic capacity in aging can be alleviated by endurance exercise. PLoS One. 2010;5:e13307. doi: 10.1371/journal.pone.0013307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee CE, McArdle A, Griffiths RD. The role of hormones, cytokines and heat shock proteins during age-related muscle loss. Clin Nutr. 2007;26:524–34. doi: 10.1016/j.clnu.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Leiter JR, Anderson JE. Satellite cells are increasingly refractory to activation by nitric oxide and stretch in aged mouse-muscle cultures. Int J Biochem Cell Biol. 2010;42:132–6. doi: 10.1016/j.biocel.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 42.Peng X, Wang J, Lassance-Soares RM, Najafi AH, Sood S, Aghili N, et al. Gender differences affect blood flow recovery in a mouse model of hindlimb ischemia. Am J Physiol Heart Circ Physiol. 2011;300:H2027–34. doi: 10.1152/ajpheart.00004.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verthelyi D. Female’s heightened immune status: estrogen, T cells, and inducible nitric oxide synthase in the balance. Endocrinology. 2006;147:659–61. doi: 10.1210/en.2005-1469. [DOI] [PubMed] [Google Scholar]
- 44.Stupka N, Tarnopolsky MA, Yardley NJ, Phillips SM. Cellular adaptation to repeated eccentric exercise-induced muscle damage. J Appl Physiol. 2001;91:1669–78. doi: 10.1152/jappl.2001.91.4.1669. [DOI] [PubMed] [Google Scholar]
- 45.Stupka N, Lowther S, Chorneyko K, Bourgeois JM, Hogben C, Tarnopolsky MA. Gender differences in muscle inflammation after eccentric exercise. J Appl Physiol. 2000;89:2325–32. doi: 10.1152/jappl.2000.89.6.2325. [DOI] [PubMed] [Google Scholar]
- 46.Grounds MD. Phagocytosis of necrotic muscle in muscle isografts is influenced by the strain, age, and sex of host mice. J Pathol. 1987;153:71–82. doi: 10.1002/path.1711530110. [DOI] [PubMed] [Google Scholar]
- 47.Glenmark B, Nilsson M, Gao H, Gustafsson JA, Dahlman-Wright K, Westerblad H. Difference in skeletal muscle function in males vs. females: role of estrogen receptor-beta. Am J Physiol Endocrinol Metab. 2004;287:E1125–31. doi: 10.1152/ajpendo.00098.2004. [DOI] [PubMed] [Google Scholar]
- 48.Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve. 2011;44:318–31. doi: 10.1002/mus.22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wood LK, Arruda EM, Brooks SV. Regional stiffening with aging in tibialis anterior tendons of mice occurs independent of changes in collagen fibril morphology. J Appl Physiol. 2011;111:999–1006. doi: 10.1152/japplphysiol.00460.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ettema GJ. Muscle efficiency: the controversial role of elasticity and mechanical energy conversion in stretch-shortening cycles. Eur J Appl Physiol. 2001;85:457–65. doi: 10.1007/s004210100464. [DOI] [PubMed] [Google Scholar]
- 51.Gajdosik RL, Vander Linden DW, Williams AK. Influence of age on length and passive elastic stiffness characteristics of the calf muscle-tendon unit of women. Phys Ther. 1999;79:827–38. [PubMed] [Google Scholar]
- 52.Brooks SV, Opiteck JA, Faulkner JA. Conditioning of skeletal muscles in adult and old mice for protection from contraction-induced injury. J Gerontol A Biol Sci Med Sci. 2001;56:B163–71. doi: 10.1093/gerona/56.4.B163. [DOI] [PubMed] [Google Scholar]
- 53.Chan YS, Li Y, Foster W, Fu FH, Huard J. The use of suramin, an antifibrotic agent, to improve muscle recovery after strain injury. Am J Sports Med. 2005;33:43–51. doi: 10.1177/0363546504265190. [DOI] [PubMed] [Google Scholar]
- 54.Turgeman T, Hagai Y, Huebner K, Jassal DS, Anderson JE, Genin O, et al. Prevention of muscle fibrosis and improvement in muscle performance in the mdx mouse by halofuginone. Neuromuscul Disord. 2008;18:857–68. doi: 10.1016/j.nmd.2008.06.386. [DOI] [PubMed] [Google Scholar]
- 55.Irintchev A, Wernig A. Muscle damage and repair in voluntarily running mice: strain and muscle differences. Cell Tissue Res. 1987;249:509–21. doi: 10.1007/BF00217322. [DOI] [PubMed] [Google Scholar]
- 56.Ontell M. Morphological aspects of muscle fiber regeneration. Fed Proc. 1986;45:1461–5. [PubMed] [Google Scholar]
- 57.Head SI. Branched fibres in old dystrophic mdx muscle are associated with mechanical weakening of the sarcolemma, abnormal Ca2+ transients and a breakdown of Ca2+ homeostasis during fatigue. Exp Physiol. 2010;95:641–56. doi: 10.1113/expphysiol.2009.052019. [DOI] [PubMed] [Google Scholar]
- 58.Pisconti A, Brunelli S, Di Padova M, De Palma C, Deponti D, Baesso S, et al. Follistatin induction by nitric oxide through cyclic GMP: a tightly regulated signaling pathway that controls myoblast fusion. J Cell Biol. 2006;172:233–44. doi: 10.1083/jcb.200507083. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Anderson JE. A role for nitric oxide in muscle repair: nitric oxide-mediated activation of muscle satellite cells. Mol Biol Cell. 2000;11:1859–74. doi: 10.1091/mbc.11.5.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leiter JR, Peeler J, Anderson JE. Exercise-induced muscle growth is muscle-specific and age-dependent. Muscle Nerve. 2011;43:828–38. doi: 10.1002/mus.21965. [DOI] [PubMed] [Google Scholar]
- 61.Beggs ML, Nagarajan R, Taylor-Jones JM, Nolen G, Macnicol M, Peterson CA. Alterations in the TGFbeta signaling pathway in myogenic progenitors with age. Aging Cell. 2004;3:353–61. doi: 10.1111/j.1474-9728.2004.00135.x. [DOI] [PubMed] [Google Scholar]
- 62.Starkey JD, Yamamoto M, Yamamoto S, Goldhamer DJ. Skeletal muscle satellite cells are committed to myogenesis and do not spontaneously adopt nonmyogenic fates. J Histochem Cytochem. 2011;59:33–46. doi: 10.1369/jhc.2010.956995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rossi CA, Pozzobon M, Ditadi A, Archacka K, Gastaldello A, Sanna M, et al. Clonal characterization of rat muscle satellite cells: proliferation, metabolism and differentiation define an intrinsic heterogeneity. PLoS One. 2010;5:e8523. doi: 10.1371/journal.pone.0008523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vettor R, Milan G, Franzin C, Sanna M, De Coppi P, Rizzuto R, et al. The origin of intermuscular adipose tissue and its pathophysiological implications. Am J Physiol Endocrinol Metab. 2009;297:E987–98. doi: 10.1152/ajpendo.00229.2009. [DOI] [PubMed] [Google Scholar]
- 65.Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–63. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12:143–52. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 67.Lee AS, Kahatapitiya P, Kramer B, Joya JE, Hook J, Liu R, et al. Methylguanine DNA methyltransferase-mediated drug resistance-based selective enrichment and engraftment of transplanted stem cells in skeletal muscle. Stem Cells. 2009;27:1098–108. doi: 10.1002/stem.28. [DOI] [PubMed] [Google Scholar]
- 68.Plant DR, Colarossi FE, Lynch GS. Notexin causes greater myotoxic damage and slower functional repair in mouse skeletal muscles than bupivacaine. Muscle Nerve. 2006;34:577–85. doi: 10.1002/mus.20616. [DOI] [PubMed] [Google Scholar]
- 69.Rowlatt C, Chesterman FC, Sheriff MU. Lifespan, age changes and tumour incidence in an ageing C57BL mouse colony. Lab Anim. 1976;10:419–42. doi: 10.1258/002367776780956917. [DOI] [PubMed] [Google Scholar]
- 70.Koopman R, Schaart G, Hesselink MK. Optimisation of oil red O staining permits combination with immunofluorescence and automated quantification of lipids. Histochem Cell Biol. 2001;116:63–8. doi: 10.1007/s004180100297. [DOI] [PubMed] [Google Scholar]
- 71.Chan S, Head SI. Age- and gender-related changes in contractile properties of non-atrophied EDL muscle. PLoS One. 2010;5:e12345. doi: 10.1371/journal.pone.0012345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chan S, Seto JT, MacArthur DG, Yang N, North KN, Head SI. A gene for speed: contractile properties of isolated whole EDL muscle from an alpha-actinin-3 knockout mouse. Am J Physiol Cell Physiol. 2008;295:C897–904. doi: 10.1152/ajpcell.00179.2008. [DOI] [PubMed] [Google Scholar]