Abstract
Changes in cell shape are one of the driving forces of tissue morphogenesis. Contractile cytoskeletal assemblies based on actomyosin networks have emerged as a main player that can drive these changes. Different types of actomyosin networks have been identified, with distinct subcellular localizations, including apical junctional and apicomedial actomyosin. A further specialization of junctional actomyosin are so-called actomyosin ‘cables’, supracellular arrangements that appear to stretch over many cell diameters. Such actomyosin cables have been shown to serve several important functions, in processes such as wound healing, epithelial morphogenesis and maintenance of compartment identities during development. In the Drosophila embryo, we have recently identified a function for a circumferential actomyosin cable in assisting tube formation. Here, I will briefly summarize general principles that have emerged from the analysis of such cables.
Keywords: Actomyosin, morphogenesis, cable, wound healing, Drosophila, anisotropy, development
Introduction
Actin and myosin form contractile assemblies that can exist either in the form of highly ordered sarcomeric ‘contraction machines’ in muscles that use muscle-specific myosin, or in the form of more loose actomyosin networks in non-muscle cells that use isoforms of non-muscle myosin II.1 The inherent ATP-driven contractility of actomyosin networks allows them to affect cell shapes when linked to cellular junctions, and they can generate tension within epithelial sheets of cells that are mechanically coupled by cell-cell junctions.2 The exact molecular nature of the link between actomyosin and cell-cell or cell-matrix junctions is unclear, but both types of junctions contain many actin-binding proteins that can provide a strong link to actin.3-5 Moreover, several regulators of myosin II contractility are recruited and localized to adherens junctions, including RhoA/Rho1, certain RhoGEFs, and the Rok-activator Shroom.4
Actomyosin linked to adherens junctions affects Cadherin clustering and turn-over,6,7 and it likely generates line tension at the level of the zonula adherens. This tension, coordinated and balanced between neighboring epithelial cells, will provide any individual cell with a stable apical domain. During several developmental processes, junctional actomyosin appears to be aligned between cell neighbors, and assembled into structures that span many cells, so called actomyosin cables (Fig. 1). Upstream pathways that specify where actomyosin cables are assembled have been identified.8-13 However, many questions remain with regards to the assembly and molecular composition of these cables. Is there a common mechanism activating cable assembly that would be triggered by all upstream signaling pathways? How is cable assembly coordinated between neighboring cells? What type of junction links the cable to the membrane, and how are these junctions linked between cells? What regulates whether a cable is assembled from cells on both sides of the cable or only one row of cells? How is a cable linked laterally (meaning perpendicular to its long axis) to the plasma membrane?
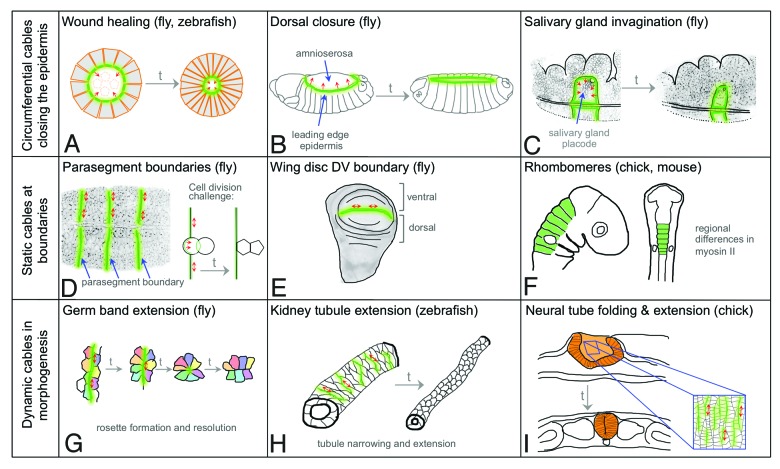
Figure 1. Actomyosin cables during development. Supracellular actomyosin cables tend to fall into three classes: circumferential cables (A-C), static cables at boundaries (D-F) and dynamic cables during morphogenesis (G-I). (A) An embryonic epithelial wound closes through contraction of an actomyosin ‘purse string’ located at the wound edge. (B) An actomyosin cable at the leading edge of the dorsal-most epithelial cells assists dorsal closure in the fly embryo. (C) A circumferential cable forms around the salivary gland placode and assists tube formation. (D) Actomyosin cables at parasegmental boundaries in the embryonic epidermis prevent cell mixing when the boundary is challenged by cell division. (E) The dorso-ventral boundary in the fly wing disc prevents cell mixing between the dorsal and ventral compartments. (F) Regional differences in myosin contractility are involved in rhombomere maintenance and ventricle formation, though whether bona fide actomyosin cables are involved is still unclear. (G) Myosin cables running vertically within the embryonic epidermis (i.e., perpendicular to the long axis of the embryo) help pull cells into multicellular rosettes that resolve horizontally, thus driving convergent extension (germ band extension). (H) Convergent extension of kidney tubules in zebrafish is also involves rosette formation driven by actomyosin cables that assemble perpendicular to the direction of the tube. (I) Folding and convergent extension of the chick neural tube involves formation of actomyosin cables perpendicular to the long axis of the tube that aid cell rearrangements and neighbor exchanges. In all panels, green lines mark the location of the actomyosin cables. Arrows in (A-C) indicate the centripetal force generated by the circumferential cables, double arrows in (D-I) indicate line tension generated by the cables.
The analysis of actomyosin cable function in Drosophila and vertebrate embryos thus far has identified commonalities and differences that suggest at least three main classes of cables exist, and I will discuss these classes in detail below.
Closing gaps in the epidermis: circumferential cables during wound healing, dorsal closure and salivary gland invagination
The first actomyosin cables to be described were identified during wound healing of embryonic epidermis and termed ‘actin purse-strings’14(Fig. 1A). These purse-strings quickly assemble in cells facing a wound, creating a taut wound edge through centripetal tension. This tension, in conjunction with other mechanisms, is important for successful wound closure.13,15 Re-organization of cell surface receptors such as Cadherins as well as upstream modulators of cytoskeletal behavior such as cdc42 are likely to be involved in directing actomyosin cable assembly at the wound edge.15,16
The morphogenetic process of dorsal closure in fly embryos closely resembles wound healing (Fig. 1B). During this process the embryonic epidermis moves in to cover a region of the embryo previously covered by a layer of extra-embryonic tissue. An actomyosin cable or purse-string forms at the leading edge of the ingressing epidermis and, in combination with further mechanisms, ensures wild-type closure.17,18 Differential expression of the homophilic adhesion molecule Echinoid between the extra-embryonic tissue and the epidermis, leading to its absence from the leading edge, as well as the anisotropic distribution of factors involved in planar cell polarity, such as Frizzled or Flamingo, are upstream activators of the accumulation of actin and myosin at the leading edge.12,19
We have shown recently that a circumferential actomyosin cable that exerts centripetal force is part of the machinery driving the invagination of a tubular organ, the salivary glands, in the Drosophila embryo (Fig. 1C).8 Though resembling both wound healing and dorsal closure, in this case the cable is assembled not by the cells on the outside that are moving in, but by the inside cells at the boundary of the tissue that is specified to form the tubes.20 Differential expression of the transmembrane protein Crumbs, a protein important for epithelial polarity that also has the ability to engage in homophilic interactions between cells,8,21,22 leads to its anisotropic distribution at the boundary of the salivary gland placode: Crumbs concentration is strongly reduced in the membrane where the myosin cable accumulates. At the internal membranes of cells at the boundary Crumbs recruits a negative regulator of myosin activation, aPKC,23 thereby leading to accumulation of Rok that phosphorylates and activates myosin at the Crumbs-free edge (Fig. 1C).8
Despite the inverse topology of the position of the cable during gland formation and dorsal closure, Crumbs is also anisotropic in the leading edge cells during dorsal closure and it is likely to be anisotropic at wound edges. Thus, a common mechanisms involving Crumbs (and additional factors) might operate in all three situations.20
‘Static’ cables that maintain compartment identity
Relatively stable or ‘static’ actomyosin cables, that are under tension but do not processively constrict, serve another important developmental function: they help to maintain compartment identity and restrict the intermixing of cells of different fates contained within such compartments. Compartment boundaries can be found in various tissues during development in the fly, and include the parasegmental boundaries present during embryogenesis (Fig. 1D) and the dorso-ventral (DV) and anterio-posterior (AP) boundaries in the larval wing disc (Fig. 1E). Compartment boundaries are also found in vertebrates, in particular during brain development, where they form between rhombomeres (Fig. 1F), and as the mid-hindbrain boundary and the zonula limitans intrathalamica (for reviews see:24,25). Studies in the fly embryo have shown that the tension generated by actomyosin cables at parasegmental boundaries leads to a smooth appearance of the boundary. This tension provides a mechanism by which events that challenge the boundary, such as cell division close to it, can be rectified and this ensures that correct compartment identity can be maintained (Fig. 1D).26 Whether vertebrate compartment boundaries between rhombomeres also employ actomyosin cables to restrict cell movement is still to be determined, but, in support if this idea, regional differences in F-actin and phosphorylated myosin II can be observed between central rhombomere cells and the boundary cells in the chick.27
In Drosophila, both in the embryo at the parasegment boundary a well as at the DV boundary in the wing disc, the boundary cable is formed by the accumulation of actomyosin in each row of cells on either side of the boundary. Although the upstream signals in the embryo are still unclear, Notch signaling has been shown to be the upstream activator of cable formation at the DV boundary in the larval wing disc (Table 1).11 Notch signaling is translated into a depletion of Bazooka/Par-3 at the boundary, which is thought to direct actomyosin cable assembly (see below).
Table 1. Upstream signals and molecular anisotropies leading to actomyosin cable formation.
| Tissue/process | Upstream signal | Molecular asymmetry/anisotropy | Refs. |
|---|---|---|---|
| wound healing (Drosophila) |
H2O2/loss of contact |
bazooka, Cadherin ?, Crumbs?, Echinoid? |
13
,
15
|
| dorsal closure (Drosophila) |
Dpp, JNK, Wg, Notch signaling |
Echinoid, Flamingo, Frizzled, Canoe, Dlg, Crumbs? |
12
,
19
|
| salivary gland tubulogenesis (Drosophila) |
hairy, hkb, fkh activated transcription |
Crumbs, aPKC, Rok |
8
,
32
|
| parasagment boundaries (Drosophila) |
Wg, Hh signaling |
unknown |
26
|
| wing disc DV boundary (Drosophila) |
Notch, Wg signaling |
Baz |
11
|
| rhombomere/ventricle formation (chick) |
unknown |
unknown |
– |
| germ band extension (Drosophila) |
pair rule gene expression/ A-P patterning |
Rok, Baz, Cadherin, Arm |
10
|
| tracheal pit invagination (Drosophila) |
EGF signaling |
p-ERK |
29
|
| kidney tubule extension (zebrafish) |
PCP: Wnt signaling |
unknown |
9
|
| neural plate extension/folding (chick) |
PCP: ? | Celsr1/Flamingo, DAAM, ROCK1 | 30 |
Actomyosin cables might therefore provide a general mechanism to restrict cells into defined compartments during development, although upstream signals will differ depending on the context.
Short cables as aids to epithelial morphogenesis
In contrast to the persistent cables observed at compartment boundaries, shorter and very dynamic actomyosin cables appear to be crucial to aid cell rearrangements within epithelial sheets during morphogenesis. During germ band extension (GBE) in the fly embryo, the length of the embryo doubles while its width halves through a process of cell convergence and tissue extension. During GBE, alignment of actomyosin into many cables perpendicular to the axis of extension can be observed.28 These actomyosin cables, extending over the aligned vertical junctions of ~3–6 cells, are under increased tension and pull cells linked to the cable into so called ‘rosettes’ that subsequently resolve perpendicular to the axis of formation, thus contributing to the change in tissue shape (Fig. 1G). The orientation of these short cables perpendicular to the axis of extension involves a mechanism whereby Rho-kinase directs Bazooka/Par-3 enrichment in horizontal edges and excludes it from vertical ones. Bazooka anisotropy in turn leads to myosin planar polarization into vertical edges and cables (Table 1), i.e., perpendicular to the AP axis.10 Dynamic myosin alignments spanning a few cells have also been observed within invaginating tracheal pits, where they help cells to align around the invagination site. These small cables are induced by differences in EGF-receptor signaling, with myosin accumulating at the membrane where levels of signaling, and thus downstream phosphorylation of ERK, change.29
Rosette formation and resolution appears to be a conserved morphogenetic ‘motor’ that drives convergent-extension not only in flies but also in vertebrate tissues. During the (convergent) extension of kidney tubules in both mouse and zebrafish, multicellular rosettes can frequently be observed. Molecular and live analysis in zebrafish has now revealed that actomyosin is enriched in cable-like structures and is necessary for rosette-formation and tubule extension (Fig. 1H).9 Myosin enrichment in cables that run perpendicular to the long tissue axis is also observed during neural tube closure and convergent extension in the chick.30 It has been proposed that the contraction of these myosin cables assists both the bending of the neural tube, but also drives tissue convergence and extension, partly through formation of multicellular rosettes (Fig. 1I). The signal that localizes myosin anisotropically in the chick neural tube is a planar polarized Cadherin known to be involved in planar cell polarity.
Thus, short actomyosin cables serve important morphogenetic functions that are conserved from flies to vertebrates.
Conclusion
The numerous examples of multicellular actomyosin cables, and the variation in their dynamic behavior (in terms of the processes they are involved in) and also scale (in terms of the number of participating cells), suggests that these cables form a basic machinery that organizes epithelial cell behavior in a tissue context. Common themes have emerged from the analysis of different actomyosin cables. One theme is that they are used as transient boundaries and local force generators; a second is that the cue for their assembly always involves the local anisotropy of upstream membrane-associated components (either peripheral or transmembrane; see Table 1). These anisotropies might be upstream of a molecular chain of events that drives cable assembly in a particular part of the cell, but they could also locally change membrane properties such as cortical tension to further assist localized anisotropic actomyosin assembly (similar feed-back from tension has been observed in other contexts, see31). In either case, the localization of the cable with respect to the cells in which its tensile properties are active seems to be strongly associated with its role in the tissue. Circumferential actomyosin cables that are likely to result in centripetal tension are assembled only on one side of the boundary or edge (wounds, dorsal closure and salivary gland tube formation), whereas linear cables acting as barriers or pulling junctions exert line tension and, where this has been analyzed, are assembled in cells on both sides of the boundary.
Many questions as to the molecular events leading to cable formation remain. Furthermore, many more sites of actomyosin cable function are likely to be discovered. Looking more closely in the fly embryo, live time-lapse analysis of the ventral embryonic epidermis during late embryogenesis using myosin-GFP reveals many previously uncharacterised sites of actomyosin cables (Fig. 2). Analysis of actomyosin distribution in flies is straightforward, nonetheless these and possibly other actomyosin cables remain unexplored. This suggests that in model organisms where the analysis is much more complex, such as in chick or mouse, we are likely to identify further functions for multicellular actomyosin assemblies in the future.
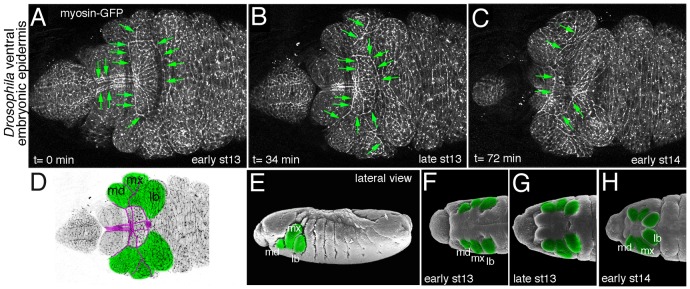
Figure 2. A multitude of actomyosin cables during morphogenesis of the ventral epidermis of the fly embryo. (A-C) Still images of a time-lapse move showing myosin-GFP in the ventral anterior epidermis during major epithelial morphogenetic movement occurring concomitant with the process of head involution (early stage 13 to early stage 14 of embryogenesis). Green arrows point to many actomyosin cables observed during this process. (D) Schematic of late stage 13 epidermis with the maximillar, mandibular and labial segments indicated. Magenta lines trace the actomyosin cables observed at this time point. (E-H) Illustrated scanning electron micrographs of the ventral epidermis corresponding to the images in (A-C) [modified from Flybase (www.flybase.org); Images from F.R. Turner. Personal Communication to Flybase, 1995]. Mandibular, maximillar and labial segments are marked in green. Anterior is to the left in all images, all panels show ventral views apart from (E) which shows a lateral view.
Acknowledgments
The author thanks members of the Röper lab for helpful comments on the manuscript and would like to apologise to all colleagues whose work could not be cited or discussed in sufficient depth due to space limitations. K.R. is supported by the Medical Research Council (MRC file reference number U105178780).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/BioArchitecture/article/25339
References
- 1.Hartman MA, Spudich JA. The myosin superfamily at a glance. J Cell Sci. 2012;125:1627–32. doi: 10.1242/jcs.094300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levayer R, Lecuit T. Biomechanical regulation of contractility: spatial control and dynamics. Trends Cell Biol. 2012;22:61–81. doi: 10.1016/j.tcb.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol. 2010;11:633–43. doi: 10.1038/nrm2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baum B, Georgiou M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J Cell Biol. 2011;192:907–17. doi: 10.1083/jcb.201009141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bulgakova NA, Klapholz B, Brown NH. Cell adhesion in Drosophila: versatility of cadherin and integrin complexes during development. Curr Opin Cell Biol. 2012;24:702–12. doi: 10.1016/j.ceb.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borghi N, James Nelson W. Intercellular adhesion in morphogenesis: molecular and biophysical considerations. Curr Top Dev Biol. 2009;89:1–32. doi: 10.1016/S0070-2153(09)89001-7. [DOI] [PubMed] [Google Scholar]
- 7.Smutny M, Cox HL, Leerberg JM, Kovacs EM, Conti MA, Ferguson C, et al. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat Cell Biol. 2010;12:696–702. doi: 10.1038/ncb2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Röper K. Anisotropy of Crumbs and aPKC drives myosin cable assembly during tube formation. Dev Cell. 2012;23:939–53. doi: 10.1016/j.devcel.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lienkamp SS, Liu K, Karner CM, Carroll TJ, Ronneberger O, Wallingford JB, et al. Vertebrate kidney tubules elongate using a planar cell polarity-dependent, rosette-based mechanism of convergent extension. Nat Genet. 2012;44:1382–7. doi: 10.1038/ng.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simões SdeM, Blankenship JT, Weitz O, Farrell DL, Tamada M, Fernandez-Gonzalez R, et al. Rho-kinase directs Bazooka/Par-3 planar polarity during Drosophila axis elongation. Dev Cell. 2010;19:377–88. doi: 10.1016/j.devcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Major RJ, Irvine KD. Localization and requirement for Myosin II at the dorsal-ventral compartment boundary of the Drosophila wing. Dev Dyn. 2006;235:3051–8. doi: 10.1002/dvdy.20966. [DOI] [PubMed] [Google Scholar]
- 12.Chang LH, Chen P, Lien MT, Ho YH, Lin CM, Pan YT, et al. Differential adhesion and actomyosin cable collaborate to drive Echinoid-mediated cell sorting. Development. 2011;138:3803–12. doi: 10.1242/dev.062257. [DOI] [PubMed] [Google Scholar]
- 13.Wood W, Jacinto A, Grose R, Woolner S, Gale J, Wilson C, et al. Wound healing recapitulates morphogenesis in Drosophila embryos. Nat Cell Biol. 2002;4:907–12. doi: 10.1038/ncb875. [DOI] [PubMed] [Google Scholar]
- 14.Martin P, Lewis J. Actin cables and epidermal movement in embryonic wound healing. Nature. 1992;360:179–83. doi: 10.1038/360179a0. [DOI] [PubMed] [Google Scholar]
- 15.Abreu-Blanco MT, Verboon JM, Liu R, Watts JJ, Parkhurst SM. Drosophila embryos close epithelial wounds using a combination of cellular protrusions and an actomyosin purse string. J Cell Sci. 2012;125:5984–97. doi: 10.1242/jcs.109066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin P, Parkhurst SM. Parallels between tissue repair and embryo morphogenesis. Development. 2004;131:3021–34. doi: 10.1242/dev.01253. [DOI] [PubMed] [Google Scholar]
- 17.Jacinto A, Wood W, Woolner S, Hiley C, Turner L, Wilson C, et al. Dynamic analysis of actin cable function during Drosophila dorsal closure. Curr Biol. 2002;12:1245–50. doi: 10.1016/S0960-9822(02)00955-7. [DOI] [PubMed] [Google Scholar]
- 18.Hutson MS, Tokutake Y, Chang MS, Bloor JW, Venakides S, Kiehart DP, et al. Forces for morphogenesis investigated with laser microsurgery and quantitative modeling. Science. 2003;300:145–9. doi: 10.1126/science.1079552. [DOI] [PubMed] [Google Scholar]
- 19.Kaltschmidt JA, Lawrence N, Morel V, Balayo T, Fernández BG, Pelissier A, et al. Planar polarity and actin dynamics in the epidermis of Drosophila. Nat Cell Biol. 2002;4:937–44. doi: 10.1038/ncb882. [DOI] [PubMed] [Google Scholar]
- 20.Thompson BJ, Pichaud F, Röper K. Sticking together the Crumbs - an unexpected function for an old friend. Nat Rev Mol Cell Biol. 2013;14:307–14. doi: 10.1038/nrm3568. [DOI] [PubMed] [Google Scholar]
- 21.Fletcher GC, Lucas EP, Brain R, Tournier A, Thompson BJ. Positive feedback and mutual antagonism combine to polarize Crumbs in the Drosophila follicle cell epithelium. Curr Biol. 2012;22:1116–22. doi: 10.1016/j.cub.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Zou J, Wang X, Wei X. Crb apical polarity proteins maintain zebrafish retinal cone mosaics via intercellular binding of their extracellular domains. Dev Cell. 2012;22:1261–74. doi: 10.1016/j.devcel.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishiuchi T, Takeichi M. Willin and Par3 cooperatively regulate epithelial apical constriction through aPKC-mediated ROCK phosphorylation. Nat Cell Biol. 2011;13:860–6. doi: 10.1038/ncb2274. [DOI] [PubMed] [Google Scholar]
- 24.Monier B, Pélissier-Monier A, Sanson B. Establishment and maintenance of compartmental boundaries: role of contractile actomyosin barriers. Cell Mol Life Sci. 2011;68:1897–910. doi: 10.1007/s00018-011-0668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Umetsu D, Dahmann C. Compartment boundaries: Sorting cells with tension. Fly (Austin) 2010;4:241–5. doi: 10.4161/fly.4.3.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monier B, Pélissier-Monier A, Brand AH, Sanson B. An actomyosin-based barrier inhibits cell mixing at compartmental boundaries in Drosophila embryos. Nat Cell Biol. 2010;12:60–5, 1-9. doi: 10.1038/ncb2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filas BA, Oltean A, Majidi S, Bayly PV, Beebe DC, Taber LA. Regional differences in actomyosin contraction shape the primary vesicles in the embryonic chicken brain. Phys Biol. 2012;9:066007. doi: 10.1088/1478-3975/9/6/066007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blankenship JT, Backovic ST, Sanny JS, Weitz O, Zallen JA. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev Cell. 2006;11:459–70. doi: 10.1016/j.devcel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Nishimura M, Inoue Y, Hayashi S. A wave of EGFR signaling determines cell alignment and intercalation in the Drosophila tracheal placode. Development. 2007;134:4273–82. doi: 10.1242/dev.010397. [DOI] [PubMed] [Google Scholar]
- 30.Nishimura T, Honda H, Takeichi M. Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell. 2012;149:1084–97. doi: 10.1016/j.cell.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez-Gonzalez R, Simoes SdeM, Röper JC, Eaton S, Zallen JA. Myosin II dynamics are regulated by tension in intercalating cells. Dev Cell. 2009;17:736–43. doi: 10.1016/j.devcel.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myat MM, Andrew DJ. Epithelial tube morphology is determined by the polarized growth and delivery of apical membrane. Cell. 2002;111:879–91. doi: 10.1016/S0092-8674(02)01140-6. [DOI] [PubMed] [Google Scholar]