Abstract
Characterization of neuronal connectivity is essential to understanding the architecture of the animal nervous system. Specific labeling and imaging techniques can visualize axons and dendrites of single nerve cells. Two-dimensional manual drawing has long been used to describe the morphology of labeled neuronal elements. However, quantitative morphometry, which is essential to understanding functional significance, cannot be readily extracted unless the detailed neuronal geometry is comprehensively reconstructed in three-dimensional space. We have recently applied an accurate and robust digital reconstruction system to cerebellar climbing fibers, which form highly dense and complex terminal arbors as one of the strongest presynaptic endings in the vertebrate nervous system. Resulting statistical analysis has shown how climbing fibers morphology is special in comparison to other axonal terminals. While thick primary branches may convey excitation quickly and faithfully to the far ends, thin tendril branches, which have a larger bouton density, form the majority of presynaptic outputs. This data set, now publicly available from NeuroMorpho.Org for further modeling and analysis, may constitute the first detailed and comprehensive digital reconstruction of the complete axonal terminal field with identified branch types and full accounting of boutons for any neuronal class in the vertebrate brain.
Keywords: axon, terminal arbor, bouton, branches, tendril, cerebellar cortex, molecular layer, biotinylated dextran amine, rat
Introduction
Neuronal presynaptic outputs are found on the arborization formed at the termination of long neuronal processes named axons. The climbing fiber in the vertebrate cerebellum is one of the densest and strongest terminal arbors in the central nervous system. We have recently reported the digital reconstruction of 68 climbing fibers in the rat cerebellum with a computer-aided system,1 including a microscope with a mounted digital camera and specialized software for computerized tracing of labeled neurons (Neurolucida from MBF Bioscience). Morphometric analysis of these digital reconstructions has extracted extensive quantitative information about the functional organization of climbing fibers. This commentary aims to discuss this study and to provide some relevant background, particularly about neuronal reconstruction and the role of climbing fibers in the cerebellar neuronal circuitry.
Reconstruction of the Nervous System Architecture
The animal nervous system supposedly has the most complex organization of any bodily organ. Neurons, the main cellular component giving rise to nervous system function, generally have multiple branching processes with specialized morphology and molecular expression. One of the processes, the axon, is a long string-like, branching structure that conveys signals, in the form of action potentials, to separate areas in both the central and peripheral nervous system. The other processes, dendrites, are also tree-like but generally shorter than axons, and receive signals from the axons of other neurons.2 A synaptic connection is formed between the axonal terminals of a neuron and the dendrites of another neuron for transmission of signals. Neurons vary considerable in axonal branching and synaptic connection patterns, making the whole nervous system tremendously complex, particularly in animals such as vertebrates, mollusks, and arthropods. Some types of neurons have axonal terminals that are uniformly arranged, such as in a flat sheet near the surface of the brain, creating a highly organized cortical structure.
Many common tissue staining procedures, such as hematoxylin-eosin, are relatively limited for quantitatively analyzing the cytological organization of the nervous system, since they cannot visualize axons, which are much thinner than the cell body of neurons. Ramón y Cajal2 utilized a staining technique developed by Golgi to visualize entire nerve cells, including axons and dendrites. This procedure has played a significant role in revealing the basic structure and connectivity of neurons in various areas of the nervous system of vertebrates (Fig. 1A). Neuronal and axonal staining methods further developed over the last century, including extrinsically injected tracer chemicals such as biotinylated dextran amine,3 intrinsically expressed fluorescent proteins or antigens. These sophisticated neuronal staining methods enable the selection of specific populations of neurons to be labeled.
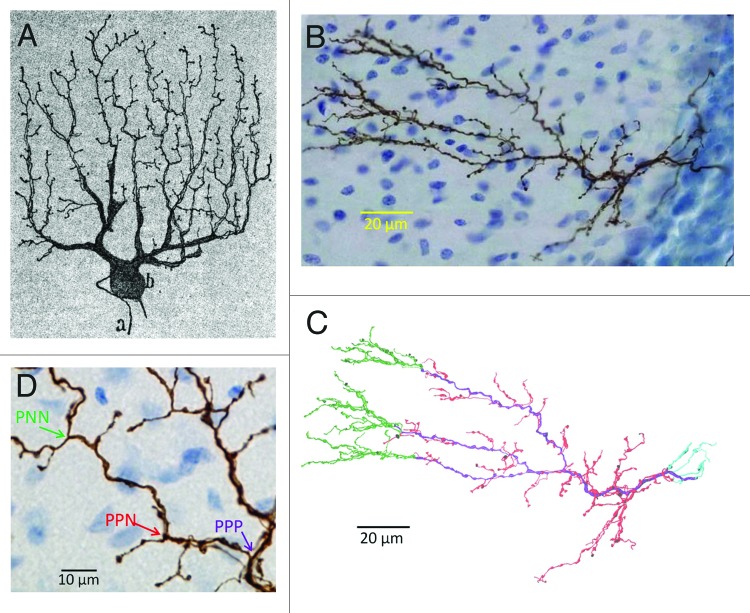
Figure 1. Reconstruction of climbing fibers. (A) Drawing of a human cerebellar climbing fiber labeled with Golgi staining by Ramón y Cajal (1911). The cell body and thick dendrites of the target Purkinje cell are also drawn. (B) Photomicrograph of a rat cerebellar climbing fiber anterogradely labeled with biotinylated dextran amine. (C) Digital reconstruction of the climbing fiber shown in (B). Colors indicate branch types: primary (purple), and non-primary, which are themselves sub-classified into tendril (red), retrograde (cyan), and distal (green). (D) Bifurcations classified into primary-to-primary-and-primary (PPP), primary-to-primary-and-non-primary (PPN), and primary-to-non-primary-and-non-primary (PNN). Panels (B–D) originally published in Brown et al., 2012. Image in panel (A) reprinted by permission of Oxford University Press.
As the functional organization of the nervous system depends so prominently on the connectivity of axons and dendrites, it is essential to quantify axonal and dendritic properties such as path, branching, and size, as well as their distribution and density of contacts, to characterize neuronal circuitry. A single photomicrograph is not usually appropriate to describe these elements, since the neuronal layout is extended in three-dimensional space. It is instead necessary to extract data across all three spatial dimensions from the histological preparation to understand the morphological organization of the nervous system.
Accurate human drawing of the labeled axons and dendrites can depict neuronal circuitry much more clearly than a photo, since the reconstructed neuron is freed from labeling artifacts and background noise. One of the earliest devices used to facilitate neuronal reconstruction since the late 19th century was the camera lucida apparatus, which was originally utilized by painters to draw portraits and landscapes. When attached to a microscope, the camera lucida enabled accurate drawing by using mirrors to reflect one’s drawing hand as overlaying the neuronal tissue seen through the microscope. Drawing can accumulate labeled neuronal elements at different z depths into a single plane as a projection, yielding complete representations as pioneered by Ramón y Cajal.2 Although drawing with pencil and paper was replaced with computer-aided tracing with illustration software in the late 20th century, the technique remained essentially the same.
In histological sections of biological preparation, which can be as thick as 100–400 µm, it is generally difficult to obtain depth information (i.e., spatial position of an object along the z-axis). A solution to this problem is to detect stage position in the z-direction with a mechanical sensor. The sensor is supposed to show the z-position of the element of interest if it is focused from eyepieces by a human. A more sophisticated and accurate method to obtain z position is to detect the best focused photo from a stack of photographs taken at every different focus depth (separated by 0.33 µm, for example).
There are several variations of computer-aided neuronal tracing tools nowadays. The system used in our study1 cannot only record X, Y and Z positions of any point of the labeled neuronal element, but also diameter along the neuronal branch path and connection pattern between branch points. With these data, it is possible to reconstruct the neuronal trees (e.g., axonal terminal arbor) in three-dimensional space and apply potentially hundreds of types of morphometric analyses. Neuroscientists have been working with and developing neuronal computer-aided digital reconstruction systems for years,4 revealing the complete three-dimensional morphology of thousands of neurons,5 including interneurons whose axons nearly span the entire span of the tissue slice.6
Climbing Fibers in the Cerebellar Cortex of the Vertebrate
Climbing fibers (Fig. 1B) may be the most complex, delicate, and densest axon terminal arbors in the vertebrate nervous system.7,8 Climbing fibers are found in the shallowest molecular layer of the vertebrate cerebellar cortex, in which neuronal components are aligned in a crystal-like regular organization within clear longitudinal, transverse, and vertical orientations. Climbing fibers are the axonal terminals of inferior olive neurons in the medulla. The axon itself bifurcates into about seven branches in its path, and each branch terminates as a climbing fiber in the cerebellar cortex.9 The climbing fiber forms a strong excitatory synaptic connection onto the dendritic arbor of a target Purkinje cell, which are the sole output neurons of the cerebellar cortex. Excitation of a climbing fiber elicits generation of a so-called complex spike, which then causes short- to long-term modulation of Purkinje cell excitability, providing powerful excitation and altering the efficacy of individual parallel fiber-Purkinje cell synapses.10 Thus, the effect of climbing fibers on Purkinje cells is central to all cerebellar functions.11
Climbing fibers synapse on the proximal dendrites of a Purkinje cell but not on its distal dendrites, where thousands of individual parallel fibers form synapses instead. Purkinje cells' dendritic arbors spread out longitudinally, while remaining relatively flat and stacked in rows in the lateral direction of the cerebellum. A climbing fiber terminal arbor appears ladder-like from the rear view since its stem and branches cling to Purkinje cell dendrites like ivy climbing around a tree. In addition, fine collaterals of a climbing fiber that are extended in the lateral direction to other targets like molecular layer cells (stellate cells, basket cells, and NG2-positive glial cells) can be observed in the rear view.9,12
Climbing fibers are present in the cerebellum of all vertebrates. The basic morphological properties of these arbors are similar among all species, although there are differences in details. The well-organized morphology of climbing fibers is gradually formed in the prenatal and postnatal periods, with distinct appearances at different developmental stages.2,13 Subtotal lesioning of climbing fibers in adult or immature stages causes compensatory reinnervation of surviving climbing fibers to Purkinje cells that lost their originally paired climbing fiber. These alterations are accompanied by abnormal morphology in the reinnervating climbing fibers.14 Alteration in climbing fiber morphology is also brought about by gene manipulation and by degeneration or hypoplasia of certain neuronal populations in the cerebellum.15 As a whole, the complex morphology of climbing fibers reflects precise functional, developmental, comparative and genetic modulation of the nervous system.
Morphometry of Climbing Fibers With Digital Reconstruction
Because of its unique structure among all neuronal elements in the vertebrate nervous system, climbing fibers are well suited to perform detailed morphometry. Since their complete morphology has not been comprehensively analyzed except for a few two-dimensional qualitative analyses,2,9 we digitally reconstructed 68 climbing fibers in the normal adult rat cerebellar cortex.1 These climbing fibers were labeled by biotinylated dextran amine injection to the inferior olive nucleus. Labeled climbing fibers were visualized using a standard diaminobenzidine technique following incubation of avidin-perroxidase complex in 50 µm-thick serial parasagittal sections of the cerebellum.
In this study, we fully characterized the morphology of climbing fiber terminal arbors throughout the finest structures that can be detected by light microscopy (Fig. 1C). The digitally reconstructed trees have shown considerable wiring density, on average 26 times higher than other compared axon types. This reflects tight packing of collaterals within the terminal arbor. Indeed, the stem axon fiber and most of the collaterals of the climbing fiber terminal arbor cling tightly onto, while covering large portions of, the thick Purkinje dendrites. Additionally, we observed low branching symmetry and high caulescence, which measures the prominence of the main path of the arbor. These values capture the distinct morphology of climbing fibers using mathematical parameters. Climbing fiber terminal arbors were also found to exhibit considerable morphological diversity (e.g., in height, width, number of branches, total tree length and so on). This diversity cannot be attributed to differences across anatomical regions or to distinct climbing fiber subclasses, indicating that variability is an irreducible population characteristic. In stark contrast, the molecular layer, in which climbing fibers are located, has as a whole a uniformly organized stereotype.
To analyze the spatial organization of the terminal arbor, we have introduced a simple morphometric definition that quantitatively classifies branches into “primary (P)” and “non-primary (N)” types (Fig. 1D). The former is thick and relatively smooth, while the latter has the appearance of beaded threads with large, dense boutons. As a result of this classification, all bifurcations can be classified into PPP, PPN, and PNN. PPP is the point where a primary branch divides into two more primaries, PPN is a point where a primary divides into one primary and one non-primary, and PNN is a primary dividing into two non-primaries. Quantitative analyses revealed that N branches arising from PPNs gave rise to significantly smaller sub-trees than N branches that coming from PNN bifurcations. N branches from PPNs and from PNNs can therefore be distinguished as “tendril”2,9 and “distal” fibers to reflect their morphological, and potentially functional, differences.
Boutons, representing the axon’s presynaptic transmission sites, are recognizable as localized increases in axonal diameter. Boutons can be found either along a branch (in which case they are called “en-passant”) or at the axonal end (terminal boutons). We found significant differences in the density, structure, and distribution of terminal and en-passant boutons across climbing fibers and branch types. Boutons were more densely located in N branches than P branches, and en-passant boutons were more ovoid (elongated along the branch) than terminal boutons. Furthermore, overlap of boutons with interneuron somata was observed beyond chance expectation, suggesting targeted circuit formation between the climbing fibers and molecular layer interneuron cell bodies.
P branches were found to be thicker than N branches, and the boutons on primary branches were more similar in diameter to inter-bouton regions of primary branches. Consequently, conduction velocity of action potentials through primary branches would likely be significantly larger than for N branches, and propagation failures would be less likely to occur than in N branches. In this manner, primary branches can be likened to communication highways, ensuring that signals reach distal territories fast and efficiently. At the same time, non-primary branches (tendril and distal), which provide the vast majority of branch length and boutons, represent the main presynaptic signal transmitters.
Impact of Digital Morphometry of Axonal Projection
The above results using digital reconstructions provide a comprehensive structural foundation for the climbing fiber terminal arborization. Many unique morphological characteristics, some of which had been qualitatively described, have now been expressed in mathematical values, which can aid future research by objectively distinguishing distinct axonal components, such as the stem axon (primary branch), tendril, distal, and retrograde fibers (which reach back to synapse on Purkinje cell bodies), and bouton types. One value of three-dimensional digital axonal reconstruction, as performed in our study,1 is that any morphological metric that may be appreciated at the optic microscopic level can be readily extracted. This method reproducibly reconstructs all branches and swellings, allowing their comprehensive morphometric analysis.
All 68 climbing fiber reconstructions are available through deposition in the public NeuroMorpho.Org database.5 To the best of our knowledge, these are the first available climbing fiber digital reconstructions with identified branch types and fully reconstructed boutons. They might serve as essential data to be included in anatomically realistic cerebellar network models. This set of digital reconstructions may also be the first detailed presentation of the complete axonal terminal field for any neuronal class in the vertebrate brain. Their comprehensive morphological analysis could reveal insights into basic function that may also apply to other regions of the animal nervous system.
Acknowledgments
Funding was provided by the National Institutes of Health under Grant R01 NS-39600 (G.A.A.).
Disclosure of Potential Conflict of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/BioArchitecture/article/24062
References
- 1.Brown KM, Sugihara I, Shinoda Y, Ascoli GA. Digital morphometry of rat cerebellar climbing fibers reveals distinct branch and bouton types. J Neurosci. 2012;32:14670–84. doi: 10.1523/JNEUROSCI.2018-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramón y Cajal S. Histologie du Systeme Nerveux de l'Homme et des Vertébrés, Vol. II. Paris: Maloine, 1911. English translation published by Oxford University Press, 1995. [Google Scholar]
- 3.Sugihara I. Bright field neuronal preparation optimized for automatic computerized reconstruction, a case with cerebellar climbing fibers. Neuroinformatics. 2011;9:113–8. doi: 10.1007/s12021-011-9099-9. [DOI] [PubMed] [Google Scholar]
- 4.Ascoli GA, Krichmar JL, Nasuto SJ, Senft SL. Generation, description and storage of dendritic morphology data. Philos Trans R Soc Lond B Biol Sci. 2001;356:1131–45. doi: 10.1098/rstb.2001.0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ascoli GA, Donohue DE, Halavi M. NeuroMorpho.Org: a central resource for neuronal morphologies. J Neurosci. 2007;27:9247–51. doi: 10.1523/JNEUROSCI.2055-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ascoli GA, Brown KM, Calixto E, Card JP, Galván EJ, Perez-Rosello T, et al. Quantitative morphometry of electrophysiologically identified CA3b interneurons reveals robust local geometry and distinct cell classes. J Comp Neurol. 2009;515:677–95. doi: 10.1002/cne.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eccles SJC, Ito M, Szentagothai J. The cerebellum as a neuronal machine. Berlin, Springer-Verlag, 1967. [Google Scholar]
- 8.Llinás RR, Walton K. Cerebellum. In: Shepherd GM, ed. The Synaptic Organization of the Brain, 4th ed. New York, Oxford University Press, 1998:255-88. [Google Scholar]
- 9.Sugihara I, Wu H, Shinoda Y. Morphology of single olivocerebellar axons labeled with biotinylated dextran amine in the rat. J Comp Neurol. 1999;414:131–48. doi: 10.1002/(SICI)1096-9861(19991115)414:2<131::AID-CNE1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 10.Ito M. Long-term depression. Annu Rev Neurosci. 1989;12:85–102. doi: 10.1146/annurev.ne.12.030189.000505. [DOI] [PubMed] [Google Scholar]
- 11.Houk JC, Buckingham JT, Barto AG. Models of the cerebellum and motor learning. Behav Brain Sci. 1996;19:368–83. doi: 10.1017/S0140525X00081474. [DOI] [Google Scholar]
- 12.Lin SC, Huck JH, Roberts JD, Macklin WB, Somogyi P, Bergles DE. Climbing fiber innervation of NG2-expressing glia in the mammalian cerebellum. Neuron. 2005;46:773–85. doi: 10.1016/j.neuron.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 13.Sugihara I. Microzonal projection and climbing fiber remodeling in single olivocerebellar axons of newborn rats at postnatal days 4-7. J Comp Neurol. 2005;487:93–106. doi: 10.1002/cne.20531. [DOI] [PubMed] [Google Scholar]
- 14.Aoki H, Sugihara I. Morphology of single olivocerebellar axons in the denervation-reinnervation model produced by subtotal lesion of the rat inferior olive. Brain Res. 2012;1449:24–37. doi: 10.1016/j.brainres.2012.02.040. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe M, Kano M. Climbing fiber synapse elimination in cerebellar Purkinje cells. Eur J Neurosci. 2011;34:1697–710. doi: 10.1111/j.1460-9568.2011.07894.x. [DOI] [PubMed] [Google Scholar]