Abstract
Background
Monocytes transmigrating to the brain play a central role in HIV neuropathology. We hypothesized that the continued existence of neurocognitive impairment (NCI) despite potent antiretroviral (ARV) therapy is mediated by the inability of such therapy to control this monocyte/macrophage reservoir.
Methods
Cross-sectional and longitudinal analyses were conducted within a prospectively enrolled cohort. We devised a monocyte efficacy (ME) score based on the anticipated effectiveness of ARV medications against monocytes/macrophages using published macrophage in vitro drug efficacy data. We examined, within an HIV neurocognitive database, its association with composite neuropsychological test scores (NPZ8) and clinical cognitive diagnoses among subjects on stable ARV medications unchanged for >6 months prior to assessment.
Results
Among 139 subjects on ARV therapy, higher ME score correlated with better NPZ8 performance (r=0.23, P<0.01), whereas a score devised to quantify expected penetration effectiveness of ARVs into the brain (CPE score) did not (r=0.12, P=0.15). In an adjusted model (adjusted r2=0.12), ME score (β=0.003, P=0.02), CD4+ T-cell nadir (β=0.001, P<0.01) and gender (β=−0.456, P=0.02) were associated with NPZ8, whereas CPE score was not (β=0.003, P=0.94). A higher ME score was associated with better clinical cognitive status (P<0.01). With a range of 12.5–433.0 units, a 100-unit increase in ME score resulted in a 10.6-fold decrease in the odds of a dementia diagnosis compared with normal cognition (P=0.01).
Conclusions
ARV efficacy against monocytes/macrophages correlates with cognitive function in HIV-infected individuals on ARV therapy within this cohort. If validated, efficacy against monocytes/macrophages may provide a new target to improve HIV NCI.
Introduction
The frequency of neurocognitive impairment (NCI) in HIV-infected individuals remains high despite the availability of potent antiretroviral (ARV) therapy [1]. NCI is identified among individuals lacking significant confounding factors for cognitive dysfunction, leading many to consider that ARV therapy is insufficient to eradicate the cognitive effect of HIV [2–4]. One emphasized hypothesis for the inadequacy of therapy relates to the variable ability of individual ARV medications to penetrate the central nervous system (CNS). The CNS penetration-effectiveness score (CPE), which rates ARV regimens based on CNS penetration and efficacy parameters, has been studied as a surrogate marker for brain concentrations of ARV drugs [5]. Studies that have assessed the value of CNS penetrating drugs have, however, shown mixed results, with some studies reporting an association between use of such penetrating drugs and less NCI [6,7] or lower cerebrospinal fluid (CSF) viral load [5], while others have reported no association [8,9], less conclusive results with associations only with use of >3 ARV drugs [10] or have reported that clinical status at time of commencing therapy may substantially confound retrospective analyses [11]. In one published randomized study designed to evaluate the strategy of increasing CPE, ARVs with good CNS penetration were more effective in controlling CSF viral replication, but were associated with poorer neurocognitive performance [12]. The lack of clarity in such clinical studies provides reason to consider alternative mechanistic indicators of the effectiveness of ARV therapy on HIV NCI.
A compelling alternative but not mutually exclusive hypothesis relates to the inability of potent ARV therapy to sufficiently suppress virus within circulating monocytes and brain macrophages. Although not universally accepted, it has been hypothesized that this cell lineage may be infected either in the bone marrow or blood and that these cells play a central role in HIV-related neurological inflammation and in seeding the CNS with virus. In contrast to HIV-infected CD4+ T-lymphocytes, which are rapidly killed by HIV, cells of the monocyte lineage are resistant to the cytopathic effects of HIV [13]. It has been hypothesized that upon infection with HIV, monocytes express an activated phenotype, traffic to brain tissue and, as macrophages, produce inflammatory molecules and seed the brain with virus, supporting a continued cycle of CNS immune activation and inflammation [14,15]. Studies by our centre (Hawaii Center for AIDS, University of Hawaii, Honolulu, HI, USA) linking NCI to higher levels of HIV DNA within circulating monocytes (CD14+ cells) are consistent with this hypothesis [16,17].
Several in vitro studies have demonstrated varying efficacies of existing ARV drugs against HIV infection in macrophages [18,19]. Building on the hypothesis that the reservoir of HIV-infected monocytes/macrophages is important in the pathogenesis of NCI, we further hypothesized that better ARV efficacy in this cell lineage, whether as monocytes peripherally or as macrophages in brain, would correlate with less HIV infection within these cells and with better cognitive function. We devised a monocyte efficacy (ME) score based on in vitro data on the effectiveness of ARV medications against macrophages and we demonstrate, within the Hawaii Aging with HIV Cohort study (HAHC), that individuals on ARV therapy with higher ME scores are less likely to have NCI.
Methods
Subject selection
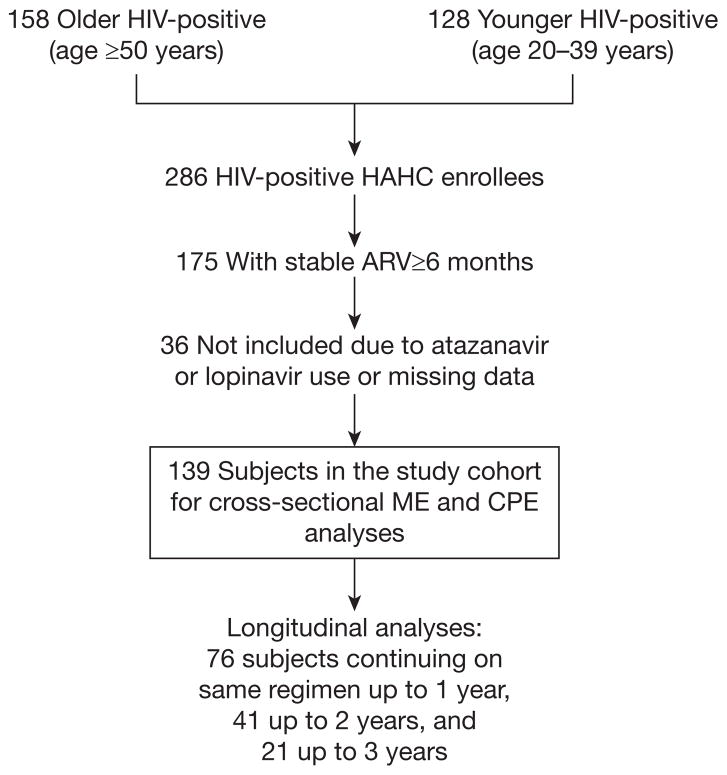
The analyses utilized baseline (entry) and longitudinal data from the HAHC study, a longitudinal cohort study assessing cognitive and neurological outcomes in older compared with younger HIV-infected individuals [20]. Briefly, 158 older (≥50 years of age) and 128 younger (20–39 years of age) HIV-infected individuals were recruited between October 2001 and October 2005 and followed up to July 2006. By study design, the HAHC did not recruit individuals who were 40–49 years of age. Exclusion criteria included major neurological or psychiatric illness, learning disability, major head injury, brain opportunistic infection and primary language other than English. Baseline and annual evaluations included demographic data, medical/medication/substance abuse histories including ARV history, neurological examination, neuropsychological testing and HIV laboratory parameters. The study was approved by the University of Hawaii Committee on Human Studies and informed consents were obtained from all subjects.
For the purposes of this analysis, the older and younger groups were combined, and individuals on stable ARV regimens unchanged for ≥6 months were selected (Figure 1). We excluded subjects with missing data and those on atazanavir or lopinavir due to lack of in vitro efficacy data in macrophages for these ARVs, resulting in a study cohort of 139 subjects used for the main analyses of the effect of ME and CPE on cognitive function. Study cohort subjects who continued on the same ARV therapy for a duration of ≥1 year and up to 3 years were used for the longitudinal analyses.
Figure 1.
Study schema
ARV, antiretroviral; CPE, central nervous system penetration effectiveness; HAHC, Hawaii Aging with HIV Cohort; ME, monocyte efficacy.
Cognitive outcome variables
All individuals completed a standardized battery of neuropsychological tests as previously described [20]. In this study, we utilized the NPZ8, a summary measure of z-scores of neuropsychological testing performance. The NPZ8 score was defined as the average of z-scores for the following tests: timed gait, Grooved Pegboard dominant hand, Grooved Pegboard non-dominant hand, Trail Making Test parts A and B, the Digit Symbol subtest from the WAIS-R, and the Choice and Sequential Reaction Time trials from the CalCap test battery. The z-score by definition has a mean in the general population of 0 with −1.0 and 1.0 representing 1 standard deviation below and above the mean, respectively.
In addition, we assessed the value of the ME score using cognitive diagnostic categorizations as determined by a consensus conference involving two neurologists, two neuropsychologists and a geriatrician in collaboration with Johns Hopkins University as previously published [20] using the American Academy of Neurology 1991 criteria [21]. These cognitive diagnostic categorizations provided an additional dimension by considering the patient’s functional status. Due to cohort enrolment dates, methodology used for clinical cognitive determination employed the older American Academy of Neurology 1991 classification and categorized subjects as having normal cognition (NC), mild cognitive motor disorder (MCMD) or HIV-associated dementia (HAD); however, these designations do not differ greatly from the currently employed schema defined in 2007 [22]. In general, the diagnosis of HAD required marked testing abnormality in ≥2 cognitive domains and the presence of some functional impairment associated with cognitive symptoms, whereas MCMD required a lesser degree of cognitive and functional impairment. For subjects with neuropsychological testing abnormalities but without functional impairment, the designation of neuropsychologically abnormal (NP abnormal) was used, in a manner similar to the methodology of asymptomatic neurocognitive impairment in the 2007 criteria [22]. All other subjects were deemed to have NC. Full details are published elsewhere [20].
Monocyte efficacy and central nervous system penetration effectiveness scores
We defined the ME scores for each ARV regimen as the summed reciprocal score (×1,000) of each component ARV’s median effective concentration (EC50) using the acute infection model in resting macrophages as summarized by Gavegnano and Schinazi [18] (Table 1). Information on EC50 (acute infection) values for some ARV drugs (atazanavir and lopinavir) utilized by study subjects were not available. Therefore, subjects utilizing these ARV drugs were not included in the analyses. The unpublished EC50 (acute infection) value for emtricitabine was provided by the laboratory of RFS. This allowed us to include in the ME score analyses a sizeable number of subjects who were on Truvada (fixed-dose tenofovir and emtricitabine). Ritonavir was not considered in the ME score when used in a low dose since its primary function was to boost plasma concentrations of the protease inhibitors in the regimen. The ME value used for fosamprenavir was that of amprenavir because fosamprenavir is a prodrug of amprenavir. The ME value of saquinavir was used for both hard-gel saquinavir (Invirase®, F Hoffman–La Roche Ltd, Basel, Switzerland) with low-dose ritonavir, and for soft-gel saquinavir (Fortovase®, F Hoffman–La Roche Ltd).
Table 1.
Published in vitro EC50 acute infection values for various ARV drugs in primary macrophage cell cultures and the calculated ME scores
| ARV drug | Acute infection in macrophages EC50, nM | ME scorea |
|---|---|---|
| NRTI | ||
| Abacavir sulfate | 300 | 3 |
| Didanosine | 50 | 20 |
| Emtricitabineb | 80 | 12.5 |
| Lamivudine | 20 | 50 |
| Stavudine | 240 | 4 |
| Tenofovir disoproxil fumarate | 20 | 50 |
| Zalcitabine | 3 | 333 |
| Zidovudine | 20 | 50 |
| NNRTI | ||
| Delavirdine | 10 | 100 |
| Efavirenz | 10 | 100 |
| Nevirapine | 50 | 20 |
| Protease inhibitor | ||
| Amprenavirc | 10 | 100 |
| Indinavir | 60 | 17 |
| Nelfinavir | 80 | 12.5 |
| Ritonavir | 120 | 8.3 |
| Saquinavir | 50 | 20 |
| Fusion inhibitor | ||
| Enfuvirtide | 20 | 50 |
(1/median effective concentration [EC50])×1,000.
Unpublished data from the laboratory of RFS.
Amprenavir values used as an surrogate for fosamprenavir, a prodrug of amprenavir. Adapted with permission from [18].
ARV, antiretroviral; ME, monocyte efficacy; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor.
We utilized the 2010 revised CNS penetration score as published by Letendre et al. [23] for the CPE analyses. In secondary analyses, we also evaluated the performance of the older three-tier CPE score because this has been more widely employed historically [5].
Statistical methods
We evaluated the relationships between various parameters of interest (ME score, CPE score, nadir CD4+ T-cell count, current CD4+ T-cell count and peripheral blood mononuclear cell [PBMC] log [HIV DNA]) using Pearson’s correlations. The nadir CD4+ T-cell count was obtained by patient report, previously validated to concur with historical records (r=0.90) [24]. We utilized multiple regression to assess the significance of ME and CPE scores at baseline after adjusting for confounders, with NPZ8 as the outcome. If additional variables affected either ME score or CPE score by ≥10%, such variables were considered confounding variables and were included in the model. Additional analyses were performed to determine whether adjusting for PBMC HIV DNA levels would improve the prediction model. To inform real-life importance of this finding among patients maximally treated, multiple linear regression analyses were repeated in a subpopulation of subjects with undetectable plasma HIV RNA, defined as a single measurement <50 copies/ml at entry visit.
We evaluated the distribution of ME scores and CPE scores by clinical cognitive status (NC, NP abnormal, MCMD and HAD) by Kruskal–Wallis test. We also completed pairwise comparisons between any two groups using the Wilcoxon rank-sum test with Bonferroni correction. In addition, the association between ME score and clinical cognitive status was analysed by multinomial logistic regression model where NC was the reference group.
Longitudinal analyses were performed to determine the relationship between ME score and NPZ8 over time among a subgroup of the study cohort who were on the same ARV at their annual follow-up visits 1–3 years following their entry visit into HAHC as shown in Figure 1. We utilized repeated measure analysis after assessment of the appropriate covariance structure to assess the ME effect where time was analysed both as a continuous or categorical variable.
Results
Cohort characteristics
The selection criteria resulted in a total of 139 subjects (59 younger and 80 older subjects) from a possible 286 enrollees into the HAHC (49%; Figure 1). Approximately two-thirds of the study cohort were virologically suppressed in plasma (Table 2). Although current CD4+ T-cell count was reasonably high, the median nadir CD4+ T-cell count was relatively low (150 cells/ml). Analyses of ARV therapy showed that 46.0% were on protease inhibitors and 58.3% on non-nucleoside reverse transcriptase inhibitors. Approximately 17% met research classification for HAD, while 37% had MCMD.
Table 2.
Patient characteristics
| Variable | Value (n=139) |
|---|---|
| Age | |
| Young, years | 37.3 (33.4–39.3) |
| Old, years | 53.7 (51.1–57.9) |
| Gender | |
| Male | 84.89 |
| Female | 15.11 |
| Education, years | 12 (12–16) |
| Ethnicity | |
| Caucasian | 61.15 |
| Asian/Pacific Islanders | 28.06 |
| Others | 10.79 |
| Log HIV RNA<50 copies/ml | 66.19 |
| Log HIV RNA in those with >50 copies/ml, copies/ml | 3.6 (2.6–4.7) |
| Current CD4+ T-cell count, cells/ml | 510.0 (318.0–647.0) |
| Nadir CD4+ T-cell count, cells/ml | 150 (50–300) |
| NPZ8 | −0.3 (−0.9–0.2) |
| Cognitive classification | |
| Normal | 20.14 |
| Neuropsycologically abnormal | 26.32 |
| Minor cognitive motor deficit | 36.69 |
| HIV-associated dementia | 16.55 |
| Estimated duration of HIV at entry, years | 9.6 (5.1–15) |
| Antiretroviral therapy | |
| Total on NRTI | 97.1 |
| Zidovudine | 45 |
| Stavudine | 29 |
| Didanosine | 6 |
| Tenofovir disoproxil fumarate | 12 |
| Abacavir | 24 |
| Lamivudine | 80 |
| Zalcitabine | 1 |
| Total on NNRTI | 58.3 |
| Efavirenz | 40 |
| Nevirapine | 15 |
| Delavirdine | 4 |
| Total on protease inhibitors | 46.0 |
| Nelfinavir | 20 |
| Ritonavira | 1 |
| Saquinavirb | 5 |
| Indinavir | 15 |
| Amprenavirc | 7 |
| Estimated duration of current ARV regimen prior to HAHC entry, years | 2.1 (1.0–3.6) |
| CPE | 7.0 (7.0–9.0) |
| Monocyte efficacy score | 120.0 (100.0–170.0) |
Data are presented as median and IQRs or as percentages.
On ritonavir in therapeutic doses and not in doses meant for boosting other protease inhibitors.
Combined total of individuals on Fortovase® (F Hoffman-La Roche Ltd., Basel, Switzerland) and hard-gel saquinavir with ritonavir boosting.
Combined total of individuals on amprenavir and fosamprenavir.
ARV, antiretroviral; CPE, central nervous system penetration effectiveness; HAHC, Hawaii Aging with HIV Cohort study; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor.
Effect of monocyte efficacy score and central nervous system penetration effectiveness score on NPZ8
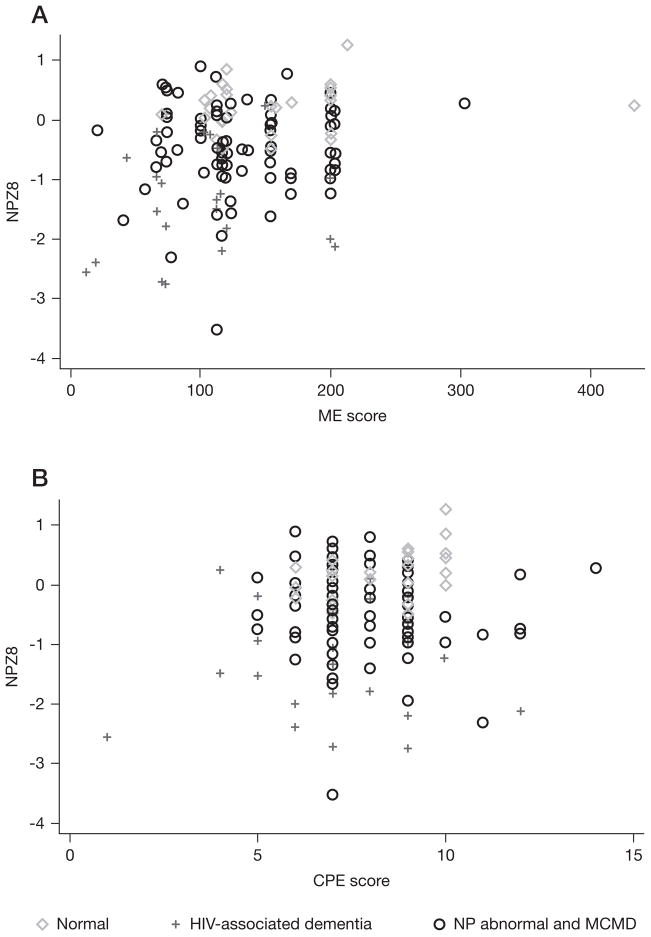
The calculated ME score for the study cohort ranged from 12.5 to 433.0 units with a mean of 133.2 and median [IQR] of 120 [100–170]. Using Pearson’s correlations, we identified an association between NPZ8 and ME score (r=0.23, P<0.01), as well as with nadir CD4+ T-cell count (r=0.24, P<0.01) and PBMC HIV DNA (r=−0.46, P<0.01), but not between NPZ8 and CPE score (r=0.12, P=0.15). A scatterplot of NPZ8 versus ME score and NPZ8 versus CPE score is depicted in Figure 2 with the extremes in clinical cognitive status (individuals with NC or those with HAD) specifically identified. A significant association was present between PBMC HIV DNA and the ME score (r=−0.24, P<0.01). Interestingly, the CPE score also correlated with the ME score (r=0.40, P<0.01) and with PBMC log HIV DNA (r=−0.18, P=0.03)
Figure 2.
Scatterplot of NPZ8 versus monocyte efficacy score and versus central nervous system penetration effectiveness score
(A) A significant correlation is present between NPZ8 and monocyte efficacy (ME) score but (B) not present between NPZ8 and central nervous system penetration effectiveness (CPE) score. MCMD, mild cognitive motor disorder; NP abnormal, neuropsychologically abnormal.
The predictive values of the ME score and CPE score were assessed using multiple linear regression with NPZ8 as the dependent variable and nadir CD4+ T-cell count and gender as significant confounders. These regression analyses demonstrated a significant predictive value of the ME score (β=0.003, P=0.02) as well as for gender (β=−0.456, P=0.02) and nadir CD4+ T-cell count (β=0.001, P=0.01), but not the CPE score (β=0.003, P=0.94), with the model explaining 11.8% of the variability in the NPZ8. By virtue of standardized z-score components, the NPZ8 is already adjusted for age and education. Adjusting for age grouping as ‘young’ versus ‘old’ did not alter the results of the analyses. Inclusion of the duration of current ARV therapy also did not change the results. In a model that included the PBMC HIV DNA, we noted that HIV DNA explained a substantial percentage (29.9%) of the variability in the NPZ8. As previously published, HIV DNA levels were associated with cognition [25]. In this model, PBMC HIV DNA was highly significant (β=−0.379, P<0.01) and the nadir CD4+ T-cell count (β=0.001, P=0.02) and gender (β=−0.590, P<0.01) remained significant; however the effect of the ME score was attenuated, and the P-value was no longer <0.05 (β=0.002, P=0.14). The CPE score remained non-significantly associated with NPZ8 (β=−0.019, P=0.62).
We repeated the Pearson’s correlations and multiple linear regression analyses among subjects with undetectable HIV RNA (<50 copies/ml; n=92) with similar results as presented above. Specifically, in these analyses, Pearson’s correlations showed moderate associations between NPZ8 and the ME score (r=0.24, P=0.02), CD4+ T-cell nadir (r=0.32, p<0.01) and PBMC HIV DNA (r=−0.43, P<0.01). An association between ME score and PBMC HIV DNA was also present (r=−0.22, P=0.04). Multiple linear regression analyses showed that ME score (β=0.004, P=0.03), nadir CD4+ T-cell count (β=0.002, P<0.01) and gender (β=−0.629, P<0.01) were significantly associated with the NPZ8. We did not identify an association between CPE score and the NPZ8 (β=−0.053, P=0.40). Using the older three-tiered score did not alter our findings (data not shown).
Effect of monocyte efficacy score and central nervous system penetration effectiveness score on clinical cognitive classification
Distributions of ME scores differed by clinical cognitive classification with a pattern of lesser ME score associated with more severe impairment (P=0.002; Figure 3A). Pairwise comparison by Wilcoxon rank-sum test demonstrated a significant difference in distribution of ME scores between NC and HAD (P=0.0004), whereas other comparisons between groups were not significant. A difference in distribution of CPE scores among the four cognitive classifications was also found (P=0.022); however, no significant pairwise comparison was found (Figure 3B).
Figure 3.
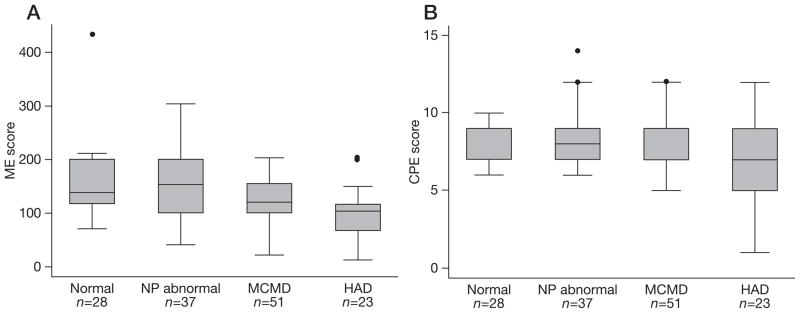
Monocyte efficacy and central nervous system penetration effectiveness scores by cognitive status
(A) Monocyte efficacy (ME) scores. (B) Central nervous system penetration effectiveness (CPE) scores. Outliers are shown as black circles. HAD, HIV-associated dementia; MCMD, minor cognitive motor disorder; NP abnormal, neuropsychologically abnormal.
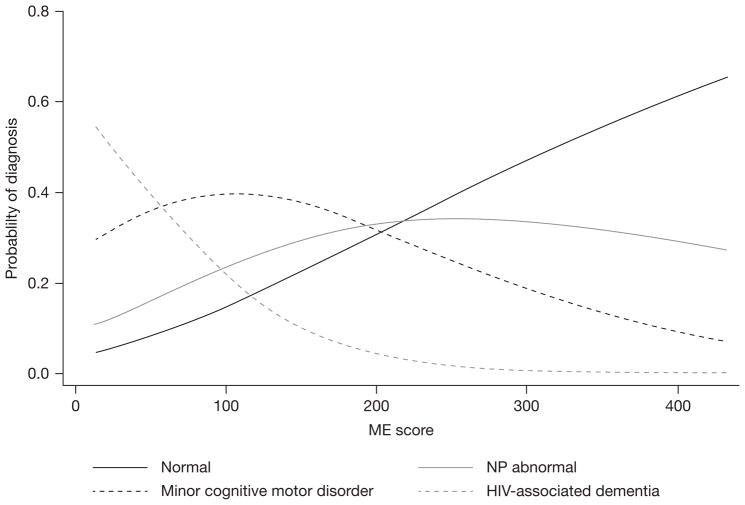
Analyses using multinomial logistic regression with clinical cognitive status as the primary outcome and the ME score as the predictor of interest demonstrated similar findings. Higher ME scores were associated with lower odds of being diagnosed with HAD (HAD versus NC OR 0.98; P<0.01) as well as lower odds of being diagnosed with MCMD (MCMD versus NC OR 0.99; P=0.04). The odds of being diagnosed with NP abnormal was not statistically different between higher and lower ME scores (NP abnormal versus NC, P=0.35). The estimated probability of each clinical diagnosis by ME score is shown graphically in Figure 4. This analysis suggests that a 100 unit increase in ME score results in a 10.63× decrease in the OR for HAD and a 2.61× decrease in the OR for MCMD.
Figure 4.
Probability of diagnosis of normal cognition, neuropsychologically abnormal, mild cognitive motor disorder and HIV-associated dementia by monocyte efficacy score
ME, monocyte efficacy; NP abnormal, neuropsychologically abnormal.
Longitudinal effect of monocyte efficacy score on NPZ8
Longitudinal analyses were performed in subjects who remained on the same ARV therapy as at entry to assess whether the association between the ME score and NPZ8 held over time. Treating time, that is, the year of visit, as a categorical variable, an interaction between year of visit and ME score was demonstrated (P=0.04) with estimated slopes of 0.0035 (baseline), 0.0035 (year 1), 0.0015 (year 2) and −0.0046 (year 3). Analyses performed with time as a continuous variable demonstrated no interaction with both ME score effect (P=0.01) and time effect (P=0.02) showing significance with magnitudes of 0.0031 and 0.0874, respectively. Thus, an association between NPZ8 and ME score was demonstrated to be present over a time duration of 3 years by either assumption.
Discussion
The results of this study suggest that the degree of effectiveness of ARV medications against HIV infection in monocytes, as assessed by in vitro assays in macrophages, is associated with cognitive impairment even among individuals with suppressed plasma HIV RNA. The results may provide insight into a potential mechanism of ongoing brain injury despite meeting standard of care treatment for HIV.
Our study was conceived based on the hypothesis that cells of the monocyte lineage are a protected reservoir for HIV and that these cells are central to the pathogenesis of NCI in the era of HAART. Most of the attention in the literature on reservoirs that support HIV replication has focused on lymphoid cell reservoirs. As a consequence, the role of myeloid cells, and in particular monocytes and macrophages, in viral replication has received far less attention. Although there is much evidence that tissue macrophages support viral replication in vivo, there is no consensus as to whether macrophages are a viral reservoir in patients on ARV therapy. A recent study from Deleage et al. [26] showed the presence of HIV in macrophages within seminal vesicles of patients on effective HAART. Similarly, there has been little focus on the extent to which circulating monocytes, the precursor to the tissue macrophage, are infected in patients on or off therapy and again, little consensus as to whether they constitute a viral reservoir in vivo [27,28]. However, recent studies from Spivak et al. [29] examined the frequency of infected monocytes in elite controllers. Although monocyte infection was undetectable in elite controllers, they reported infected monocytes in a few subjects on HAART. Collectively, these studies implicate monocyte and macrophage in viral persistence and additional studies are warranted in order to fully determine the extent to which myeloid lineage cells support viral persistence in the presence and absence of suppressive therapy.
The potential role of monocytes and macrophages in CNS disease is suggested by human autopsy studies, which have shown that there is continued CNS neuroinflammation with high numbers of macrophages in brain despite potent ARV therapy [30,31] and from studies in the simian model, which suggest that these macrophages originate from bone-marrow-derived monocytes that traffic through the bloodstream into the brain [14,32]. Studies by our group have further linked NCI to higher levels of HIV DNA within monocytes (CD14+ T-cells) in ARV-naive subjects as well as among subjects with suppressed plasma HIV RNA using typical ARV regimens [16,17]. As currently available ARV medications vary in their efficacy in macrophages, we hypothesized that the degree of effectiveness of these ARV medications in blood monocytes and tissue macrophages may relate to the degree of NCI in HIV-infected subjects on ARV therapy.
Our model made use of the in vitro EC50 values of ARVs measured using the ‘acute infection’ macrophage model published by Gavegnano and Schinazi [18] for correlation with neurocognitive end points. This assay methodology, which makes use of the standardized patient viral isolates M-R5 HIV-1BaL and M-R5 HIV-1SRA1433 macrophages from a pooled aggregate of donors, and hyperactivation using M-CSF, has typically produced an assay variability of approximately 5% in the laboratory of RFS. Specification of the model is important because EC50 values differ greatly between the ‘acute’ and ‘chronic’ in vitro macrophage models [19]. The terminology of ‘acute infection’ and ‘chronic infection’ used in published literature in reference to in vitro EC50 assays in macrophages is unfortunate as it does not equate to acute infection or chronic infection in the clinical sense. In the in vitro acute infection model, macrophages are incubated with drug prior to exposure to HIV. This model therefore is meant to provide information about how effective the drug is in preventing HIV infection of macrophages. By contrast, in the in vitro chronic infection model, macrophages are incubated with HIV prior to exposure to drug, providing theoretical information of whether HIV infection can be inhibited or eradicated after cells are already infected. Although both may be important in chronically infected patients, we hypothesized that prevention of monocytes/macrophages may be the central factor; hence our use of the acute infection in vitro EC50 data. It should also be pointed out that most ARVs, as a general rule, are ineffective in the in vitro chronic infection model, making EC50s from this chronic infection model impractical as a sole measure of efficacy. It is possible that a formula combining aspects of both the acute and chronic in vitro model may improve accuracy in estimating clinical efficacy.
The strong negative association between the ME score and the PBMC HIV DNA lends theoretical support that the ARV regimens with higher ME scores might affect cognition at least partially by preventing HIV infection of peripheral blood monocytes. Our study utilized HIV DNA within PBMCs as the only available data and not specifically within monocytes. However, PBMC HIV DNA appears to strongly correlate with HIV DNA values specifically within CD14+ T-cells when assessed in the context of cognitive impairment [17].
Our study also suggested a weak association between the CPE score and NCI, but only in the cognitive diagnoses and without pairwise differences between groups. This is not inconsistent with our finding that the ME score is associated with cognition as the two hypotheses are not mutually exclusive. Both hypotheses are based on scientifically sound principles that current ARV therapies do not sufficiently target reservoirs that are important to the CNS. The CPE hypothesis posits that ARV medications must be present in the CSF at levels that exceed the median inhibitory concentration as defined in plasma or in vitro models [5]. The inference is that these levels are reflective of effectiveness in CNS. The ME hypothesis similarly targets effectiveness of therapy but against monocytes/macrophages whether in the bloodstream or brain as the focus of interest, with the rationale that these cells upon transmigration to the CNS are the primary ongoing source for proinflammatory cytokines and viral replication responsible for synapto-dendritic injury and cognitive consequences [14]. Since the effect of ARV therapy on monocytes/macrophages in relation to CNS outcomes may also require that drugs adequately penetrate the CNS, it is plausible that the most effective treatments will be those that both minimize HIV intracellular reservoir in monocytes/macrophages and penetrate well into the CNS. It is conceivable that combining the elements of both the ME score and the CPE score into a single score may improve the predictive value above each score independently.
Together, our new data presented here and our past work related to intracellular HIV DNA provide a mechanistic framework for further investigation based on the hypothesis that current ARV therapy is not able to completely prevent HIV infection of monocytes/ macrophages and that this has relevance to NCI. HIV infection of these cells can be hypothesized to lead to an activated phenotype that would support increased monocyte transmigration, increased concentration of HIV infected macrophages in brain, immune activation and synapto-dendritic dysfunction resulting in cognitive compromise [14]. With persistent use, combinations of ARV medications with higher effectiveness in this reservoir may slowly decrease intracellular burden over time in this cell lineage by preventing new infections prior to leaving bone marrow, while circulating in the blood stream, or among macrophages and microglial cells in CNS tissue.
This study has some important limitations. The study entry criteria of stable ARV therapy ≥6 months was chosen based on the expected duration of time needed to result in maximal repression of plasma HIV RNA. However, this inclusion criteria, together with the lack of EC50 (acute infection) values for atazanavir and lopinavir resulted in exclusion of approximately half of the original HAHC population, which may have introduced bias into the study. Furthermore, the EC50 data comes from in vitro assays performed in macrophages and not blood monocytes and the ME score formula does not account for the unique pharmacodynamics/ kinetics of each drug. The HAHC study was conducted between 2001 and 2006, at a time when newer ARV medications such as etravirine, darunavir, maraviroc and raltegravir were not commercially available. Future studies will need to validate whether the efficacy of the ME score in predicting cognitive status continues to apply in an HIV-infected population on more current medications.
The results of this study are intriguing but require validation before its premises can be accepted as valid. If validated, however, our findings may have clinical significance for strategies on how to address the neurological complications of HIV. These findings may also have relevance to attempts to eradicate HIV. Recent review articles focus on clearance of HIV from the latent CD4+ T-cell infected reservoir; yet equal attention may be required for the monocyte/macrophage reservoir [33,34]. As these cells serve as primary antigen presenting cells for CD4+ T-lymphocytes and a primary source of new infection for these cells, control of monocyte/ macrophage infection may be a crucial part of reservoir eradication. Prospective studies designed to determine if ARV therapy with a higher ME score will have a superior effect on cognition may need to consider the extended half-life of activated cells of the monocyte lineage, estimated to be from months to years [35].
Acknowledgments
We thank our study participants and community physicians for their roles in this study.
This work was supported by NIH grants U54NS43049, U19MH081835, U54RR026136, R01NS053345 (BS), R01NS061696 (VGV), K23AG032872 (VGV), 2P30AI050409 (RFS) and the Department of Veterans Affairs (RFS).
Footnotes
Disclosure statement
The authors declare no competing interests.
References
- 1.Heaton RK, Clifford DB, Franklin DR, Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: mind the gap. Ann Neurol. 2010;67:699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- 4.Simioni S, Cavassini M, Annoni JM, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24:1243–1250. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- 5.Letendre S, Marquie-Beck J, Capparelli E, et al. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cysique LA, Vaida F, Letendre S, et al. Dynamics of cognitive change in impaired HIV-positive patients initiating antiretroviral therapy. Neurology. 2009;73:342–348. doi: 10.1212/WNL.0b013e3181ab2b3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tozzi V, Balestra P, Salvatori MF, et al. Changes in cognition during antiretroviral therapy: comparison of 2 different ranking systems to measure antiretroviral drug efficacy on HIV-associated neurocognitive disorders. J Acquir Immune Defic Syndr. 2009;52:56–63. doi: 10.1097/qai.0b013e3181af83d6. [DOI] [PubMed] [Google Scholar]
- 8.Giancola ML, Lorenzini P, Balestra P, et al. Neuroactive antiretroviral drugs do not influence neurocognitive performance in less advanced HIV-infected patients responding to highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2006;41:332–337. doi: 10.1097/01.qai.0000197077.64021.07. [DOI] [PubMed] [Google Scholar]
- 9.Tozzi V, Balestra P, Bellagamba R, et al. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr. 2007;45:174–182. doi: 10.1097/QAI.0b013e318042e1ee. [DOI] [PubMed] [Google Scholar]
- 10.Smurzynski M, Wu K, Letendre S, et al. Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS. 2011;25:357–365. doi: 10.1097/QAD.0b013e32834171f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garvey L, Winston A, Walsh J, et al. Antiretroviral therapy CNS penetration and HIV-1-associated CNS disease. Neurology. 2011;76:693–700. doi: 10.1212/WNL.0b013e31820d8b0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marra CM, Zhao Y, Clifford DB, et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS. 2009;23:1359–1366. doi: 10.1097/QAD.0b013e32832c4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho DD, Rota TR, Hirsch MS. Infection of monocyte/ macrophages by human T lymphotropic virus type III. J Clin Invest. 1986;77:1712–1715. doi: 10.1172/JCI112491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.González-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 15.Gras G, Kaul M. Molecular mechanisms of neuroinvasion by monocytes-macrophages in HIV-1 infection. Retrovirology. 2010;7:30. doi: 10.1186/1742-4690-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kusao I, Liang C-Y, Grove J, et al. Cognitive performance related to HIV-1-infected monocytes. J Neuropsychiatry Clin Neurosci. 2012;24:71–80. doi: 10.1176/appi.neuropsych.11050109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valcour VG, Shiramizu BT, Sithinamsuwan P, et al. HIV DNA and cognition in a Thai longitudinal HAART initiation cohort: the SEARCH 001 Cohort Study. Neurology. 2009;72:992–998. doi: 10.1212/01.wnl.0000344404.12759.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gavegnano C, Schinazi RF. Antiretroviral therapy in macrophages: implication for HIV eradication. Antivir Chem Chemother. 2009;20:63–78. doi: 10.3851/IMP1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perno CF, Svicher V, Schols D, Pollicita M, Balzarini J, Aquaro S. Therapeutic strategies towards HIV-1 infection in macrophages. Antiviral Res. 2006;71:293–300. doi: 10.1016/j.antiviral.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Valcour V, Shikuma C, Shiramizu B, et al. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology. 2004;63:822–827. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Academy of Neurology. Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection. Report of a Working Group of the American Academy of Neurology AIDS Task Force. Neurology. 1991;41:778–785. doi: 10.1212/wnl.41.6.778. [DOI] [PubMed] [Google Scholar]
- 22.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Letendre SL, Ellis RJ, Ances BM, McCutchan JA. Neurologic complications of HIV disease and their treatment. Top HIV Med. 2010;18:45–55. [PMC free article] [PubMed] [Google Scholar]
- 24.Valcour V, Yee P, Williams AE, et al. Lowest ever CD4 lymphocyte count (CD4 nadir) as a predictor of current cognitive and neurological status in human immunodeficiency virus type 1 infection –The Hawaii Aging with HIV Cohort. J Neurovirol. 2006;12:387–391. doi: 10.1080/13550280600915339. [DOI] [PubMed] [Google Scholar]
- 25.Shiramizu B, Williams AE, Shikuma C, Valcour V. Amount of HIV DNA in peripheral blood mononuclear cells is proportional to the severity of HIV-1-associated neurocognitive disorders. J Neuropsychiatry Clin Neurosci. 2009;21:68–74. doi: 10.1176/appi.neuropsych.21.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deleage C, Moreau M, Rioux-Leclercq N, Ruffault A, Jegou B, Dejucq-Rainsford N. Human immunodeficiency virus infects human seminal vesicles in vitro and in vivo. Am J Pathol. 2011;179:2397–2408. doi: 10.1016/j.ajpath.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almodóvar S, Del CCM, Maldonado IM, et al. HIV-1 infection of monocytes is directly related to the success of HAART. Virology. 2007;369:35–46. doi: 10.1016/j.virol.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Josefsson L, King MS, Makitalo B, et al. Majority of CD4+ T-cells from peripheral blood of HIV-1-infected individuals contain only one HIV DNA molecule. Proc Natl Acad Sci U S A. 2011;108:11199–11204. doi: 10.1073/pnas.1107729108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spivak AM, Salgado M, Rabi SA, O’Connell KA, Blankson JN. Circulating monocytes are not a major reservoir of HIV-1 in elite suppressors. J Virol. 2011;85:10399–10403. doi: 10.1128/JVI.05409-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Influence of HAART on HIV-related CNS disease and neuroinflammation. J Neuropathol Exp Neurol. 2005;64:529–536. doi: 10.1093/jnen/64.6.529. [DOI] [PubMed] [Google Scholar]
- 31.Zink MC, Brice AK, Kelly KM, et al. Simian immunodeficiency virus-infected macaques treated with highly active antiretroviral therapy have reduced central nervous system viral replication and inflammation but persistence of viral DNA. J Infect Dis. 2010;202:161–170. doi: 10.1086/653213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burdo TH, Soulas C, Orzechowski K, et al. Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog. 2010;6:e1000842. doi: 10.1371/journal.ppat.1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewin SR, Rouzioux C. HIV cure and eradication: how will we get from the laboratory to effective clinical trials? AIDS. 2011;25:885–897. doi: 10.1097/QAD.0b013e3283467041. [DOI] [PubMed] [Google Scholar]
- 34.Chomont N, El-Far M, Ancuta P, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montaner LJ, Crowe SM, Aquaro S, Perno CF, Stevenson M, Collman RG. Advances in macrophage and dendritic cell biology in HIV-1 infection stress key understudied areas in infection, pathogenesis, and analysis of viral reservoirs. J Leukoc Biol. 2006;80:961–964. doi: 10.1189/jlb.0806488. [DOI] [PubMed] [Google Scholar]